Abstract
Low adherence to cardiac rehabilitation (CR) might be improved by remote monitoring systems that can be used to motivate and supervise patients and tailor CR safely and effectively to their needs. The main objective of this study was to evaluate the feasibility of a smartphone-guided training system (GEX) and whether it could improve exercise capacity compared to CR delivered by conventional methods for patients with coronary artery disease (CAD). A prospective, randomized, international, multi-center study comparing CR delivered by conventional means (CG) or by remote monitoring (IG) using a new training steering/feedback tool (GEx System). This consisted of a sensor monitoring breathing rate and the electrocardiogram that transmitted information on training intensity, arrhythmias and adherence to training prescriptions, wirelessly via the internet, to a medical team that provided feedback and adjusted training prescriptions. Exercise capacity was evaluated prior to and 6 months after intervention. 118 patients (58 ± 10 years, 105 men) with CAD referred for CR were randomized (IG: n = 55, CG: n = 63). However, 15 patients (27 %) in the IG and 18 (29 %) in the CG withdrew participation and technical problems prevented a further 21 patients (38 %) in the IG from participating. No training-related complications occurred. For those who completed the study, peak VO2 improved more (p = 0.005) in the IG (1.76 ± 4.1 ml/min/kg) compared to CG (−0.4 ± 2.7 ml/min/kg). A newly designed system for home-based CR appears feasible, safe and improves exercise capacity compared to national CR. Technical problems reflected the complexity of applying remote monitoring solutions at an international level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiac rehabilitation (CR) aims at restoring exercise performance [1] in patients after a cardiac event like myocardial infarction (MI) [1], peripheral arterial disease [2, 3] or heart failure (HF) [3–7]. CR uses multifactorial intervention, including aggressive risk factor modification [7–11]. Traditionally, these programs have tried to improve physical health and individual attitude through exercise-only based CR or comprehensive CR (e.g., smoking cessation, dietary counseling as well as exercise) [8]. Meta-analyses on exercise-based CR showed a significant reduction in cardiac mortality of about 27 % compared to patients receiving conventional care [9, 12]. Still, there are relevant national differences in how CR programs are organized [9]. Unfortunately, despite efficacy and cost-effectiveness CR, is pursued by less than one-third of eligible patients [13]. As beneficial effects of CR largely depend on continuation of a lifetime exercise program after a structured CR, patients should be encouraged to continue with an individual exercise program to preserve an improvement in exercise capacity [8, 10, 11, 13–18].
Reasons for non-participation in CR included unavailability of hospital-based CR, lack of motivation or other reasons, e.g., excessive travel distance [19]. Quality performance criteria, automated referral systems and options for home-based CR services may increase adherence to therapies in some patients. Besides center-based CR, home-based CR offers a potentially valuable alternative for many individuals [20–22], and has proved to introduce similar improvements compared to center-based programs across a range of measures [23] at lower [24] or comparable cost.
These findings would support an extension of home-based CR as an attempt to widen access and participation [25–27]. Furthermore, the evolution of the technology has led to the design of mobile-based solutions which could facilitate home-based CR and overcome patient safety concerns [28–32]. The GEx system [33–35], designed under those requirements, introduces a closed-loop disease management system supporting the prescription and administration of CR. A feasibility study was previously performed to compare signals of this new system with standard cardiac exercise testing (CPX) during inpatient phase II-CR that showed the suitability of the whole system for monitoring of home-based CR [36] with accurate heart rate (HR) measurements. In this present study improvements obtained due to long-term home-based CR (CR—phase III) were determined. Guided exercise training supervised by the GEx system was compared to the standard care in three different countries (Great Britain, Spain, Germany).
