Abstract
Polyamines are cationic molecules synthesized via a highly regulated pathway, obtained from the diet or produced by the gut microbiota. They are involved in general molecular and cellular phenomena that play a role also in vascular disease. Bicuspid aortic valve (BAV) is a congenital malformation associated to a greater risk of thoracic ascending aorta (TAA) aneurysm, whose pathogenesis is not yet well understood. We focused on differential analysis of key members of polyamine pathway and on polyamine concentration in non-dilated TAA samples from patients with either stenotic tricuspid aortic valve (TAV) or BAV (diameter ≤ 45 mm), vs. normal aortas from organ donors, with the aim of revealing a potential involvement of polyamines in early aortopathy. Changes of gene expression in TAA samples were evaluated by RT-PCR. Changes of ornithine decarboxylase 1 (ODC1), a key enzyme in polyamine formation, and cationic amino acid transporter 1 (SLC7A1/CAT-1) expression were analyzed also by Western blot. ODC1 subcellular localization was assessed by immunohistochemistry. Polyamine concentration in TAA samples was evaluated by HPLC. BAV TAA samples showed an increased concentration of putrescine and spermidine vs. TAV and donor samples, together with a decreased mRNA level of polyamine anabolic enzymes and of the putative polyamine transporter SLC7A1/CAT-1. The catabolic enzyme spermidine/spermine N1-acetyltransferase 1 showed a significant mRNA increase in TAV samples only, together with a decreased concentration of spermine. The decreased expression of SLC7A1/CAT-1 and ODC1 mRNAs in BAV corresponded to increased or unchanged expression of the respective proteins. ODC was located mainly in smooth muscle cell (SMC) nucleus in TAV and donor samples, while it was present also in SMC cytoplasm in BAV samples, suggesting its activation. In conclusion, BAV, but not TAV non-dilated samples show increased polyamine concentration, accompanied by the activation of a regulatory negative feedback mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyamines (PAs) are small aliphatic cationic molecules synthesized via a highly regulated pathway in almost all living cells. Alternatively, cells may also, under certain conditions, e.g., when endogenous synthesis is inhibited, take up PAs from either the diet or PAs produced by gut microbiota [1]. The PAs putrescine (PUT), spermidine (SPD) and spermine (SPM) are best known for their cell proliferation promoting effect and for their influence on cell differentiation and apoptosis [2], but they regulate also diverse other functions at the levels of chromatin condensation, gene transcription, mRNA processing, stability and translation [3].
With particular reference to the vascular tissue in physiological and pathophysiological conditions, including aortopathy, PAs have been demonstrated to be involved in smooth muscle cell (SMC) proliferation, migration and phenotypic changes [4,5,6,7], as well as in vascular remodeling [8], in actin cytoskeleton dynamics [9] and in modulation of epithelial-to-mesenchymal transition (EMT) [10, 11]. A bridge is also emerging between PAs and epigenetic regulation of gene expression in different biological settings [12]. This is of particular interest in the context of aortopathy, where alterations in the epigenetic machinery are suggested to play a role in pathogenesis of thoracic ascending aorta (TAA) aneurysm [13, 14]. Aortopathy is mediated also by a mechanism of matrix remodeling, characterized by collagen alterations, in terms of fiber architecture, maturity and relative balance of type content. As a consequence, the potential relevance of the PA pathway in the context of aortopathy progression relies also on its tight connections with the production of nitric oxide (NO) from the common substrate arginine, and with the production of proline, a major component of collagen, from the common substrate ornithine (Fig. 1).
Main players of the polyamine pathway. Target molecules analyzed by RT-PCR, Western blot, immunohistochemistry and HPLC in this study are marked by an asterisk. AMD1 adenosylmethionine decarboxylase 1, Arg1 arginase 1, Arg2 arginase 2, dcSAM decarboxylated s-adenosylmethionine, eNOS nitric oxide synthase 3, NO nitric oxide, OAT ornithine aminotransferase, ODC1 ornithine decarboxylase 1, P5C pyrroline-5-carboxylate, PUT putrescine, PYCR1 pyrroline-5-carboxylate reductase 1, SAMe s-adenosylmethionine, SAT1 spermidine/spermine N1-acetyltransferase 1, SLC7A1/CAT-1 solute carrier family 7 member 1/cationic amino acid transporter 1, SMS spermine synthase, SPD spermidine, SPM spermine, SRM spermidine synthase
Bicuspid aortic valve BAV is the most common cardiovascular congenital malformation (0.5–2% of live births), associated with an increased risk for dilatation, aneurysm and dissection of TAA [15]. BAV aortopathy is characterized by heterogeneous clinical manifestations, and the debate on its pathogenesis, i.e., whether it is determined by genetics or by haemodynamics, has given way to the consideration that these two causative factors may not necessarily exclude each other [16]. Aortic wall remodeling processes may therefore be concomitantly affected by congenital defects and by an altered biomechanical environment secondary to the long-standing haemodynamic disorder.
Surprisingly, despite its potential involvement in pathophysiological alterations of the vascular wall, including those affecting matrix remodeling, SMC phenotype and secretory activity, the PA pathway has not been investigated so far in TAA aortopathy in TAV and/or BAV patients. Additionally, the large majority of previous studies on BAV aortopathy focused mainly on severe dilatations, and only recently an increasing number of studies are analyzing also non-dilated TAA samples from BAV/TAV patients, to catch the early mechanisms/alterations at the basis of aortopathy.
