Abstract
Although intravenous diuretics have been mainstay drugs in patients with acute heart failure (AHF), they have been suggested to have some deleterious effects on prognosis. We postulated that renal function may modify their deleterious effects in AHF patients. The study population consisted of 1094 AHF patients from three hospitals. Renal dysfunction (RD) was defined as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 on admission, and the cohort was divided into a high-dose furosemide (≥100 mg/48 h) and low-dose furosemide group according to the amount of intravenous furosemide used within 48 h from admission. In the whole cohort, in-hospital mortality rate was higher in the high-dose furosemide group than the low-dose furosemide group (12.5 vs. 6.6 %, respectively, P = 0.001). However, this difference in the in-hospital mortality rates was significant only in the RD subgroup (15.6 vs. 7.0 %, respectively, P < 0.001), and not in the non-RD subgroup (2.5 vs. 5.9 %, respectively, P = 0.384). Propensity score-matched analysis was performed to evaluate the impact of high-dose furosemide on prognosis. After propensity score matching, high-dose furosemide was not associated with in-hospital mortality (OR 1.25, 95 % CI 0.73–2.16, P = 0.408). However, there was a qualitative difference in OR for in-hospital mortality between AHF with RD (OR 1.77, 95 % CI 0.96–3.28, P = 0.068) and without RD (OR 0.23, 95 % CI 0.05–1.10, P = 0.064), and there was a significant interaction between eGFR and prognostic impact of high-dose furosemide (P for OR interaction = 0.013). An inverse relationship was observed between eGFR and OR for in-hospital death in the group treated with high-dose furosemide (decreasing OR with better eGFR). The deleterious effect of diuretics was significantly modified with renal function in AHF. This association may be one reason for poorer prognosis of AHF patients complicated with renal impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute heart failure (AHF) is a worldwide public health issue with high rates of morbidity and mortality. The prognosis of patients with AHF is poor, and around half of all patients die or are rehospitalized due to heart failure within 6 months [1–3]. The in-hospital mortality rate of AHF ranges from 4 to 30 % [4, 5]. The number of patients requiring hospital admission due to AHF will continue to increase with increases in number of AHF patients in percentages of elderly patients [6].
Intravenous diuretics represent the cornerstone of AHF treatment, and around 80 % of AHF patients are treated with these drugs [4]. Indeed, diuretics are the main form of therapy in cases of AHF with congestive symptoms. However, unfavorable effects of diuretics have been reported, including neurohormonal activation in heart failure (HF) patients, and several observational studies have indicated deleterious effects of diuretics on prognosis in both acute and chronic HF patients [7–11]. Although the precise mechanisms underlying the negative effects of diuretics in HF patients remain to be elucidated, the direct effects of furosemide on kidney macula densa and subsequent renin release and neurohormonal activation have been well documented [12, 13]. This mechanism and the observation that renin activity is activated to a greater extent in patients with renal impairment suggest that this neurohormonal effect of diuretics may be influenced by renal function. Indeed, patients with AHF and renal dysfunction have prolonged length of hospital stay and high in-hospital and long-term mortality rates [14]. No therapy, thus far tested, has improved either short-term or long-term outcomes in these patients, including recently reported therapies such as ultrafiltration, standard and low-dose nesiritide, and low-dose dopamine [15, 16].
To clarify whether renal function modifies the prognostic impact of diuretics in AHF, we investigated an observational cohort of patients hospitalized for AHF.