Methods
Study design and study objectives, ethical considerations
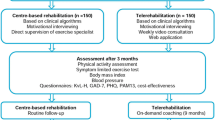
In this open, prospective, randomized, parallel group, German, British and Spanish, three-center, Phase I study, the standard national approach to CR was compared to an approach adding the new training steering and feedback GEx System. All patients were recruited during phase II cardiac rehabilitation in Spain, GB and Germany to compare national difference in CR. The study was performed after discharge from rehabilitation facilities at home (phase III rehabilitation) with individual training prescription. Patients were randomized to an intervention group (IG) and a control group (CG). Overall, treatment was according to national CR recommendations. Additionally, patients randomized to the IG were equipped with the GEx System.
The main objective of this study was to evaluate, whether the GEx System can improve physical exercise capacity at 6-month follow-up during home-based, during phase III CR compared to national CR standards. Secondary objectives were to evaluate the effect of the GEx System on compliance, total exercise time, fear and anxiety, physical fitness, symptoms and whether home training was safe as well as serum cholesterol, LDL and fastening glucoses blood pressure (BP) control, QoL and ejection fraction (EF).
The Regional Ethical Review Board at the University of Aachen (00017326, EK218/11), Hospital Clínico San Carlos of Madrid (C.I. 11/232-E) and University of Hull (12/YH/0072) approved the study. Principles according to the Helsinki declaration (WMA 2008) were followed. Written informed consent was obtained from all participants prior to inclusion in the study. Also, the study was registered at ClinicalTrials.gov (identifier NCT01761448).
Inclusion and exclusion criteria
Inclusion criteria were: presence of CAD after acute MI or elective coronary intervention, EF ≥30 %, willing to exercise, eligible for CR and ability to use computer and Internet. Respectively, exclusion criteria were: EF <30 %, HF with NYHA IV, inability to exercise, severe valve disease, recent cardiac surgery <4 weeks, implantable devices (ICD or CRT-device, pacemaker) or open thorax wound.
Study phases and data acquisition
All patients underwent an initial baseline evaluation consisting of a careful history taking (with activity profile evaluation) and a physical examination. A standard ECG, laboratory analysis, 2D echocardiography and exercise testing with additional lactate samples was carried out. All individuals were asked to fill in QoL questionnaires.
During the 6-month training phase, patients in the IG performed training under guidance of the GEx system. Individual training performance was closely monitored and exercise prescriptions were continuously reviewed and adjusted as needed. This was done by a dedicated team of sport physicians and exercise scientists, the so-called Central Training Committee (CTC). Patients in the IG were evaluated with respect to usability of the system, knowledge about heart-related health, exercise habits and adaption efforts. In contrast, patients in the CG were asked to report on daily physical activities on a paper dairy.
At 6-month FU, all baseline examinations were repeated in both groups.
Laboratory parameters
Urea clearance, potassium, sodium, white/red blood cell count, platelet count, total cholesterol, LDL-cholesterol, HDL, cholesterol, HbA1c, hsCRP, ntBNP were analyzed.
ECG
A standard electrocardiography was performed using a commercial system at each site.
Echocardiography
A standard 2D-echocardiography was performed using available systems at the different sites. All standard views (2 and 4-chamber view) were acquired. Left ventricular EF was then calculated according to Simpson’s method.
Quality of life
All patients were instructed to complete questionnaires regarding QoL (EQ5D) [37] and anxiety and depression (HADS) [38].
Cardiopulmonary exercise testing (CPX)
A cardiopulmonary exercise testing (cycle ergometer) starting at 25 W, with increments of 25 W per 2 min (incremental), was performed. Peak oxygen uptake (VO2peak) at peak exercise was defined as the highest oxygen consumption measured during the last 30 s of the symptom-limited exercise test and expressed as ml/kg/min. VO2 (ml/kg/min), VCO2 (L/min), and VE (L/min) were collected throughout the test. VE and VCO2 were designated as the y- and x-axis variables, respectively. VE/CO2 slope was calculated with the slope calculation option of the software package. In Aachen Carefusion Masterscreen CPX, Lab Manager Version 4.67 was used, in Hull Innocor by Innovision and in Spain Ergostik Geratherm were applied. HR was taken through 12 lead ECG. BP was taken using an automated BP machine with integrated microphone (Getemed). During testing, relevant ischemia, arrhythmias, HR and BP were evaluated. For anaerobic threshold v-slope method was used [39, 40]. Also HR reserve (HRR, normal < 20 bpm), oxygen pulse (O2/HR, ml/beat) and aerobic workrate dO2/dW (normal > 10 ml/min/W) [41, 42] were determined.