In this study, we aimed to investigate the differential expression and the subcellular localization of key molecules belonging to the PA pathway, as well as the differential PA concentration in non-dilated TAA samples collected from TAV and BAV cohorts of patients (aortic diameter ≤ 45 mm) submitted to elective surgery for replacement of stenotic aortic valve, compared with samples from organ donors without aortic disease.
Our results demonstrate alterations of PA concentration in BAV patients only, accompanied by the tentative activation of a regulatory feedback mechanism, indicating the involvement of PAs in the pathogenesis of BAV-associated aortopathy.
Methods
Patients
Aortic wall samples were collected from TAV and BAV patients with aortic valve stenosis undergoing valve replacement surgery and from heart transplant donors (“controls”) with negative personal and familial history of BAV and/or aortopathy.
The study was approved by the Ethics Committee of the Università della Campania “L. Vanvitelli”, Naples, Italy, and performed in accordance with the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
BAV morphology was defined by the raphe location among the left (L), right (R) or non-coronary (N) cusps. Aortic valve stenosis was echocardiographically defined and graded on the basis of morphological evaluation and Doppler-derived measurements of gradients and velocity. All patients had a severe echocardiographic degree of aortic valve stenosis. Mild dilatation of the ascending aorta, when present, was limited to the tubular ascending tract (ascending phenotype). Diameters at the root and ascending tract were measured by echocardiography at end-diastole by the leading-edge method.
The study included 41 patients with a stenotic TAV and with normal or mildly dilated ascending aorta (echocardiography-measured aortic diameter ≤ 45 mm), 50 patients with a stenotic congenital BAV (non-familial, non-syndromic) and with normal or mildly dilated aorta (echocardiography-measured maximal aortic diameter ≤ 45 mm), and 27 organ donors.
Clinical and demographic characteristics of the patients are summarized in Table 1. We selected only patients with predominant aortic valve stenosis because differences have been previously demonstrated in gene expression and aortic histology between BAV patients with valve stenosis vs. regurgitation [17, 18]. Patients with impaired systolic ventricular function, other cardiac diseases or aortic dissection were excluded.
Aortic wall sample collection
At surgery, an aortic sample was retrieved from each ascending aorta (41 TAV, 50 BAV), from the so-called convexity (CVX: greater curvature) region, 1–2 cm distal to the sino-tubular junction (from the ends of the transverse aortotomy in patients without dilatation). Samples were immediately stored in RNALater (Qiagen) or in RIPA buffer at − 80 °C for subsequent RNA or protein extraction, respectively, or in 4% buffered formaldehyde at room temperature for subsequent histological and immunohistochemical analysis.
RNA extraction and reverse-transcription-polymerase chain reaction analysis
Total RNA was extracted from whole aortic samples using the RNAeasy minikit (Qiagen). RNA was treated with DNase (Qiagen) to remove DNA contamination, its concentration was measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies) and its integrity was verified by electrophoresis on denaturing 1% agarose gel.
The absence of residual DNA was verified by PCR on total RNA without reverse transcription. GeneBank® sequences for human mRNAs and the Primer Express software (Applied Biosystems) were used to design primer pairs for the target genes (Table 2). Reverse-transcription-polymerase chain reaction (RT-PCR) experiments were performed as described previously [19], using semi-quantitative RT-PCR and real-time RT-PCR with SYBR Green for selected targets. RT-PCR targets comprised genes involved in polyamine anabolism [arginase 1 (ARG1), arginase 2 (ARG2), ornithine decarboxylase 1 (ODC1), adenosylmethionine decarboxylase 1 (AMD1)], transport [solute carrier family 7 member 1/cationic amino acid transporter 1 (SLC7A1/CAT-1)], polyamine catabolism [spermidine/spermine N1-acetyltransferase 1 (SAT1)], as well as nitric oxide synthesis [nitric oxide synthase 3 (eNOS)] and proline biosynthesis [ornithine aminotransferase (OAT)].
Protein extraction and Western blot detection of target proteins
Proteins were extracted from whole aortic samples by homogenization in RIPA buffer containing a protease inhibitor cocktail (Sigma-Aldrich). For each sample, 20 µg of proteins were separated in reducing conditions by 12% SDS-PAGE and transferred to PVDF membrane (Millipore).
Membrane was blocked for 1 h at room temperature with 5% powdered skim milk (Bio-Rad) in Tris-buffered saline pH 7.4 with 0.1% Tween (T-TBS). The membrane was then incubated with antibodies for ODC1 (#ab66067; dil. 1:500; Abcam), SLC7A1/CAT-1 (#HPA039721; dil. 1:200; Sigma-Aldrich) or GAPDH (#G8795; dil. 1:20.000; Sigma-Aldrich). Antibodies were diluted in T-TBS with 3% bovine serum albumin (BSA, Sigma-Aldrich) and incubated overnight at 4 °C. Blots were washed 3 times with T-TBS and incubated with HRP-conjugated anti-mouse secondary antibody (#sc-2005; dil. 1:4000; Santa Cruz Technology) in T-TBS with 3% BSA for 1 h at room temperature. Bound antibodies were detected through enhanced chemiluminescence (ECL) (Millipore). Bands were analyzed by densitometry, using the ChemiDoc Imaging system (Bio-Rad).
