Abstract
To propose a clinical prognostic index for death and heart failure in patients with ischemic cardiomyopathy implanted with an ICD. This prospective study included 192 consecutive patients (age 68 ± 10) recruited from 2004 to 2009 and implanted with an ICD for MADIT II criteria. All patients performed 24-h ambulatory blood pressure monitoring after discharge and common haematological samples. The prognostic index (PI) was built according to the formula: 120 − age + mean 24 h systolic blood pressure − (creatinine × 10). Other variables were assessed: EF, haemoglobin concentration, mean 24 h heart rate and diastolic blood pressure, sodium level, pacing mode and diabetes. Non-arrhythmic cardiac death and new hospitalizations for heart failure during 1-year follow-up were the combined end point. A total of 48 events (25 %) occurred during the follow-up: 7 cardiac deaths and 41 hospitalizations for acute heart failure. Cox proportional-hazards model showed that PI was the only predictor of events (HR = 0.96; CI 95 % 0.944–0.976, p < 0.0001). ROC curve showed that PI best cut-off was 144, with AUC 0.79, p < 0.0001; sensitivity 77 %, specificity 74 %, positive predictive value 50 %, negative predictive value 90 %. PI was predictive of events in a clinical setting where EF had no predictive value. PI works according to the rule “the lower the worse”. The high negative predictive value (90 %) of PI allows to identify subjects at lower risk for death and heart failure. PI can be a practical tool to stratify risk in ischemic cardiomyopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subjects with ischemic cardiomyopathy and low ejection fraction (EF) who receive a defibrillator to prevent sudden death are still at high risk of heart failure and non-arrhythmic death [1].
Prediction tools are particularly helpful in this context in guiding medical decision-making.
Unfortunately, methods for predicting heart failure and non-arrhythmic death in these subjects are lacking and left ventricular ejection fraction (LVEF), the well-known gold standard method in predicting cardiovascular events, seems to be an uncertain predictor when applied in a cohort of subjects with depressed systolic function (all with LVEF ≤35 %).
In our previous studies it was found that age, creatinine concentration, mean 24-h systolic blood pressure but not ejection fraction were independent predictors in subjects with ischemic cardiomyopathy and ICD [2, 3].
In present study, we propose a new prognostic index (PI) built with the same variables linked in a formula according to 120 − age + m24hSBP − (creatinine × 10).
The new index has been tested to predict new hospitalizations for acute heart failure and cardiac non-arrhythmic death (the combined end point) in 1-year follow-up in 192 subjects who implanted an ICD for MADIT II criteria [4, 5].
Methods
Study population included 192 consecutive subjects with previous myocardial infarction and left ventricular systolic dysfunction (EF ≤ 35 %) who just received an ICD, for primary prevention of sudden death.
Exclusion criteria were relevant comorbidities, permanent atrial fibrillation and inability to follow the study protocol. The study was approved by institutional review committee and the informed consent was given by the subjects.
PI, left ventricular ejection fraction, pacing mode (CRT or not), mean 24-h diastolic blood pressure, mean 24-h heart rate, haemoglobin concentration (Hb) (g/dl), sodium level (mEq/l) and diabetes were evaluated.
The general features of the study population are reported in Table 1.
Mean 24-h blood pressure values and m24-h heart rate were obtained by ambulatory blood pressure monitoring (ABPM) with the Spacelabs 90207 recorded 2 weeks after discharge.
ABPM system records blood pressure for 24 h or longer with subjects doing their normal daily activities and during sleep. All the monitors, fully automatic, used the oscillometric technique and were programmed to take readings every 15 (daytime) or 30 min (night).
ABPM can provide an estimate of the true blood pressure and the recorded values do not significantly differ from intra-arterial measures [6]. A mean of 81 measurements/ABPM were recorded with 15.552 total readings.
LVEF was assessed by 2D-echo according to the Simpson method [7] and performed before ICD implantation and about 2 weeks before ABPM recordings.