Lactate concentration was measured at baseline and at every exercise step. Specifically, HR and watt at 2 mmol lactate and at 4 mmol lactate were obtained.
Guided exercise system (GEx)
The GEx system is a closed-loop disease management system facilitating prescription and administration of CR therapies [36] made up of three main components: Professional System, Patient Station and Portable Station.
The Professional System is a Web-based tool for medical professionals (Fig. 2) providing information on the patients’ profile with respect to medical history and CR prescription and performance in the past. Furthermore, data from the patients’ systems (Portable Station and Patient Station) including HR, BP, notifications on events during exercise session and ECG tracings are displayed.
The Patient Station acts as the gateway between the Portable Station and the Professional System, as it is able to synchronize exercise plans prescribed by professionals and extract monitored data from the portable station and upload them to the professional system. It is also in charge of providing educational material and motivational feedback generated from actual exercise performances.
The Portable Station (Fig. 1) is used by the patient during prescribed exercise sessions. It includes a sensor for acquisition of vital signs and a smartphone for interaction with patients. The smartphone software contains algorithms that process the vitals collected from the sensor and, based on the individual exercise plan, provide immediate feedback with respect to training intensity (Fig. 2).
Web-based training database. Web-based training data base. A status bar on the bottom of the screen where specific information about the logged user is displayed: user name, status message on the actions the user performs in the system, language selection. Patients training data and progress of training process are visible
In order to ensure patients’ safety and to avoid hazardous situations, a series of verifications were implemented prior to each CR session (cardiac symptoms (angina pectoris, dyspnea), well-being, blood pressure). Patients received immediate feedback on whether it was safe to exercise or if it was better to reschedule the exercise at a later point in time.
Training prescription/central training committee (CTC)
The CTC was created to obtain individual training prescription for all patients at all trial sites based on a homogeneous strategy. The information gathered during initial exercise testing was sent to the Institute for Cardiology and Sports Medicine, German Sports University Cologne, Germany, for all patients. This held also true for retesting performed during the training phase. At the CTC, individual exercise prescriptions for each patient participating in IG of the study were elaborated and sent back to the study site. Importantly, all changes in medication, especially beta-blockers and calcium channel blockers had to be announced to the training committee.
In case a patient failed to follow the training prescription or was at risk of over-exercising, the CTC had to be notified to assess whether a change in the training prescription was needed.
Description of training content
Patients were instructed to perform training sessions containing endurance but also resistance training. The goal of the endurance training (e.g., cycling, walking) was to make the patient work out in a safe but also effective HR zone. Consequently, patients’ HR was supposed to stay in an individual, pre-defined target zone [43].
Resistance training was a combination of both isometric and isotonic exercises using a rubber band.
Intensification strategy
The intensification strategy followed the FITT [44, 45] (frequency, intensity, time and type of activity) principles model. GEx patients indeed followed a training plan consisting of three stages as described in Fig. 3.
Power calculation, randomization and statistical analysis
According to previous literature [46, 47], it is feasible to expect a 3 ml/min/kg standard deviation of the main variable (VO2max). A sample size of 130 analyzable patients (65 per group) would therefore have adequate power (80 %) at a 5 % level of significance (one-tailed) to show a 1.3 ml/min/kg difference. In order to meet the minimum sample size requirements for the objectives, accounting for possible study attrition (e.g., subject death, withdraw of consent), the study was meant to enroll a total of 150 subjects. More specifically, 25 patients per group were expected for each study site, thus, 50 patients were meant to be included at each site. Overall, 75 patients for each randomization group had to be allocated. All analyses of secondary endpoints were regarded as exploratory. The explorative approach was concomitant to the explanation and not to adjust for multiple comparisons. The p-values were therefore merely descriptive.