Immunohistochemical analysis
Aortic samples were fixed in 4% buffered formaldehyde, dehydrated and embedded in paraffin. Consecutive 5 μm cross-sections were analyzed. Antigen retrieval was done in a microwave through incubation in 10 mM Citrate buffer pH 6.0 (Dako). Blocking was done in 5% donkey serum (Jackson ImmunoResearch), followed by incubation with the primary antibody against anti-human mouse monoclonal ODC1 (#ab66067; dil. 1:500; Abcam) at 4 °C overnight. The primary antibody was diluted in 1% BSA. Endogenous peroxidases were blocked with 4% H2O2 in methanol. After washing, slides were incubated with biotin-conjugated secondary antibody (#sc-2039; dil. 1:200; Santa Cruz Technology) in 1% BSA for 1 h at room temperature. Staining was done through incubation with peroxidase streptoavidin (Vector Laboratories) for 30′ at room temperature, followed by incubation with 3,3′-diaminobenzidine (DAB) (Vector Laboratories). Specific primary antibody was omitted in negative control of the reactions. Nuclei were counterstained with Mayer’s haematoxylin (Sigma-Aldrich).
Determination of polyamine concentration
The concentrations of PUT, SPD and SPM were determined in aortic tissue homogenate by HPLC and normalized to total protein as described by Seiler et al. [20]. Cellular PA concentration was expressed as nmol/mg total protein.
Statistical analysis
Statistical analysis was performed using the GraphPad software (Prism 4.0) and the SPSS version 21.0. Significant differences in RT-PCR, Western blot and HPLC data obtained in BAV and TAV TAA samples and in normal aortas from organ donors as baseline, were assessed by one-way ANOVA followed by Bonferroni post hoc test. Correlations were analyzed by Spearman’s test. P values < 0.05 were considered significant.
Summaries for continuous variables are presented as mean ± SEM, for categorical variables as numbers and percentages.
Results
TAA samples from BAV patients exhibit different changes in the expression of genes involved in polyamine pathway vs. TAV patients and donors
RT-PCR analysis was performed on total RNA extracted from whole BAV (n = 30), TAV (n = 23) and donor (n = 10) TAA samples with an aortic diameter ≤ 45 mm, and revealed a significant decrease of mRNAs coding for the anabolic enzymes of the PA pathway (Fig. 1) ODC1, ARG1, ARG2 and AMD1 in BAV vs. donor TAA samples (Fig. 2a–d), as well as a significant decrease of AMD1, ODC1 and ARG1 in BAV vs. TAV TAA samples (Fig. 2a–c). ARG 2 showed a slight, but significant decrease also in TAV vs. donor TAA samples (Fig. 2d), but no significant difference between BAV and TAV TAA samples.
Reverse transcription polymerase chain reaction gene expression data of target molecules in TAA samples harvested from organ donors and from TAV and BAV groups of patients. a AMD1, b ODC1, c ARG1, d ARG2, e eNOS, f OAT, g SLC7A1/CAT-1, h SAT1. Results were normalized to GAPDH levels. Values are expressed as mean ± SEM of data from n = 10 donor, n = 23 TAV and n = 30 BAV TAA samples. *P < 0.05 vs. donor TAA data; # P < 0.05 vs. TAV TAA data
eNOS mRNA showed no change in TAA samples from BAV and TAV patients in comparison with donors (Fig. 2e). OAT mRNA analysis revealed an increased expression in both BAV and TAV patients vs. donor TAA samples, but the increase did not reach significance (Fig. 2f). Conversely the SLC7A1/CAT-1 mRNA, coding for the putative polyamine transporter SLC7A1/CAT-1 [21], showed a marked decrease in all patients vs. donors, which reached significance only in the BAV cohort, with about 50% decrease (Fig. 2g).
Finally, the mRNA coding for the catabolic enzyme of the PA pathway SAT1 showed a significant 2.78-fold increase in TAV vs. donor TAA samples and a significant decrease in BAV vs. TAV TAA samples (Fig. 2h).
None of the gene expression data exhibited a significant correlation with either age or aortic diameter in any of the groups we examined. However, we highlighted a significant positive correlation between gene expression datasets for SAT1 and AMD (r = 0.70, p = 0.014) and for SAT1 and ODC1 (r = 0.72, p = 0.008) in BAV TAA samples, suggesting a co-regulation at mRNA level of the catabolic enzyme SAT1 and of the anabolic enzymes ODC1 and AMD1 in BAV early aortopathy.
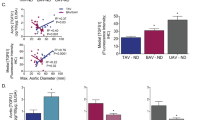
Western blot analysis was performed on proteins extracted from whole BAV, TAV and donor TAA samples (n = 5 for each group) with an aortic diameter ≤ 45 mm and revealed a significant increase of SLC7A1/CAT-1 in both TAV and BAV patients vs. donor TAA samples (3.06- and 2.36-fold increase, respectively), but no significant difference in BAV vs. TAV TAA samples (Fig. 3b, c). The opposite trends showed by SLC7A1/CAT-1 at protein (Fig. 3b) and mRNA level (Fig. 2g) suggest a negative feedback mechanism of regulation of this amino acid transporter at mRNA level that will be discussed further below.
Western blot analysis of ODC1 (a) and SLC7A1/CAT-1 (b) expression in TAA samples harvested from organ donors and from TAV and BAV groups of patients. Results were normalized to GAPDH levels. ODC1 and SLC7A1/CAT-1 immunoreactivity was observed at the expected monomer molecular weight of 51 and 68 kDa, respectively. Values are expressed as mean ± SEM of data from n = 5 TAA samples in each group. *P < 0.05 vs. donor TAA data. Column graphs in a and b: densitometric analysis of Western blots, as shown in c
Western blot analysis revealed a differential mechanism of regulation at mRNA and protein level also for ODC1, with the ODC1 protein remaining unchanged in all the cohorts of subjects we analyzed (Fig. 3a, c), while the corresponding mRNA showed a significant 30% decrease in TAA samples from BAV patients vs.TAV and organ donors (Fig. 2b).