Materials and methods
Patient population
The study population consisted of consecutive AHF patients aged >18 years diagnosed by the attending cardiologists according to the Framingham criteria [17] presenting with acute onset or worsening of symptoms and admitted to one of the three participating hospitals—Kameda Medical Center (965 patients-bed inpatient facility, around 250 AHF admission annually, and functions as one of the core centers in Chiba prefecture), Awa Regional Medical Center (149 patients-bed inpatient facility, around 100 AHF admission annually, and functions as one of the core centers in Chiba prefecture), and Kawasaki Medical School Hospital (965 patients-bed inpatient facility, around 120 AHF admission annually, and functions as one of the core center in Okayama prefecture)—between January 2011 and July 2013. AHF patients with acute coronary syndrome, primary pulmonary hypertension, pericardial disease, or with a history of maintained hemodialysis were excluded. On analysis, cases with B-type natriuretic peptide (BNP) <100 pg/mL on admission were also excluded because the primary diagnosis of these cases may not have been heart failure [18, 19]. Medical records were reviewed by the attending cardiologists, and baseline data, including patient characteristics, medical history, and all initial treatments provided within 48 h after admission, including the total intravenous furosemide dose, were collected. The values of all biomarkers on admission were collected. Estimated glomerular filtration rate (eGFR) was calculated by the Japanese coefficient-modified Chronic Kidney Disease Epidemiology Collaboration equation. In this equation, the Japanese coefficient of 0.813 was used to calculate eGFR. The internal and external validation of this equation has already been satisfactorily performed [20]. A diagnosis of renal dysfunction (RD) on admission was made in cases in which eGFR calculated from the creatinine level on admission was <60 mL/min/1.73 m2. The primary outcome in this study was all-cause in-hospital mortality. This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee and institutional review board of each participating hospital. Due to the retrospective and observational nature of the present study, written informed consent was not required under current Japanese guidelines.
Statistical analysis
Data are expressed as the mean ± standard deviation for normally distributed variables and as the medians with interquartile range for non-normally distributed data. The distribution of data was examined by Kolmogorov–Smirnov test, and variables were transformed for further analyses when necessary. Categorical data are expressed as numbers and percentages. We divided the whole cohort into four groups according to quartiles of the total furosemide diuretic dose, and baseline characteristics were compared by one-way analysis of variance (ANOVA), Kruskal–Wallis test, or Chi squared test where appropriate. We calculated the odds ratio (OR) with 95 % confidence interval (95 % CI) derived from the logistic regression model to evaluate the prognostic effect of high-dose furosemide. Multiple imputation generated 2000 data sets of complemented missing values [1 case (0.1 %) in heart rate; 1 case (0.1 %) in prescription at admission of angiotensin converting enzyme inhibiter (ACE-I) or angiotensin receptor II blocker (ARB); 10 cases (1.2 %) in prescription of beta blocker; 1 case (0.1 %) in amount of prescribed oral furosemide; 26 cases (2.4 %) in left ventricular ejection fraction; 10 cases (0.9 %) in hemoglobin; and 2 cases (0.2 %) in serum potassium] [21]. To investigate the relationship between renal function on admission and the prognostic impact of diuretic dose, we defined high-dose furosemide as a total dose of ≥100 mg within 48 h, corresponding to the highest quartile dose in this cohort [22]. To adjust for differences in patient backgrounds, we constructed a logistic model for calculating the propensity score (PS) for each individual based on the following variables: age; gender; systolic blood pressure; heart rate; left ventricular ejection fraction; hemoglobin; albumin; serum creatinine; blood urea nitrogen (BUN); serum sodium; serum potassium; brain natriuretic peptide (BNP); dose of prescribed furosemide at admission; prescription at admission of ACE-I or ARB, HMG-CoA inhibitor, and beta blocker; history of atrial fibrillation, hypertension, and diabetes. PS matching was performed in a one-to-one fashion between high-dose furosemide and low-dose furosemide groups. The balancing of variables that could potentially affect the prognosis before and after PS matching was checked by comparing standardized mean differences. An absolute standardized difference <0.1 was considered to indicate successful balancing [23].
The OR of in-hospital mortality for patients treated with high-dose furosemide was compared to patients treated with low-dose furosemide (total furosemide dose <100 mg within 48 h) and was graphically described at each level of eGFR. The interaction between renal function and the prognostic impact of high-dose diuretics was examined for in-hospital all-cause death. Statistical analyses were performed using R version 3.2.0 (R foundation for Statistical Computing, Vienna, Austria) and the graphical user interface EZR [24]. In all analyses, P < 0.05 was taken to indicate statistical significance.