All data were stored in an eCRF using the number of randomized patient as identification.
Randomization stratified by ‘gender’ and ‘center’ was done electronically by the system (eCRF). To evaluate the number of patients that matched with the inclusion and exclusion criteria, a screening list was carried. Due to the kind of intervention, the study had to be performed open-labeled. The un-blinded design seemed to be appropriate to reach primary objectives. However, physicians/technicians performing and evaluating echocardiography or functional testing were unaware of the study group.
Continuous data were summarized by the median (interquartile range); categorical data by percentages. A two-tailed Student’s t test was used for comparison between groups. Linear regression was used to adjust for baseline covariates (age and sex as a minimum). Residuals were checked for normality. Either Fisher’s exact test or Chi-squared test were used for categorical variables with nominal scales and the Mann–Whitney U test for categorical variables with ordinal scales. Missing values were not imputed but reported in tables. All tests were assessed at the 5 % statistical significance level. The ‘Stata’ statistical computer package was used to analyze the data.
Results
Patients’ characteristics
Starting on 4/2011, 132 patients (59 ± 14 years, 11 % females) gave consent (33 in Aachen, Germany, 44 in Hull, GB and 55 in Madrid, Spain). Of these, 89 % were randomized and included in the study: 63/53 % were assigned to the CG and 55/47 % to the IG (Table 1). The patients’ characteristics are stated in Table 2. Patients were recruited during the phase II rehabilitation. This allowed all patients to enter the study at the same stage, thus, delivering the same information to all patients at the same point in time. Furthermore, the phase II could be used to make patients familiar with the new training tool and provide time for further instructions.
All patients were on standard medication for CAD (Table 2). Table 2 shows the baseline exercise characteristics of the patients consented. Furthermore, quality of life characteristics at the beginning of the study are stated in Table 2, showing no difference between anxiety, depression and EQ-5D at baseline.
Randomization and follow-up
In the IG, 15 patients (27 %) dropped out before starting the guided rehabilitation. Of those actually participating in the program 12 (30 %) finished all interventions. However, 17 (8 %) showed poor compliance and 21 patients (53 %) stopped during the training phase due to several reasons: lack of time, issues with Internet connectivity, demotivation because of safety algorithms delaying or stopping exercise too often, follow-up was already too late, or no training was allowed due to chronic infections or chronic back pain.
In the CG, n = 15 patients denied participation in the study and n = 3 patients cancelled follow-up investigations.
Training sessions and safety algorithm
In effect, only 43 % of the exercise sessions were fully completed, other 37 % were interrupted by the safety algorithms, 20 % where abandoned because of technical errors (e.g., poor signal quality or poor communication with sensor), and 2 % were explicitly stopped by the users.
This may explain why some users were annoyed by the safety measurements. More concretely, of these interruptions, 63 % were because of high BP, 26 % because of HR values out of range for too long and the remaining 2 % because of other reported symptoms.
Safety of cardiac rehabilitation
Adverse events were reported in 6 patients (31 %) in the IG and 3 patients (8 %) in the CG. However, there was no complication directly associated with CR. More specifically, in the IG 2 patients complained of chest pain based on chest infection after CABG, 2 patients were admitted to hospital with consecutive angiography due to new onset of angina pectoris which was not related to training and 2 patients contacted the study center because of chest pain before training and were sent to hospital for further investigation resulting in CABG because of progression of disease. In the CG, reasons for adverse event were new onset of atrial fibrillation (n = 1), new angina at rest (n = 1), which resulted in angiography without intervention and pseudo-aneurysm of the right femoral arteries after PCI (n = 1) with surgery intervention.