TAA samples BAV patients exhibit different alterations of polyamine concentration vs. TAV patients and donors
HPLC analysis was performed on tissue homogenates of whole BAV (n = 9), TAV (n = 7) and donor (n = 6) TAA samples with an aortic diameter ≤ 45 mm and revealed a significant 3.02-fold increase of PUT and a twofold increase of SPD in BAV vs. TAV and donor TAA samples (Fig. 4a, b) only, while SPM showed a significant 30% decrease in TAV vs. donor samples and a significant increase in BAV vs. TAV TAA samples (Fig. 4c).
Polyamine concentration in TAA samples harvested from reference organ donors and from TAV and BAV groups of patients. Polyamines were analyzed by high-performance liquid chromatography and normalized to total protein concentration in each sample. a PUT, b SPD, c SPM. Values are expressed as mean ± SEM of data from n = 6 donor, n = 7 TAV and n = 9 BAV TAA samples. *P < 0.05 vs. donor TAA data; # P < 0.05 vs. TAV TAA data
We also performed a correlation analysis between PA concentration and age or aortic diameter of BAV/TAV patients. A moderate, non-significant inverse correlation between aortic diameter and PA concentration data for all three PAs was highlighted in BAV TAA samples (r = − 0.60 for PUT).
A moderate, non-significant positive correlation between SPM concentration and age was observed in BAV TAA samples (r = 0.61).
ODC1 exhibits a different subcellular localization in BAV vs. TAV and donor TAA samples
We evaluated the subcellular localization of ODC1 in TAA samples from donors (n = 6) and from BAV (n = 6) and TAV (n = 6) TAA samples with mild aortic dilation, as ODC1 shuttling between nucleus and cytoplasm can affect the activity of this rate-limiting enzyme.
The preliminary histological evaluation of aortic cross-sections confirmed our previous observations in other sets of samples with analogous clinical and demographic characteristics [19, 40], mainly revealing a thinner, altered intima, and a slight cell loss in the media layer in both TAV and BAV TAA samples. We also observed an altered orientation of SMCs in BAV TAA samples and, to a lesser extent, also in TAV TAA samples, with a shift from a circumferential to a longitudinal direction (data not shown).
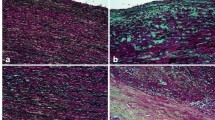
The immunohistochemical detection of ODC1 on TAA cross-sections revealed a marked expression of ODC1 by a large number of SMCs in the media layer of all the samples we analyzed (Fig. 5). High magnification analysis revealed that ODC1 was localized mainly in the nucleus of SMCs in donor and TAV TAA samples (Fig. 5a, b, e, f), while it also showed a cytoplasmatic localization in SMCs of BAV TAA samples (Fig. 5c, g).
Immunohistochemical detection of ODC1 in the media layer of TAA samples harvested from reference organ donors (n = 6) and from TAV (n = 6) and BAV (n = 6) patients. a, e, d, h Donor TAA cross-sections; b, f TAV TAA cross-section; c, g BAV TAA cross-section. ODC1: brown staining. Hematoxylin nuclei counterstaining: light blue. a–d ×40 magnification. e–h × 100 magnification of selected representative areas. d, h TAA cross-section adjacent to a, e, immunolabeled with a nonspecific IgG antibody (negative control)
Discussion
Multiple potential interconnections for polyamine pathway in aortopathy
We investigated for the first time the potential involvement of the PA pathway in aortopathy progression in patients with stenotic TAV or BAV, through a multilevel analysis suggested by its complex regulation, aiming at the preservation of homeostasis of intracellular PAs [22].
PAs could play a key role in TAV and BAV aortopathy, characterized by changes in SMC phenotype [19], together with alterations in collagen turnover and cross-linking [23], oxidative stress [24], EMT [25], cell loss and medial degeneration [26]. Some studies tried to analyze the differences at cellular level between BAV and TAV diseased TAA, suggesting a maturation defect of SMCs in BAV vs. TAV TAA samples [27], together with a loss of SMCs with contractile-phenotype and the emergence of a differentiated myofibroblast line [19], and a lower proliferation rate of BAV vs. TAV endothelial cells in vitro [28].
Overall results indicate an increase of PA concentration only in TAA samples from BAV patients vs. TAV patients and organ donors (Fig. 4), suggesting a link between the structural changes observed in BAV TAA and increased PAs. The increased concentration of PAs corresponded to a decreased expression of mRNAs coding for enzymes involved in PA biosynthesis, suggesting the activation of a negative feedback mechanism aiming at the preservation of intracellular PA homeostasis, as described elsewhere [29].
The PA pathway could be of particular relevance in aortopathy in view of the competitive utilization of aortic arginine by ARG and by eNOS (Fig. 1). The dysregulation of the arginine/nitric oxide balance has been demonstrated to be involved in several pathological vascular conditions [30], and could play a major role also in aortopathy, in concurrence with other pathogenetic mechanisms. The decreased expression of ARG1/2 mRNA in BAV TAA samples could represent a feedback mechanism to counteract the increased concentration of PUT and SPD and may suggest a greater availability of the substrate arginine for eNOS in this cohort of patients. Differences in the expression of ARG2 between TAV and BAV patients could be related to the different cellular localization and regulation of ARG isoenzymes [31]. Several studies have analyzed the expression and the activation level of eNOS in BAV vs. TAV TAA samples, dilated or not, producing largely contrasting data. Among them, a recent study by Kotlarczyk et al. [32] revealed an increased eNOS expression and activation in BAV vs. TAV aortic samples, but no change in NO bioavailability. eNOS “uncoupling” is a contributing factor to reduced local NO concentration in cardiovascular disease [33]. Additional studies would be necessary to assess the NO bioavailability in our collection of TAA samples to verify a potential disequilibrium as a reaction to increased PA concentration in BAV non-dilated TAA samples.