Results
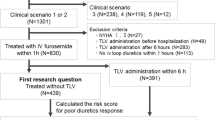
Of the total of 1278 potentially eligible AHF patients admitted to one of the three participating hospitals, 1094 patients were finally included in the analyses (Fig. 1). No patients were treated with any intravenous diuretics other than furosemide. The median dose of intravenous furosemide used within 48 h of admission was 60 mg and the interquartile range was 20–100 mg. The whole cohort was divided into high- and low-dose furosemide groups according to the definition outlined above (Table 1). The high-dose furosemide group included more males, had less history of hypertension and atrial fibrillation, and higher prescribed furosemide dose at admission, lower hemoglobin and albumin, and higher potassium level. There were also pronounced differences in renal function (creatinine and BUN) and BNP at admission between the two groups.
There were 92 all-cause in-hospital deaths (8.4 %), and Fig. 2 shows in-hospital mortality in the whole cohort and subgroups. In the whole cohort, the in-hospital mortality rate was higher in the high-dose furosemide than the low-dose furosemide group (12.5 vs. 6.6 %, respectively, P = 0.001). However, in-hospital mortality rates were significantly different between groups only in the RD subgroup (15.6 vs. 7.0 %, respectively, P < 0.001) but not in the non-RD subgroup (2.5 vs. 5.9 %, respectively, P = 0.384).
To investigate the impact of interaction between RD and deleterious effect of furosemide, we performed PS matching in high-dose and low-dose furosemide groups. The characteristics of patients treated with low- and high-dose furosemide after PS matching are listed in Table 1. After PS matching, the high- and low-dose furosemide groups were well balanced (Fig. 3). In the PS-matched cohort, high-dose furosemide was not associated with high in-hospital mortality in the whole cohort (OR 1.25, 95 % CI 0.73–2.16, P = 0.408). However, OR for in-hospital mortality was qualitatively different in the RD subgroup (OR 1.77, 95 % CI 0.96–3.28, P = 0.068) and non-RD subgroup (OR 0.23, 95 % CI 0.05–1.10, P = 0.064), and there was a significant interaction between eGFR and prognostic impact of high-dose furosemide (P for OR interaction = 0.013). These results did not change if we used RD (eGFR <60 mL/min/1.73 m2) as an interacting factor of prognostic impact of high-dose furosemide as a sensitivity analysis (P for OR interaction = 0.018). Figure 4 describes the OR of in-hospital death for patients treated with high-dose furosemide compared to those receiving low-dose furosemide estimated at each level of eGFR. An inverse relationship was observed between eGFR and OR for in-hospital death in the group treated with high-dose furosemide (decreasing OR with better eGFR).
We also did a sensitivity analysis by applying ≥160 mg as a definition of high-dose furosemide. After propensity score matching, 133 pairs were obtained. Although inverse relationship was retained between eGFR and OR for in-hospital death in the group treated with high-dose furosemide (decreasing OR with better eGFR) even in this analysis (data not shown), P for OR interaction lost its significance (P for OR interaction = 0.433).
Discussion
In our multicenter retrospective AHF dataset, high-dose intravenous furosemide use within 48 h of admission seemed to be associated with poor prognosis only before but not after PS matching. However, there was a significant qualitative interaction in prognostic impact of high-dose furosemide use between AHF patients with and without RD in well-matched groups. We also confirmed that there was an inverted linear relationship between eGFR at admission and OR of high-dose furosemide use for in-hospital mortality in the PS matched cohort. These results suggested that renal function modifies the prognostic impact of furosemide independent of other confounding factors. This novel finding may explain why AHF patients with renal impairment have poorer prognosis than those with conserved renal function.
Prognostic implications of furosemide in AHF patients
Treatment of AHF patients with loop diuretics has been shown to activate the renin–angiotensin–aldosterone system and sympathetic nervous activity [8, 25], which are the factors that are most likely involved in the poor prognosis in such cases.