Outcome of the primary endpoint
If compared between CG and IG, the use of the GEx system results in a statistically higher improvement in VO2 peak (Table 3).
In both groups, CR resulted in reduction of HR at rest and at anaerobic threshold, reduction of VE/VCO2-slope, increased maximum watts, reduction of lactate levels at 4, 6, 8 and 10 min during exercise. However, there was no significant difference with respect to increase in the latter parameters between the two groups.
Outcome of the secondary endpoints
Clinical parameters
BMI in the CG and IG showed no significant reduction (Table 3).
Echocardiography parameters
Additionally, training in the CG group showed a significant decrease in EF, whereas in the IG group the EF was increased. Also, the there was a significant difference between the group regarding change in EF (p = 0.004) (Table 3).
Laboratory parameters
Training results in both groups in a non-significant reduction of total cholesterol, LDL and fastening glucose, reduction of hsCRP and NT-pro-BNP levels. Statistically, there was no significant difference between the two groups (Table 4).
Quality of life
Comparison of anxiety and depression and EQ-5D showed no significant difference between CG and IG (Table 5).
Discussion
The main finding of this study is that home CR was feasible and safe. Furthermore, it seems that adequate use of the GEx system had a substantial effect on exercise capacity compared to standard CR in patients with cardiac disease.
Unexpectedly, some problems were encountered with the safety mechanism implemented in the portable device, which completely prevented patient participation or caused frequent delays leading to patient frustration and demotivation. There may be several reasons for it: patients started exercising with too high intensity during the warm-up or the target HR limit was so low during warm-up that it was difficult to keep HR there. Several sessions were cancelled or delayed also because of the safety measures used with BP values. That safety mechanism included research algorithms that had not been extensively tested with CAD patients and therefore were not optimized. An important note for the future is that the target HR zone should be wide enough so that the user is able to keep HR within the target and BP safety thresholds should be better tailored to patient’s conditions. These issues would have been readily surmountable had a prototype safety algorithm been clinically tested in advance with a sufficient number of patients or had there been more time to conduct the study.
Although there were technical problems, the system was accepted by users as also confirmed by a specific acceptance questionnaire.
In conclusion, for those patients who were not affected by the security algorithms, quality of the HR signal was good and the patients were able to keep their HR within the prescribed HR zone.
Limitations
Due to some technical problems and the strict behavior in the safety mechanisms, only a limited number of patients were able to reach the final study point. Still, the GEx Study demonstrated a considerable improvement in cardiopulmonary performance with a remotely supported training program compared to standard CR. Indeed, the magnitude of effect appeared substantially greater than with other exercise training programs that depend on patients attending classes and complying with exercise prescriptions at home, between classes, without remote support. The number of dropouts was disappointing but does not detract from remotely supported rehabilitation as a proof-of-concept. Some technical issues can be fixed, more lax safety algorithm can be implemented and the methods and implementation of the exercise prescription can certainly be improved. This trial provides the impetus to scale up the intervention to manage much larger patient groups, potentially in the context of further randomized controlled trials.
Conclusion
The GEx system for home-based CR showed to be feasible and successful as compared to three different national CR systems (GB, Spain, Germany). It improved exercise capacity, was associated with reduction of weight, levels of hsCRP and cholesterol levels. Training sessions were safely performed. Still, a more mature technological solution is required to make it an alternative for today’s standard CR approach.
Unfortunately the major loss to follow-up weakens any conclusions that can be drawn from the study. However, the study could be viewed as an important proof-of-concept that can be refined for deployment in more substantial clinical trials.