Haematological samples were collected the same day of the ABPM recordings. Serum creatinine, sodium level and haemoglobin concentration were measured.
The follow-up was 12 months, with ICD controls every 3 months.
Cardiac death and symptomatic left ventricular dysfunction, defined as acute pulmonary oedema or development of signs and symptoms of heart failure requiring hospitalization, have been the combined end point.
Two or more hospitalizations for one subject during the follow-up were considered a single event.
The appropriate classification of events was obtained checking the single clinical documents.
The choice of the pharmacological treatments was left to the attending cardiologists; during the study most subjects received standard medical therapy for heart failure with 96 % with diuretics, 94 % ACE-inhibitors or angiotensin receptor blockers, 90 % statins, 85 % β-blockers and 16 % digoxin.
Means ± SD were calculated for continuous variables, whereas frequencies were measured for categorical variables (Table 1).
Multivariate Cox proportional hazard regression analysis was used to identify risk factors for time-related occurrence of the combined end point during the follow-up.
The results of the Cox proportional-hazards model are presented as the hazard ratio (HR) and 95 % confidence interval (CI).
ROC curve was built for the PI as independent predictor.
The survival curves were assessed with the Kaplan–Meier method. Survival curves of the subgroups were compared using the log-rank test.
Data analysis was performed using the R statistical software package (R 2.9.1 version The R Foundation for Statistical Computing). A p value <0.05 was considered statistically significant.
Results
During 1-year follow-up the primary combined end point was registered in 48 subjects (25 %) with 7 cardiac deaths and 41 hospitalizations for acute heart failure.
The Cox multivariate analysis showed that PI for its low values is the only independent predictor of events (HR = 0.96; CI 95 % 0.944–0.976, p < 0.0001) (Table 2).
None of the other variables and particularly LVEF was predictive (p = 0.61).
The ROC curve of the PI (Fig. 1) showed the best cut-off value equal to 144, (AUC 0.79, p < 0.0001. Sensitivity 77 %, specificity 74 %, positive predictive value 50 %, negative predictive value 90 %).
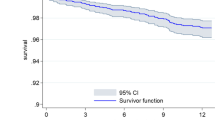
The Kaplan–Meier survival analysis confirmed a significant cumulative risk for groups with lower PI (Fig. 2).
Discussion
The identification of subjects at high risk of heart failure is an extremely important issue in cardiology and particularly in people with previous myocardial infarction.
LVEF evaluated by 2D-echo is universally known as an important prognostic variable in this context [8].
However, LVEF is not able to stratify risk in a cohort of subjects suffering from ischemic cardiomyopathy with left ventricular systolic function uniformly depressed (all with LVEF ≤35 %).
In fact, LVEF does not predict the outcome in our study (p = 0.61), but it shows (see Table 2) a very large confidence index (0.001–88.46).
The explanation is in the wide range value of this variable, in our study; from 11 to 35 %.
Possibly, LVEF is predictive only for the lower values of this range. The small sample of the study and the number of the events does not allow to validate this hypothesis.
Several attempts have been done in the congestive heart failure population with or without ICD to identify predictors of life expectancy [9].
Multiples studies found that systolic blood pressure, serum creatinine and age were independent predictors of death and heart failure in subjects with ischemic and non-ischemic cardiomyopathy [2, 3, 10–15].
Our prognostic index was built supposing that the synergistic action of the independent variables, linked in a formula, could be useful in predicting non-arrhythmic death and new hospitalizations for heart failure. Examining the single variables and particularly the systolic blood pressure, we can point out immediately the interesting result concerning blood pressure; in fact the well-known dogma “the lower the better”, true in primary prevention can be uncertain in secondary prevention and particularly in heart failure.
This finding has been previously described in high-risk subjects with or without ischemic heart disease [16] and in acute or chronic heart failure. In fact, the Acute Decompensated Heart Failure National Registry (ADHERE) developed a risk-stratification model to predict in-hospital mortality, using the data collected at admission of the first 33.046 hospitalizations with primary diagnosis of acutely decompensated heart failure, in 263 hospitals in the United States [17].