In the present study, high-dose furosemide in AHF patients was not associated with poorer prognosis in the whole cohort after balancing for differences in some patient characteristics at baseline by PS. These results were consistent with some previous studies but not others. Peacock et al. analyzed the ADHERE registry and suggested that high-dose furosemide was associated with poorer prognosis [26]. However, this study potentially did not fully adjust for difference in patient background between high- and low-dose furosemide groups, because some important variables associated with prognosis, including BNP, were missed and were not included in PS analysis. We used all of the important prognostic markers for PS analysis and these factors were well balanced between high- and low-dose furosemide groups. Conversely, Yilmaz et al. reported that there was no difference in prognosis between AHF patients treated with intravenous furosemide at doses above and below 1.0 mg/kg within 24 h of admission in the ALARM-HF registry [27]. In this study, the authors dichotomized the whole cohort into high- and low-dose groups according to the 75th percentile of furosemide dose (1.0 mg/kg) used within 24 h of admission and performed propensity score-matched analysis.
Similarly, there was no significant difference in 30-day outcome between high- and low-dose furosemide groups in the DOSE trial [28]. However, it may not be possible to simply generalize this result to Japanese AHF patients because only those treated with oral furosemide at a dose of >80 mg daily were included in the DOSE study. The median dose of oral furosemide prescribed in our cohort was 10 mg, consistent with the recently published results of the Japanese Heart Failure Registry [29], and less than 10 % of patients in the present study fulfilled this inclusion criterion. The consistency of our study results with the DOSE study makes it possible to validate these results even for a Japanese AHF cohort.
Interaction between renal function and prognostic implications of diuretic dose
Significant interaction between renal function and prognostic implication of diuretics could be explained in the context of neurohormonal activity. Plasma renin activity is activated to a greater extent in HF patients with renal impairment than in those with conserved renal function, and it was shown to be an independent prognostic predictor [30, 31]. Impaired renal function in AHF patients on admission may be due to preadmission worsening renal function caused by decompensation of HF and subsequent neurohormonal activation. This is supported by the results of two observational studies regarding improvement of renal function in AHF. In the first of these studies, patients with improvement of renal function had significantly higher preadmission eGFR, and the change in eGFR from preadmission to admission was significantly greater in those experiencing improvement of renal function, while prognosis was poorer compared to those without improvement of renal function [32]. In the other study, the BUN/Cr ratio, which is recognized as an indicator of neurohormonal activation, on admission was shown to be an independent predictor of improvement of renal function [33]. These observations suggest that neurohormonal activation may be activated to a greater extent in AHF patients with impaired than conserved renal function, and it is natural to suggest that the prognosis would be poorer with activation of renin and sympathetic nervous activity by diuretics especially in AHF patients with high levels of neurohormonal activation.
This hypothesis was supported by the results of an observational study by Testani et al., which showed that high-dose furosemide therapy worsened the prognosis of chronic heart failure patients only in those with high BUN level [22]. This suggests that there is a significant interaction between the degree of neurohormonal activation and prognostic implications of furosemide. The BUN level was significantly higher in the high-dose furosemide group in the present study; however, the interaction between prognostic impact of high-dose furosemide and BUN was not significant in our post-PS matched cohort (P = 0.226). These observations suggest that renal function itself influences the prognostic implications of furosemide independent from BUN. As patients with RD are treated with high-dose diuretics in both acute and chronic heart failure, the deleterious effects of diuretics on prognosis must be enhanced synergistically in this high-risk population.