Abbreviations
- BF:
-
Breathing frequency
- BP:
-
Blood pressure
- CAD:
-
Coronary artery disease
- CG:
-
Control group
- CPX:
-
Cardiac exercise testing
- CR:
-
Cardiac rehabilitation
- CTC:
-
Central Training Committee
- dO2/HR:
-
Aerobic workrate
- EF:
-
Ejection fraction
- GEx:
-
Guided exercise
- HF:
-
Heart failure
- HR:
-
Heart rate
- HRR:
-
Heart rate reserve
- IG:
-
Intervention group
- MI:
-
Myocardial infarction
- O2/HR:
-
Oxygen pulse
- PC:
-
Personal computer
- QoL:
-
Quality of life
- VO2 peak:
-
Peak oxygen uptake
References
Kurose S, Iwasaka J, Tsutsumi H, Yamanaka Y, Shinno H, Fukushima Y, Higurashi K, Imai M, Masuda I, Takeda S, Kawai C, Kimura Y (2016) Effect of exercise-based cardiac rehabilitation on non-culprit mild coronary plaques in the culprit coronary artery of patients with acute coronary syndrome. Heart Vessels 31(6):846–854
Otsuka S, Morisawa T, Yuguchi S, Hojo Y, Matsuo T, Nakajima M, Ishida A, Takahashi T (2016) Clinical importance of change in physical activity after endovascular treatment combined with exercise training in patients with peripheral arterial disease. Heart Vessels
Yamauchi F, Adachi H, Tomono JI, Toyoda S, Iwamatsu K, Sakuma M, Nakajima T, Oshima S, Inoue T (2015) Effect of a cardiac rehabilitation program on exercise oscillatory ventilation in Japanese patients with heart failure. Heart Vessels
Menezes AR, Lavie CJ, Forman DE, Arena R, Milani RV, Franklin BA (2014) Cardiac rehabilitation in the elderly. Prog Cardiovasc Dis 57(2):152–159
Grace SL, Bennett S, Ardern CI, Clark AM (2014) Cardiac rehabilitation series: Canada. Prog Cardiovasc Dis 56(5):530–535
Humphrey R, Guazzi M, Niebauer J (2014) Cardiac rehabilitation in Europe. Prog Cardiovasc Dis 56(5):551–556
Madan K, Babu AS, Contractor A, Sawhney JP, Prabhakaran D, Gupta R (2014) Cardiac rehabilitation in India. Prog Cardiovasc Dis 56(5):543–550
Harb BM, Wonisch M, Brandt D, Muller R (2011) Long-term risk factor management after inpatient cardiac rehabilitation by means of a structured post-care programme. Eur J Cardiovasc Prev Rehabil 18(6):843–849
Heran BS, Chen JM, Ebrahim S, Moxham T, Oldridge N, Rees K, Thompson DR, Taylor RS (2011) Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev (7):CD001800
Schwaab B, Waldmann A, Katalinic A, Sheikhzadeh A, Raspe H (2011) In-patient cardiac rehabilitation versus medical care—a prospective multicentre controlled 12 months follow-up in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil 18(4):581–586
Reeves GR, Whellan DJ (2010) Recent advances in cardiac rehabilitation. Curr Opin Cardiol 25(6):589–596
Monte IP, Mangiafico S, Buccheri S, Bottari VE, Lavanco V, Arcidiacono AA, Leggio S, Deste W, Tamburino C (2015) Myocardial deformational adaptations to different forms of training: a real-time three-dimensional speckle tracking echocardiographic study. Heart Vessels 30(3):386–395
Bjarnason-Wehrens B, McGee H, Zwisler AD, Piepoli MF, Benzer W, Schmid JP, Dendale P, Pogosova NG, Zdrenghea D, Niebauer J, Mendes M, Cardiac Rehabilitation Section European Association of Cardiovascular P, Rehabilitation (2010) Cardiac rehabilitation in Europe: results from the European Cardiac Rehabilitation Inventory Survey. Eur J Cardiovasc Prev Rehabil 17(4):410–418
Hambrecht R, Walther C, Mobius-Winkler S, Gielen S, Linke A, Conradi K, Erbs S, Kluge R, Kendziorra K, Sabri O, Sick P, Schuler G (2004) Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation 109(11):1371–1378