The data were subjected to classification and regression tree analysis to identify the best predictors to develop the model. The validity was then independently assessed using a second validation cohort consisting of the subsequent 32.229 hospitalization episodes.
The majority of the subjects (58 %) of the ADHERE registry had coronary artery disease and 52 % were women [18].
Of the 39 variables evaluated the method identified blood urea nitrogen level (≥43 mg/dl) at admission as the best single discriminator between hospital survivors and non-survivors. The next best predictor of in-hospital mortality in both, the higher and lower blood urea nitrogen, was low systolic blood pressure, clinically measured, at a discriminator level of 115 mmHg.
The finding of the low systolic blood pressure, clinically measured, as one of the independent predictors for mortality, in a long-term follow-up (1, 2 and 3 years) was also confirmed in the Seattle Heart Failure model, a modifiable score, prospectively validated by data of 9,942 subjects with heart failure, and obtained calculating clinical, laboratory variables and medical or devices tools [19].
Thus the above-mentioned studies involving 75.217 episodes of heart failure, with ischemic and non-ischemic cardiomyopathy, in a short and long time, prove the efficacy of low systolic blood pressure, also clinically measured, to predict mortality.
Our study is the first to have employed the ABPM in stable subjects with ischemic cardiomyopathy and ICD (the MADIT II cohort), to evaluate the risk of mortality and acutely decompensated heart failure and confirms the relevance, in a small cohort, of low systolic blood pressure as a predictor of outcome.
Why do subjects with ischemic cardiomyopathy and with low systolic blood pressure can be at higher risk of acutely decompensated heart failure and death?
We can hypothesize that low systolic blood pressure, in these subjects, is a consequence of the left ventricular systolic dysfunction, frequently associated with right ventricular involvement. Moreover, low systolic blood pressure can be an expression of a damaged neurohormonal pathway, resulting in an unfit response to usual and unusual stressors [20].
The second question is: what are the consequences of the blood pressure reduction caused by ACE-inhibitors, angiotensin receptor blockers and β-blockers in these patients?
These drugs should increase stroke volume and the blood pressure in subjects with heart failure, reducing the peripheral resistances and heart rate; thus an important reduction of the systolic blood pressure can indicate that lower peripheral resistances and lower heart rate are not followed by an increase of the stroke volume, unmasking a severe systolic ventricular dysfunction.
Another predictive independent variable of the study is the serum creatinine concentration for which we can say: “the lower the better”.
The serum creatinine concentration, in fact, is a rough marker of the renal function and several studies have demonstrated that low kidney function is a significant risk factor for mortality and acutely decompensated heart failure in a short and long time, in subjects with left ventricular dysfunction [21, 22].
The above-mentioned ADHERE study found that a high serum creatinine concentration (≥2.75 mg/dl) provided additional prognostic value for subjects having high blood urea nitrogen (≥43 mg/dl) and low systolic blood pressure (<115 mmHg).
Thus in ADHERE registry, two of the three predictors (high blood urea nitrogen and high serum creatinine) are concerning the renal function, demonstrating the strong linkage between heart and kidney, particularly in this context.
Multiple other evaluations have demonstrated an association between clinical outcome and indices of renal function in subjects hospitalized for heart failure.
The Digitalis Investigation Group trial, a multivariate model for predicting mortality in patients with heart failure and systolic dysfunction [21] found that serum creatinine was one of the independent predictors of mortality, in a short and long time, in a validation cohort of 2,145 subjects with heart failure and depressed LVEF (28 ± 8 %).
A worsening of renal function was associated with a 7.5-fold increase in the adjusted risk of in-hospital mortality in a retrospective review of 1,004 consecutive subjects hospitalized for heart failure at 11 geographically diverse hospitals [22].