Ornithine is not only a precursor of PAs, but is also a precursor amino acid, via the enzyme OAT, for the synthesis of proline, a major component of collagen. Accordingly, OAT and its substrate ornithine might be important in remodeling associated with aortopathy. As expected, OAT expression increased in both TAV and BAV samples (Fig. 2f), even if the data were not-significant, possibly because of the mild dilation of the TAA samples included in this study.
For what concerns the other members of the PA pathway and genes associated with cellular PA homeostasis we analyzed, SLC7A1/CAT-1 was the first amino acid/PA transporter to be cloned and characterized [34], but other membrane solute carrier transporters have been identified since then as potential PA transporters [21]. The discrepancy between the SLC7A1/CAT-1 protein increase in both TAV and BAV TAA samples (Fig. 3b), and the increased PA concentration in BAV TAA samples only (Fig. 4) suggests that other transporters are in charge of PA/amino acid uptake in BAV TAA samples, or that other regulative mechanisms prevail.
The multilevel analysis of ODC1 highlights its differential regulation in BAV TAA samples
ODC1 catalyzes the first and rate-limiting step of PA biosynthesis in humans, i.e., the decarboxylation of ornithine to PUT (Fig. 1). While the RT-PCR analysis revealed a decreased expression of ODC1 mRNA in BAV TAA samples (Fig. 2b), possibly as a negative feedback to the increased concentration of PUT and SPD in this group (Fig. 4), the Western blot analysis revealed unchanged level of the ODC1 monomer in all the sample groups we analyzed, possibly suggesting the lack of its degradation primed by the PA sensor Antizyme1 (AZ1) [22]. ODC subcellular localization also plays a key role in ODC1 regulation, as this enzyme is able to shuttle between nucleus and cytoplasm to modulate its activity in response to different stimuli as well as during cell cycle and development [35, 36]. To the best of our knowledge, ODC immunohistochemical detection in human aorta has been previously performed only in a study focusing on chronic renal failure [37], but without any high magnification analysis of its subcellular localization. Our data suggest a trend for ODC shuttling from the nucleus to the cytoplasm in SMCs of BAV TAA samples (Fig. 5c, g), which could be responsible, at least in part, for the increased ODC activity and PA concentration in this specific cohort of patients. Additional experiments will be necessary to confirm this hypothesis. However, this finding is consistent with other studies in different pathological settings, showing a shift towards a cytoplasmatic localization of ODC1 vs. the nuclear localization observed in normal tissues [38].
Increased concentration of polyamines could imply specific effects in BAV vs. TAV TAA samples
The increased concentration of PUT and SPD we observed in BAV TAA samples could derive from increased PA biosynthesis, decreased catabolism and/or increased uptake from the circulation. Our results provide some indications in this sense, but, due to high complexity of the regulation of the PA pathway, other experiments will be necessary to gain additional information. Of interest, Durante et al. [39] suggested a major role for TGF-β1 in stimulating the production of PAs and proline in SMCs from extracellular arginine. This observation is consistent with the increased expression of TGF-β1 we detected in previous studies on BAV patients with non-dilated aortas [19, 40].
Two at least partially contrasting hypotheses could be formulated to define the potential effects of increased PA concentration in BAV TAA samples. According to the first hypothesis, increased PAs could be an early sign/cause of a more detrimental aortopathy associated with BAV. This hypothesis is supported by previous studies indicating that excessive accumulation of PAs results in induction of cell apoptosis [41].
Conversely, according to a second hypothesis, increased PA concentration in non-dilated BAV aortas could represent an early balancing reaction against increased susceptibility to dilation that is not efficiently triggered in TAV patients. Recently, other studies also hypothesized the activation of early protective mechanisms against aortopathy in BAV patients, including the increase of SOD1 [42]. This second hypothesis is strongly supported by recent experimental evidence revealing several protective roles played by PAs in different contexts. In particular, following the initial study by Eisenberg et al. showing that SPD promotes cardioprotective autophagy [43,44,45,46], all confirmed that SPD plays a protective role by promoting autophagy, a conserved mechanism that targets damaged organelles and proteins for degradation. Fan et al. concluded that SPD is able to rescue skeletal muscle atrophy through enhanced autophagy [47]. Thus, elevated PA levels may influence the aortopathy progression through stimulation of autophagy, but also via previously mentioned altered NO bioavailability, both representing alternative protective mechanisms of action. In addition, SPM and SPD are able to reverse and inhibit age-related cardiac deterioration in a murine model [48]. SPD plays a critical role also in protection against oxidative stress in inflammation models [49], and PA depletion is necessary for TGF-β1-induced EMT in vitro [10, 11]. Depending on its concentration, SPM is also able to protect against stress and damage by scavenging reactive oxygen species [50]. The significant decrease of SPM concentration in TAV TAA samples (Fig. 4b) could suggest a loss of its protective role against oxidative stress in TAV patients, possibly with the contribution of the increased expression of the PA catabolic enzyme SAT1 (Fig. 2h). This finding could also be consistent with the more severe histological abnormalities associated with TAV compared with BAV described by Heng et al. [26], especially when stratified by aortic diameter.