Taylor RS, Davies EJ, Dalal HM, Davis R, Doherty P, Cooper C, Holland DJ, Jolly K, Smart NA (2012) Effects of exercise training for heart failure with preserved ejection fraction: a systematic review and meta-analysis of comparative studies. Int J Cardiol 162(1):6–13
Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV (2009) Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc 84(4):373–383
Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN (2008) Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil 15(3):239–246
Munk PS, Breland UM, Aukrust P, Ueland T, Kvaloy JT, Larsen AI (2011) High intensity interval training reduces systemic inflammation in post-PCI patients. Eur J Cardiovasc Prev Rehabil 18(6):850–857
Fitzpatrick P, Fitz-Simon N, Lonergan M, Collins C, Daly L (2011) Heartwatch: the effect of a primary care-delivered secondary prevention programme for cardiovascular disease on medication use and risk factor profiles. Eur J Cardiovasc Prev Rehabil 18(1):129–135
Collins L, Scuffham P, Gargett S (2001) Cost-analysis of gym-based versus home-based cardiac rehabilitation programs. Aust Health Rev 24(1):51–61
Jolly K, Lip GY, Taylor RS, Raftery J, Mant J, Lane D, Greenfield S, Stevens A (2009) The Birmingham Rehabilitation Uptake Maximisation study (BRUM): a randomised controlled trial comparing home-based with centre-based cardiac rehabilitation. Heart 95(1):36–42
Briffa TG, Eckermann SD, Griffiths AD, Harris PJ, Heath MR, Freedman SB, Donaldson LT, Briffa NK, Keech AC (2005) Cost-effectiveness of rehabilitation after an acute coronary event: a randomised controlled trial. Med J Aust 183(9):450–455
Dalal HM, Zawada A, Jolly K, Moxham T, Taylor RS (2010) Home based versus centre based cardiac rehabilitation: Cochrane systematic review and meta-analysis. BMJ 340:b5631
Carlson JJ, Johnson JA, Franklin BA, VanderLaan RL (2000) Program participation, exercise adherence, cardiovascular outcomes, and program cost of traditional versus modified cardiac rehabilitation. Am J Cardiol 86(1):17–23
Taylor RS, Dalal H, Jolly K, Moxham T, Zawada A (2010) Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev (1):CD007130
Sandesara PB, Lambert CT, Gordon NF, Fletcher GF, Franklin BA, Wenger NK, Sperling L (2015) Cardiac rehabilitation and risk reduction: time to “rebrand and reinvigorate”. J Am Coll Cardiol 65(4):389–395
Lavie CJ, Arena R, Franklin BA (2016) Cardiac Rehabilitation and Healthy Life-Style Interventions: rectifying Program Deficiencies to Improve Patient Outcomes. J Am Coll Cardiol 67(1):13–15
Beatty AL, Fukuoka Y, Whooley MA (2013) Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. J Am Heart Assoc 2(6):e000568
Shaw DK, Sparks KE, Jennings HS 3rd, Vantrease JC (1995) Cardiac rehabilitation using simultaneous voice and electrocardiographic transtelephonic monitoring. Am J Cardiol 76(14):1069–1071
Squires RW, Miller TD, Harn T, Micheels TA, Palma TA (1991) Transtelephonic electrocardiographic monitoring of cardiac rehabilitation exercise sessions in coronary artery disease. Am J Cardiol 67(11):962–964
Scalvini S, Zanelli E, Comini L, Tomba MD, Troise G, Giordano A (2009) Home-based exercise rehabilitation with telemedicine following cardiac surgery. J Telemed Telecare 15(6):297–301
Worringham C, Rojek A, Stewart I (2011) Development and feasibility of a smartphone, ECG and GPS based system for remotely monitoring exercise in cardiac rehabilitation. PLoS One 6(2):e14669
Maglaveras N, Reiter H (2011) Towards closed-loop personal health systems in cardiology: the HeartCycle approach. Conf Proc IEEE Eng Med Biol Soc 2011:892–895