Our study had some limitations. First, this was a retrospective study, and, therefore, the accuracy of some variables relied on the accuracy of medical records. We evaluated furosemide only with regard to the dose administered within 48 h of admission and not later, and we did not evaluate the means of administration (i.e., continuous vs. bolus). A recent study showed that continuous furosemide infusion was associated with greater increases in plasma renin activity than bolus furosemide [34], and this may have affected our results. We did not take oral diuretic administration into consideration. However, the bioavailability of furosemide can vary, and is lower when prescribed orally, so we cannot speculate on the effects of per os furosemide in each patient [35, 36]. The number of events in non-RD group was low, and lack of association between mortality and furosemide dose might have been attributed to type II error. We evaluated only all-cause in-hospital mortality as an endpoint and, therefore, could not investigate the relations between furosemide dose and either cause-specific death or long-term mortality rate. However, this made our study results more robust because no patients were lost to follow-up and the endpoint was both objective and clinically relevant. We did not have data regarding serial changes in renal function because of the retrospective nature of this study, and creatinine was not measured according to predefined regimens. In our sensitivity analysis, P value for OR interaction was not significant; however, as higher cut-off resulted in lower number of patients with high-dose furosemide group, number of events was also reduced (only 34 in-hospital death). This is clearly under powered to detect significant interaction. Moreover, we define high-dose furosemide according to the highest quartile dose in this cohort. As this definition was used in another study and not defined arbitrarily [22], we believe that using the dose corresponding to the 75th percentile (i.e. 100 mg) in our cohort as a cutoff is reasonable. We used propensity score matching to adjust for confounders to evaluate the association between amount of diuretics and mortality. However, as propensity score cannot adjust for unknown confounders, and prognostic factors of AHF are multifactorial and complex. There are still possibilities of our results and conclusions confounded by other covariates.
In conclusion, the results of the present study indicated that there is an interaction between impact of furosemide on prognosis and renal function in AHF patients. These observations may partially explain the association between renal function and worse prognosis in AHF patients. Clinically, this study indicated that reducing furosemide dose is warranted in AHF patients with renal impairment.
References
Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF Jr, Gheorghiade M, O’Connor CM (2004) Risk stratification after hospitalization for decompensated heart failure. J Card Fail 10:460–466
Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV (2003) Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 290:2581–2587
Setoguchi M, Hashimoto Y, Sasaoka T, Ashikaga T, Isobe M (2015) Risk factors for rehospitalization in heart failure with preserved ejection fraction compared with reduced ejection fraction. Heart Vessels 30:595–603
Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CS, Sato N, Shah AN, Gheorghiade M (2014) The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63:1123–1133
Sakata Y, Shimokawa H (2013) Epidemiology of heart failure in Asia. Circ J 77:2209–2217
Sato N (2013) Critical issue in the cardiovascular field: hospitalization for heart failure. J Cardiol 62:140–141
Scott JE, Bosworth TR (1990) The comparative chemical morphology of the mammalian cornea. Basic Appl Histochem 34:35–42
Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN (1985) Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med 103:1–6
Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ (1999) Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation 100:1311–1315
Ahmed A, Husain A, Love TE, Gambassi G, Dell’Italia LJ, Francis GS, Gheorghiade M, Allman RM, Meleth S, Bourge RC (2006) Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J 27:1431–1439
Nakada Y, Takahama H, Kanzaki H, Sugano Y, Hasegawa T, Ohara T, Amaki M, Funada A, Yoshida A, Yasuda S, Ogawa H, Anzai T (2015) The predictability of renin–angiotensin–aldosterone system factors for clinical outcome in patients with acute decompensated heart failure. Heart Vessels. doi:10.1007/s00380-015-0688-7
Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J (2009) The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54:1747–1762
Sarraf M, Masoumi A, Schrier RW (2009) Cardiorenal syndrome in acute decompensated heart failure. Clin J Am Soc Nephrol 4:2013–2026
Butler J, Chirovsky D, Phatak H, McNeill A, Cody R (2010) Renal function, health outcomes, and resource utilization in acute heart failure: a systematic review. Circ Heart Fail 3:726–745