The inverse correlation between aortic diameter and PA concentration we found in BAV samples, even if non-significant, could be consistent with the hypothesis of an early protective role of PAs against aortopathy in BAV patients, and deserves the analysis of larger cohorts of patients to confirm and consolidate this data. A comparison with BAV patients with larger TAA aneurysms could also help clarifying the interpretation of the present evidence.
The increased PA concentration in non-dilated TAA samples from BAV patients suggests a role for these molecules as potential early biomarkers of BAV aortopathy, assuming that the elevated tissue concentrations reflect enhanced plasma/serum levels of PAs. Unfortunately, the detection of circulating PAs can be difficult and potentially biased by several factors such as cell lyses occurring when the blood samples are collected and processed. Nonetheless, some studies set up methods for PA detection in human serum [51], thus encouraging additional investigations in this context.
Study limitations
This is a first report on a novel aspect of BAV aortopathy and, of course, it has some limitations to consider. The PA pathway is complex and highly regulated at multiple levels; in addition, some components (e.g., PA transporters) have not yet been fully identified and characterized in man. This observation implies that an exhaustive, multilevel differential analysis of all mechanisms leading to final differences in PA concentration in TAA samples from TAV/BAV/donor cohorts is challenging, also considering the very limited size of aortic samples harvested from non-dilated aortas during valve surgery. For this reason, the different analyses have been performed on distinct sets of patients, however, homogeneous for clinical and demographic characteristics. As a consequence, the number of patients for some analyses was relatively small, implying that some of our results should be interpreted with caution, with particular reference to the possible inverse correlation between PA concentration and aortic diameter in the BAV group. Additional larger studies will be performed to consolidate these data.
Conclusions
To the best of our knowledge, this study reveals for the first time that PA concentration increases specifically in BAV patients, accompanied by the activation of a regulatory negative feedback mechanism that, however, failed to preserve PA intracellular homeostasis. It is conceivable that increased PA concentrations in BAV TAA samples might contribute to detrimental progression of aortopathy or represent an early protective reaction to excessive aortopathy in this cohort. The results we obtained in this first study suggest an association of PAs specifically with BAV aortopathy, but not a specific role, that will be clarified by additional investigations comprising a larger number of samples and based on the results reported above. Additional investigations will also evaluate the PA pathway as a potential therapeutic target or as early biomarker in BAV aortopathy.
References
Bhat MI, Kapila R (2017) Dietary metabolites derived from gut microbiota: critical modulators of epigenetic changes in mammals. Nutr Rev 75:374–389
Pegg AE (2016) Functions of polyamines in mammals. J Biol Chem 291:14904–14912
Newton GL, Aguilera JA, Ward JF, Fahey RC (1996) Polyamine-induced compaction and aggregation of DNA—a major factor in radioprotection of chromatin under physiological conditions. Radiat Res 145:776–780
Forte A, Hellstrand P, Nilsson BO, Grossi M, Rossi F, Cipollaro M (2011) The polyamine pathway as a potential target for vascular diseases: focus on restenosis. Curr Vasc Pharmacol 9:706–714
Grossi M, Persson L, Sward K, Turczynska KM, Forte A, Hellstrand P, Nilsson BO (2014) Inhibition of polyamine formation antagonizes vascular smooth muscle cell proliferation and preserves the contractile phenotype. Basic Clin Pharmacol Toxicol 115:379–388
Grossi M, Phanstiel O, Rippe C, Sward K, Alajbegovic A, Albinsson S, Forte A, Persson L, Hellstrand P, Nilsson BO (2016) Inhibition of polyamine uptake potentiates the anti-proliferative effect of polyamine synthesis inhibition and preserves the contractile phenotype of vascular smooth muscle cells. J Cell Physiol 231:1334–1342
Grossi M, Rippe C, Sathanoori R, Sward K, Forte A, Erlinge D, Persson L, Hellstrand P, Nilsson BO (2014) Vascular smooth muscle cell proliferation depends on caveolin-1-regulated polyamine uptake. Biosci Rep 34:e00153
Forte A, Grossi M, Turczynska KM, Svedberg K, Rinaldi B, Donniacuo M, Holm A, Baldetorp B, Vicchio M, De Feo M, Sante P, Galderisi U, Berrino L, Rossi F, Hellstrand P, Nilsson BO, Cipollaro M (2013) Local inhibition of ornithine decarboxylase reduces vascular stenosis in a murine model of carotid injury. Int J Cardiol 168:3370–3380
Kucharzewska P, Welch JE, Svensson KJ, Belting M (2010) Ornithine decarboxylase and extracellular polyamines regulate microvascular sprouting and actin cytoskeleton dynamics in endothelial cells. Exp Cell Res 316:2683–2691