Vera-Munoz C, Arredondo MT, Ottaviano M, Salvi D, Stut W (2013) HeartCycle: user interaction and patient education. Conf Proc IEEE Eng Med Biol Soc 2013:6988–6991
Ottaviano M, Vera-Munoz C, Arredondo MT, Salvi D, Salvi S, Paez JM, de Barrionuevo AD (2011) Innovative self management system for guided cardiac rehabilitation. Conf Proc IEEE Eng Med Biol Soc 2011:1559–1562
Skobel E, Martinez-Romero A, Scheibe B, Schauerte P, Marx N, Luprano J, Knackstedt C (2013) Evaluation of a newly designed shirt-based ECG and breathing sensor for home-based training as part of cardiac rehabilitation for coronary artery disease. Eur J Prev Cardiol
Pihl E, Cider A, Stromberg A, Fridlund B, Martensson J (2011) Exercise in elderly patients with chronic heart failure in primary care: effects on physical capacity and health-related quality of life. Eur J Cardiovasc Nurs 10(3):150–158
Allsup SJ, Gosney MA (2002) Anxiety and depression in an older research population and their impact on clinical outcomes in a randomised controlled trial. Postgrad Med J 78(925):674–677
Beaver WL, Wasserman K, Whipp BJ (1986) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 60(6):2020–2027
Wasserman K, Beaver WL, Whipp BJ (1990) Gas exchange theory and the lactic acidosis (anaerobic) threshold. Circulation 81(1 Suppl):II14–II30
Hansen JE, Sue DY, Oren A, Wasserman K (1987) Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. Am J Cardiol 59(6):669–674
Hansen JE, Casaburi R, Cooper DM, Wasserman K (1988) Oxygen uptake as related to work rate increment during cycle ergometer exercise. Eur J Appl Physiol Occup Physiol 57(2):140–145
Wenger NK (2008) Current status of cardiac rehabilitation. J Am Coll Cardiol 51(17):1619–1631
Pescatello LS (2001) Exercising for health: the merits of lifestyle physical activity. West J Med 174(2):114–118
Thompson PD, Arena R, Riebe D, Pescatello LS, American College of Sports M (2013) ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep 12(4):215–217
Swank AM, Horton J, Fleg JL, Fonarow GC, Keteyian S, Goldberg L, Wolfel G, Handberg EM, Bensimhon D, Illiou MC, Vest M, Ewald G, Blackburn G, Leifer E, Cooper L, Kraus WE, Investigators H-A (2012) Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail 5(5):579–585
O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL, Investigators H-A (2009) Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 301(14):1439–1450
Acknowledgments
The data collection would not have been possible without the help of Mrs. Nadine Kruckow, Clinical Trial Center Aachen, Mr. Salvador Domínguez del Río, Clinical Research Associate, Medtronic, and furthermore Mr. Jean-Marc Koller (Centre Suisse d’Electronique et de Microtechnique SA) and Mr. Harald Reiter (Philips technology) for the technical assistance and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Erik Skobel and Christian Knackstedt have received consultant honoraria from Philips, coordinator of the Project HeartCycle.
Funding
This work leading to these results has been supported by the European Community’s 7th FP project Heart Cycle (Grant agreement no. FP7-216695), coordinated by Philips.
Additional information
Erik Skobel and Christian Knackstedt contributed equally.
Rights and permissions
About this article
Cite this article
Skobel, E., Knackstedt, C., Martinez-Romero, A. et al. Internet-based training of coronary artery patients: the Heart Cycle Trial. Heart Vessels 32, 408–418 (2017). https://doi.org/10.1007/s00380-016-0897-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-016-0897-8