Bart BA, Goldsmith SR, Lee KL, Givertz MM, O’Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E, Heart Failure Clinical Research Network (2012) Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 367:2296–2304
Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, Bart BA, Bull DA, Stehlik J, LeWinter MM, Konstam MA, Huggins GS, Rouleau JL, O’Meara E, Tang WH, Starling RC, Butler J, Deswal A, Felker GM, O’Connor CM, Bonita RE, Margulies KB, Cappola TP, Ofili EO, Mann DL, Davila-Roman VG, McNulty SE, Borlaug BA, Velazquez EJ, Lee KL, Shah MR, Hernandez AF, Braunwald E, Redfield MM, Network NHFCR (2013) Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 310:2533–2543
McKee PA, Castelli WP, McNamara PM, Kannel WB (1971) The natural history of congestive heart failure: the Framingham study. N Engl J Med 285:1441–1446
Maisel A (2002) B-type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what’s next? Circulation 105:2328–2331
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, ESC Committee for Practice Guidelines (2012) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33:1787–1847
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S (2010) Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis 56:32–38
Rubin DB (1987) Multiple imputation for nonresponse in surveys. Wiley, New York, pp 1–250
Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE (2011) Interaction between loop diuretic-associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol 58:375–382
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46:399–424
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
He XR, Greenberg SG, Briggs JP, Schnermann J (1995) Effects of furosemide and verapamil on the NaCl dependency of macula densa-mediated renin secretion. Hypertension 26:137–142
Peacock WF, Costanzo MR, De Marco T, Lopatin M, Wynne J, Mills RM, Emerman CL, ADHERE Scientific Advisory Committee and Investigators (2009) Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology 113:12–19
Yilmaz MB, Gayat E, Salem R, Lassus J, Nikolaou M, Laribi S, Parissis J, Follath F, Peacock WF, Mebazaa A (2011) Impact of diuretic dosing on mortality in acute heart failure using a propensity-matched analysis. Eur J Heart Fail 13:1244–1252
Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O’Connor CM, Network NHFCR (2011) Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 364:797–805
Miura M, Shiba N, Nochioka K, Takada T, Takahashi J, Kohno H, Shimokawa H, Investigators C- (2012) Urinary albumin excretion in heart failure with preserved ejection fraction: an interim analysis of the CHART 2 study. Eur J Heart Fail 14:367–376
Vergaro G, Emdin M, Iervasi A, Zyw L, Gabutti A, Poletti R, Mammini C, Giannoni A, Fontana M, Passino C (2011) Prognostic value of plasma renin activity in heart failure. Am J Cardiol 108:246–251
Poletti R, Vergaro G, Zyw L, Prontera C, Passino C, Emdin M (2013) Prognostic value of plasma renin activity in heart failure patients with chronic kidney disease. Int J Cardiol 167:711–715
Testani JM, McCauley BD, Chen J, Coca SG, Cappola TP, Kimmel SE (2011) Clinical characteristics and outcomes of patients with improvement in renal function during the treatment of decompensated heart failure. J Card Fail 17:993–1000
Brisco MA, Coca SG, Chen J, Owens AT, McCauley BD, Kimmel SE, Testani JM (2013) Blood urea nitrogen/creatinine ratio identifies a high-risk but potentially reversible form of renal dysfunction in patients with decompensated heart failure. Circ Heart Fail 6:233–239
Mentz RJ, Stevens SR, DeVore AD, Lala A, Vader JM, AbouEzzeddine OF, Khazanie P, Redfield MM, Stevenson LW, O’Connor CM, Goldsmith SR, Bart BA, Anstrom KJ, Hernandez AF, Braunwald E, Felker GM (2015) Decongestion strategies and renin–angiotensin–aldosterone system activation in acute heart failure. JACC Heart Fail 3:97–107
Vasko MR, Cartwright DB, Knochel JP, Nixon JV, Brater DC (1985) Furosemide absorption altered in decompensated congestive heart failure. Ann Intern Med 102:314–318
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL (2013) 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128:1810–1852
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Y. M. received an honorarium from Otsuka Pharmaceuticals. K. Y. receives tuition support jointly from Japan Student Services Organization and Harvard T. H. Chan School of Public Health (partially supported by training grants from Pfizer, Takeda, Bayer, and PhRMA). M. S. has received honoraria from Otsuka Pharmaceutical Co., Bayer HealthCare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Medtronic, Boston Scientific, and Fukuda Denshi. Other authors have nothing to disclose.
Rights and permissions
About this article
Cite this article
Matsue, Y., Shiraishi, A., Kagiyama, N. et al. Renal function on admission modifies prognostic impact of diuretics in acute heart failure: a propensity score matched and interaction analysis. Heart Vessels 31, 1980–1987 (2016). https://doi.org/10.1007/s00380-016-0817-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-016-0817-y