Compagnone A, Bandino A, Meli F, Bravoco V, Cravanzola C, Parola M, Colombatto S (2012) Polyamines modulate epithelial-to-mesenchymal transition. Amino Acids 42:783–789
Prunotto M, Compagnone A, Bruschi M, Candiano G, Colombatto S, Bandino A, Petretto A, Moll S, Bochaton-Piallat ML, Gabbiani G, Dimuccio V, Parola M, Citti L, Ghiggeri G (2010) Endocellular polyamine availability modulates epithelial-to-mesenchymal transition and unfolded protein response in MDCK cells. Lab Investig 90:929–939
Baardman J, Licht I, de Winther MP, Van den Bossche J (2015) Metabolic-epigenetic crosstalk in macrophage activation. Epigenomics 7:1155–1164
Albinsson S, Della Corte A, Alajbegovic A, Krawczyk KK, Bancone C, Galderisi U, Cipollaro M, De Feo M, Forte A (2017) Patients with bicuspid and tricuspid aortic valve exhibit distinct regional microRNA signatures in mildly dilated ascending aorta. Heart Vessels 32:750–767
Forte A, Galderisi U, Cipollaro M, De Feo M, Della Corte A (2016) Epigenetic regulation of TGF-beta1 signalling in dilative aortopathy of the thoracic ascending aorta. Clin Sci (Lond) 130:1389–1405
Michelena HI, Della Corte A, Prakash SK, Milewicz DM, Evangelista A, Enriquez-Sarano M (2015) Bicuspid aortic valve aortopathy in adults: incidence, etiology, and clinical significance. Int J Cardiol 201:400–407
Michelena HI, Prakash SK, Della Corte A, Bissell MM, Anavekar N, Mathieu P, Bosse Y, Limongelli G, Bossone E, Benson DW, Lancellotti P, Isselbacher EM, Enriquez-Sarano M, Sundt TM 3rd, Pibarot P, Evangelista A, Milewicz DM, Body SC, Investigators BA (2014) Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon). Circulation 129:2691–2704
Cotrufo M, Della Corte A, De Santo LS, Quarto C, De Feo M, Romano G, Amarelli C, Scardone M, Di Meglio F, Guerra G, Scarano M, Vitale S, Castaldo C, Montagnani S (2005) Different patterns of extracellular matrix protein expression in the convexity and the concavity of the dilated aorta with bicuspid aortic valve: preliminary results. J Thorac Cardiovasc Surg 130:504–511
Della Corte A, De Santo LS, Montagnani S, Quarto C, Romano G, Amarelli C, Scardone M, De Feo M, Cotrufo M, Caianiello G (2006) Spatial patterns of matrix protein expression in dilated ascending aorta with aortic regurgitation: congenital bicuspid valve versus Marfan’s syndrome. J Heart Valve Dis 15:20–27
Forte A, Della Corte A, Grossi M, Bancone C, Provenzano R, Finicelli M, De Feo M, De Santo LS, Nappi G, Cotrufo M, Galderisi U, Cipollaro M (2013) Early cell changes and TGFbeta pathway alterations in the aortopathy associated with bicuspid aortic valve stenosis. Clin Sci (Lond) 124:97–108
Seiler N, Knodgen B (1980) High-performance liquid chromatographic procedure for the simultaneous determination of the natural polyamines and their monoacetyl derivatives. J Chromatogr 221:227–235
Abdulhussein AA, Wallace HM (2014) Polyamines and membrane transporters. Amino Acids 46:655–660
Wu D, Kaan HY, Zheng X, Tang X, He Y, Vanessa Tan Q, Zhang N, Song H (2015) Structural basis of ornithine decarboxylase inactivation and accelerated degradation by polyamine sensor Antizyme1. Sci Rep 5:14738
Wagsater D, Paloschi V, Hanemaaijer R, Hultenby K, Bank RA, Franco-Cereceda A, Lindeman JH, Eriksson P (2013) Impaired collagen biosynthesis and cross-linking in aorta of patients with bicuspid aortic valve. J Am Heart Assoc 2:e000034
Billaud M, Phillippi JA, Kotlarczyk MP, Hill JC, Ellis BW, St Croix CM, Cantu-Medellin N, Kelley EE, Gleason TG (2017) Elevated oxidative stress in the aortic media of patients with bicuspid aortic valve. J Thorac Cardiovasc Surg. https://doi.org/10.1016/j.jtcvs.2017.05.065
Kostina AS, Uspensky Vcapital Ie C, Irtyuga OB, Ignatieva EV, Freylikhman O, Gavriliuk ND, Moiseeva OM, Zhuk S, Tomilin A, Kostareva capital A CAC, Malashicheva AB (2016) Notch-dependent EMT is attenuated in patients with aortic aneurysm and bicuspid aortic valve. Biochim Biophys Acta 1862:733–740
Heng E, Stone JR, Kim JB, Lee H, MacGillivray TE, Sundt TM (2015) Comparative histology of aortic dilatation associated with bileaflet versus trileaflet aortic valves. Ann Thorac Surg 100:2095–2101
Grewal N, Gittenberger-de Groot AC, Poelmann RE, Klautz RJ, Lindeman JH, Goumans MJ, Palmen M, Mohamed SA, Sievers HH, Bogers AJ, DeRuiter MC (2014) Ascending aorta dilation in association with bicuspid aortic valve: a maturation defect of the aortic wall. J Thorac Cardiovasc Surg 148:1583–1590
Malashicheva A, Kostina D, Kostina A, Irtyuga O, Voronkina I, Smagina L, Ignatieva E, Gavriliuk N, Uspensky V, Moiseeva O, Vaage J, Kostareva A (2016) Phenotypic and functional changes of endothelial and smooth muscle cells in thoracic aortic aneurysms. Int J Vasc Med 2016:3107879
Persson L (2009) Polyamine homoeostasis. Essays Biochem 46:11–24
Allen JD, Giordano T, Kevil CG (2012) Nitrite and nitric oxide metabolism in peripheral artery disease. Nitric Oxide 26:217–222
Durante W (2013) Role of arginase in vessel wall remodeling. Front Immunol 4:111