Other studies confirm the relevance of the renal function in heart failure, evaluated as serum creatinine concentration or estimated as glomerular filtration rate (eGFR) which incorporates serum creatinine, age, gender and race [15, 23]. Moreover, a retrospective analysis of the MADIT II study showed that low eGFR or higher creatinine level (≥1.4 mg/dl) were independent predictors of hospitalization for heart failure in subjects with ischemic cardiomyopathy [24].
How does kidney impairment work to worsen heart failure? As a player, as a marker or both?
As a player, renal dysfunction causes further congestion and neurohormonal activation which are factors associated with adverse outcome [25].
As a marker, it is well known that higher serum creatinine levels can be caused by higher diuretic dosages, usually prescribed in subjects suffering from a more severe left ventricular dysfunction [26].
The age, the third component of the index is a progressive degenerative condition and an unquestionable powerful predictor for mortality and cardiovascular events.
All these risk factors can exist contemporarily in the same subject; thus the prediction risk model (to be meaningful) have to consider all the variables in combination providing a practical prediction tool as our prognostic index can be.
In conclusion, left ventricular ejection fraction evaluated by 2D-echo is not predictive, in our study, for acutely decompensated heart failure and cardiac non-arrhythmic death.
Conversely, the PI obtainable with simple variables is predictive for the same events and low systolic blood pressure disappoints the well-known dogma about blood pressure and cardiovascular risk.
The concept “the lower the better”, demonstrated in primary prevention seems to be not true in heart failure.
An important systolic blood pressure lowering during intensive pharmacological therapy can unmask a more compromised ventricular function.
Renal dysfunction evaluated as serum creatinine concentration is confirmed, in our study, as predictor of adverse outcome in subjects with heart failure.
The very high negative predictive value (90 %) of the PI can be useful to stratify people with heart failure at low risk to apply safely the home-based follow-up.
Although drug treatment was similar in subjects with and without events (almost all patients received β-blockers, ACE-inhibitors, angiotensin receptor blockers and diuretics), it was not possible to perform a detailed analysis of the drug doses in individuals; so we cannot exclude that differences in dosage may have influenced our results.
The number of the subjects studied and mostly men and in one centre, are the major limits of the study; but the high rate of events (25 % in 1 year) reduces the burden of the small size cohort.
Other studies should be performed but PI could be considered to be applied in existing databases of heart failure to be validated in a larger population.
References
Cevik C, Perez-Verdia A, Nugent K (2009) Implantable cardioverter defibrillators and their role in heart failure progression. Europace 11:710–715
Antonini L, Colivicchi F, Pasceri V, Greco S, Varveri A, Turani L, Kol A, Santini M (2008) A prognostic index relating 24-hour ambulatory blood pressure to cardiac events in ischemic cardiomyopathy following defibrillator implantation. Pacing Clin Electrophysiol 31:1089–1094
Antonini L, Pasceri V, Mollica C, Ficili S, Poti G, Aquilani S, Santini M, La Rocca S (2011) Ambulatory blood pressure monitoring, 2D-echo and clinical variables to cardiac events in ischaemic cardiomyopathy following cardioverter-defibrillator implantation. J Cardiovac Med 12:334–339
MADIT Executive Committee Multicenter Automatic Defibrillator (1991) Implantation trial (MADIT):design and clinical protocol. Pacing Clin Electrophysiol 14:920–927
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML (2002) Multicenter automatic defibrillator implantation trial investigators: prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346:877–883
White WB, Lund-Johansen P, Omvik P (1990) Assessment of four ambulatory blood pressure monitors and measurements by clinicians versus intraarterial blood pressure at rest and during exercise. Am J Cardiol 65:60–65
Otterstad JE, Froeland G, Sutton M, Holme J (1997) Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimension and function. Eur Heart J 18:507–513