Kotlarczyk MP, Billaud M, Green BR, Hill JC, Shiva S, Kelley EE, Phillippi JA, Gleason TG (2016) Regional disruptions in endothelial nitric oxide pathway associated with bicuspid aortic valve. Ann Thorac Surg 102:1274–1281
Kietadisorn R, Juni RP, Moens AL (2012) Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab 302:E481–E495
Hatzoglou M, Fernandez J, Yaman I, Closs E (2004) Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr 24:377–399
Murakami Y, Suzuki J, Samejima K, Oka T (2010) Developmental alterations in expression and subcellular localization of antizyme and antizyme inhibitor and their functional importance in the murine mammary gland. Amino Acids 38:591–601
Schipper RG, Cuijpers VM, De Groot LH, Thio M, Verhofstad AA (2004) Intracellular localization of ornithine decarboxylase and its regulatory protein, antizyme-1. J Histochem Cytochem 52:1259–1266
Fassot C, Briet M, Rostagno P, Barbry P, Perret C, Laude D, Boutouyrie P, Bozec E, Bruneval P, Latremouille C, Laurent S (2008) Accelerated arterial stiffening and gene expression profile of the aorta in patients with coronary artery disease. J Hypertens 26:747–757
Nilsson T, Bogdanovic N, Volkman I, Winblad B, Folkesson R, Benedikz E (2006) Altered subcellular localization of ornithine decarboxylase in Alzheimer’s disease brain. Biochem Biophys Res Commun 344:640–646
Durante W, Liao L, Reyna SV, Peyton KJ, Schafer AI (2001) Transforming growth factor-beta(1) stimulates l-arginine transport and metabolism in vascular smooth muscle cells: role in polyamine and collagen synthesis. Circulation 103:1121–1127
Forte A, Bancone C, Cobellis G, Buonocore M, Santarpino G, Fischlein TJM, Cipollaro M, De Feo M, Della Corte A (2017) A possible early biomarker for bicuspid aortopathy: circulating transforming growth factor beta-1 to soluble endoglin ratio. Circ Res 120:1800–1811
Takao K, Rickhag M, Hegardt C, Oredsson S, Persson L (2006) Induction of apoptotic cell death by putrescine. Int J Biochem Cell Biol 38:621–628
Phillippi JA, Hill JC, Billaud M, Green BR, Kotlarczyk MP, Gleason TG (2017) Bicuspid aortic valve morphotype correlates with regional antioxidant gene expression profiles in the proximal ascending aorta. Ann Thorac Surg 104:79–87
Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, Tong M, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K, Carmona-Gutierrez D, Buttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, Horsch M, Moreth K, Beckers J, Fuchs H, Gailus-Durner V, Neff F, Janik D, Rathkolb B, Rozman J, de Angelis MH, Moustafa T, Haemmerle G, Mayr M, Willeit P, von Frieling-Salewsky M, Pieske B, Scorrano L, Pieber T, Pechlaner R, Willeit J, Sigrist SJ, Linke WA, Muhlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, Madeo F (2016) Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 22:1428–1438
Yue F, Li W, Zou J, Jiang X, Xu G, Huang H, Liu L (2017) Spermidine prolongs lifespan and prevents liver fibrosis and hepatocellular carcinoma by activating MAP1S-mediated autophagy. Cancer Res 77:2938–2951
Bhukel A, Madeo F, Sigrist SJ (2017) Spermidine boosts autophagy to protect from synapse aging. Autophagy 13:444–445
Yang Y, Chen S, Zhang Y, Lin X, Song Y, Xue Z, Qian H, Wang S, Wan G, Zheng X, Zhang L (2017) Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis 8:e2738
Fan J, Yang X, Li J, Shu Z, Dai J, Liu X, Li B, Jia S, Kou X, Yang Y, Chen N (2017) Spermidine coupled with exercise rescues skeletal muscle atrophy from d-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 8:17475–17490
Zhang H, Wang J, Li L, Chai N, Chen Y, Wu F, Zhang W, Wang L, Shi S, Zhang L, Bian S, Xu C, Tian Y, Zhao Y (2017) Spermine and spermidine reversed age-related cardiac deterioration in rats. Oncotarget. https://doi.org/10.18632/oncotarget
Jeong JW, Cha HJ, Han MH, Hwang SJ, Lee DS, Yoo JS, Choi IW, Kim S, Kim HS, Kim GY, Hong SH, Park C, Lee HJ, Choi YH (2017) Spermidine protects against oxidative stress in inflammation models using macrophages and zebrafish. Biomol Ther (Seoul). https://doi.org/10.4062/biomolther.2016.272
Grancara S, Zonta F, Ohkubo S, Brunati AM, Agostinelli E, Toninello A (2015) Pathophysiological implications of mitochondrial oxidative stress mediated by mitochondriotropic agents and polyamines: the role of tyrosine phosphorylation. Amino Acids 47:869–883
Byun JA, Choi MH, Moon MH, Kong G, Chul Chung B (2009) Serum polyamines in pre- and post-operative patients with breast cancer corrected by menopausal status. Cancer Lett 273:300–304
Acknowledgements
We are grateful to Dr. Angela Ferone for technical assistance and to Ms. Maria Rosaria Cipollaro for administrative assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study has been partially supported by The Greta and Johan Kock Foundation (BON).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Forte, A., Grossi, M., Bancone, C. et al. Polyamine concentration is increased in thoracic ascending aorta of patients with bicuspid aortic valve. Heart Vessels 33, 327–339 (2018). https://doi.org/10.1007/s00380-017-1087-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-017-1087-z