Lewis EF, Moye LA, Rouleau JL, Sacks FM, Arnold JM, Warnica JW (2003) Predictors of late development of heart failure in stable survivors of myocardial infarction: the CARE study. J Am Coll Cardiol 42:1446–1453
Colonna P, Antonelli G (2011) In search of the best prognostic factor in patients with congestive heart failure: the paradox of ejection fraction without prognostic significance. J Cardiovasc Med 12:314–317
Lee KL, Woodlief LH, Topol EJ, Weaver WD, Betriu A, Col J, Simoons M, Aylward P, Van de Werf F, Califf RM (1995) Predictors of 30 day mortality in the era of reperfusion for acute myocardial infarction. Results from an international trial of 41021 patients GUSTO I investigators. Circulation 91:1659–1668
Goldenberg I, Moss AJ, McNitt S, Zareba W, Hall WJ, Andrews ML (2007) MADIT II Investigators. Inverse relationship of blood pressure levels to sudden cardiac mortality and benefit of the implantable cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol 49:1427–1433
Raphael CE, Whinnett ZI, Davies JE, Fontana M, Ferenczi EA, Manisty CH, Mayet J, Francis DP (2009) Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart 95(1):56–62
Cheng RK, Horwich TB, Fonarow GC (2008) Relation of systolic blood pressure to survival in both ischemic and non ischemic systolic heart failure. Am J Cardiol 102:1698–1705
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R (2008) Cardiorenal syndrome. J Am Coll Cardiol 52:1527–1539
Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ (2001) Reduced kidney function and anemia as risk factor for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 38:955–962
ONTARGET investigators (2009) Prognostic value of blood pressure in patients with high vascular risk in the Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial study. J Hypertens 27:1360–1369
Storrow AB, Emerman CL, Abraham WT, Wagoner LE, Collins S, Lindsell C, Wynne J (2004) Clinical risk factors for adverse outcomes in emergency department patients with acute decompensated heart failure: an ADHERE registry analysis. Ann Emerg Med 44(4):S72
Fonarow GC, Adams KF, Abrahan WT (2005) Risk stratification for in hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 293(5):572–580
Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB (2006) The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 113:1424–1433
Kallistratos, Poulimenos L, Pavlidis AN, Dritsas A, Laoutaris ID, Manolis AJ, Cokkinos DV (2012) Prognostic significance of blood pressure response to exercise in patients with systolic heart failure. Heart Vessels 27(1):46–52
Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW (2000) The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol 35:681–689
Smith GL, Vaccarino V, Kosiborod M, Lichtman JH, Cheng S, Watnick SG, Krumholz HM (2003) Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure? J Card Fail 9(1):13–25
Brophy JM, Dagenais GR, McSherry F, Williford W, Yusuf S (2004) A multivariate model predicting mortality in patients with heart failure and systolic dysfunction. Am J Med 116(5):300–304
Forman DE, Butler J, Wang Y (2004) Incidence, predictors at admission and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol 43:61–67
Kimura M, Hiramitsu S, Miyagishima K, Mori K, Yoda R, Kato S, Kato Y, Morimoto S, Hishida H, Ozaki Y (2010) Cardio-renal interaction: impact of renal function and anemia on the outcome of chronic heart failure. Heart Vessels 25(4):306–312
Sze E, Moss AJ, McNitt S, Barsheshet A, Andrews ML, Zareba W, Goldenberg I (2010) For the Multicenter Automatic Defibrillator Implantation Trial II Investigator. J Cardiovas Electrophysiol 21:1217–1223
Blackledge HM, Tomlinson J, Squire IB (2003) Prognosis for patients newly admitted to hospital with heart failure; survival trends in 12220 index admissions in Leicestershire 1993–2001. Heart 89:615–620
Rich MW, Bec Kham V, Wittenberg C, Leven CL, Freedland KE, Carney RM (1995) A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 333:1190–1195
Acknowledgments
This research did not receive specific funding.
Conflict of interest
There are no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antonini, L., Mollica, C., Auriti, A. et al. A prognostic index for risk stratification for acute heart failure and death in subjects with ischemic cardiomyopathy and cardiac defibrillator. Heart Vessels 30, 325–330 (2015). https://doi.org/10.1007/s00380-014-0494-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-014-0494-7