Abstract
C57BL/6J (B6) mice were demonstrated to be the most susceptible and C3H/HeJ (C3H) mice the most resistant to development of atherosclerosis. We hypothesized, whether pro-atherogenic (P-selectin, VCAM-1, and ICAM-1) and anti-atherogenic (endoglin and eNOS) proteins are expressed differently in aorta before the onset of atherosclerosis in these two mouse strains. B6 mice (n = 16) and C3H mice (n = 16) sustained on either chow or cholesterol (1 %) diet for 8 weeks. Biochemical analysis of lipoprotein profile and Western blot analysis of P-selectin, VCAM-1, ICAM-1, eNOS, endoglin, peNOS and TGF-βRII in aorta were performed. Western blot analysis revealed a lower expression of P-selectin by 7 %, VCAM-1 by 51 %, ICAM-1 by 6 %, and a higher expression of eNOS (by 18 %) in C3H mice in comparison with B6 mice after cholesterol diet. Further analysis revealed that cholesterol diet significantly increased the expression of endoglin (by 97 %), TGF-βRII (by 50 %), eNOS (by 21 %) and peNOS (by 122 %) in C3H mice, but not in B6 mice. We propose that lower expression of P-selectin, VCAM-1 and ICAM-1 and higher expression of eNOS in vivo in aorta of C3H mice might represent another potential mechanism for C3H mice being less susceptible to atherosclerosis when compared to B6 mice. In addition, endoglin seems to be involved in an upregulation of eNOS only in C3H mice. Thus, we propose that aorta of C3H mice is less prone to the expression of pro-inflammatory and endothelial dysfunction markers when compared to B6 mice, regardless of lipoprotein profile and before any signs of atherosclerotic process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mice are generally resistant to atherosclerosis; however, significant inter-strain differences in atherosclerosis susceptibility have been demonstrated in various studies. The most studied mouse strains with different predispositions to atherosclerosis are C57BL/6J (B6) and C3H/HeJ (C3H) mice. C57BL/6J mouse strain was shown to be the most susceptible and C3H/HeJ mouse strain the most resistant [1–3]. Several hypotheses for different susceptibility have been proposed, including variances in lipoprotein particle sizes and apolipoprotein composition between both strains [4]. However, it was also suggested that the most important factors determining different susceptibility to atherosclerosis seem to be endothelial cells, smooth muscle cells and macrophages [5–7]. It was demonstrated that oxidized LDL affects differently cultured mouse aortic endothelial cells and smooth muscle cells from B6 or C3H mice [5, 7]. Inflammatory molecules such as monocyte chemoattractant protein-1 (MCP-1), macrophage colony-stimulating factor (M-CSF), and vascular cell adhesion molecule-1 (VCAM-1) were activated in these cultured cells from hypercholesterolemic B6/apoE−/−, but not from C3H/apoE−/− mice [7].
Cell adhesion molecules play a crucial role in the whole process of atherogenesis. The expression of P-selectin, VCAM-1 and ICAM-1 (intercellular adhesion molecule-1) is a hallmark of endothelial inflammation and/or endothelial dysfunction [8]. In general, these adhesion molecules were demonstrated to be crucial for activation of endothelium and plaque formation [9–11].
Endoglin is an accessory TGF-β receptor (CD 105, TGF-β receptor III), with ability to regulate TGF-β signaling [12]. It has been demonstrated that it plays a role in various physiological and pathological processes, including cardiovascular system development, angiogenesis, vascular homeostasis [13], coronary artery disease [14] and atherosclerosis [15]. Endoglin interacts with TGF-β1 and TGF-β3 cytokines, but only when it is associated with TGF-β receptor II (TGF-βRII) [16]. Endoglin is able to regulate NO-dependent vasodilatation, as well as the expression of endothelial NO synthase (eNOS) and its activity. This suggests its important role in the function of endothelium and possibly in the protection against development of endothelial dysfunction [17, 18]. In addition, TGF-βRII has an anti-inflammatory activity and improves endothelial dysfunction via eNOS [19]. Our previous studies showed that atorvastatin increases endoglin and eNOS expression in apoE/LDLR deficient mice in atherosclerotic aorta together with reduced atherosclerosis [20, 21], suggesting a possible beneficial (atheroprotective) role of endoglin in atherosclerosis [15].
As mentioned above, ex vivo studies with endothelial cells and smooth muscle cells from C57BL/6J and C3H/HeJ aorta showed that these cells might be responsible for the different predispositions to atherosclerosis in these mice. On the other hand, to the best of our knowledge, a study showing that expression of P-selectin, VCAM-1, ICAM-1, endoglin and eNOS in mouse aorta (in vivo) of B6 and C3H mice might be involved in different susceptibility to atherosclerosis in these mice has never been done. Therefore, we wanted to quantify possible differences in the expression of P-selectin, VCAM-1, ICAM-1, endoglin and eNOS in aortic endothelium of B6 and C3H mice on both chow and cholesterol diets. We hypothesized, whether pro-atherogenic (P-selectin, VCAM-1, ICAM-1) and anti-atherogenic (endoglin, eNOS) proteins are expressed differently in aorta before the onset of atherosclerosis in these two mouse strains.
Materials and methods
Animals
Animal studies met the accepted criteria for human care and experimental use of laboratory animals. All protocols were approved by the Ethical Committee for the protection of animals against cruelty at Faculty of Pharmacy, Charles University in Prague and all experiments were carried out in accordance with Czech law No. 246/1992.
Three-month-old female C57BL/6J (B6) mice (n = 16) (Velaz s.r.o., Czech Republic) and C3H/HeJ (C3H) mice (n = 16) (Jackson Laboratories, USA) were both randomly subdivided into two groups and sustained on either chow or cholesterol diet with water (both ad libitum throughout the study) for 8 weeks. AIN-93 purified diet [22] for laboratory rodents was used as chow diet, while cholesterol diet was AIN-93 enriched with 1 % of cholesterol. Food consumption was monitored every day. No differences in the food consumption were visible, either between animals of one experimental group or between experimental groups. Finally, four groups of animals were created, according to mouse strain and diet used—B6 chow/cholesterol diet (B6 chow/chol) and C3H chow/cholesterol diet (C3H chow/chol).
At the end of the treatment period, all animals were fasted overnight and euthanized.
Blood samples were taken from vena cava inferior into heparin-coated tubes and centrifuged at 9000 rpm for 15 min. Collected plasma samples were subsequently stored at −80 °C before biochemical analysis. Descending aortas from the mice were taken for Western blot analysis. The aortas were snap frozen in liquid nitrogen and stored at −80 °C before homogenization.
Biochemistry
Total cholesterol concentrations were measured enzymatically by conventional enzymatic diagnostic kits (Lachema, Czech Republic) and spectrophotometric analysis (cholesterol at 510 nm, triglycerides at 540 nm, ULTROSPECT III, Pharmacia LKB Biotechnology, Sweden).
Western blot analysis
Samples of mouse aorta (7 animals for each group due to space limitation of sample application) were homogenized in RIPA lysis buffer (Sigma-Aldrich, USA) as described previously [23]. Homogenates (30 μg of aorta proteins) were used for membranes preparation as described previously [23]. The membranes were blocked for 1 h with 5 % nonfat dry milk in Tris buffered saline containing 0.1 % Tween-20 (TBST), and then incubated with primary antibodies at the following concentrations: rabbit polyclonal antibody anti-CD62P (P-selectin, 84 kDa) at 1:200 (Abcam plc, UK), goat polyclonal anti-VCAM-1 (110 kDa) at 1:500 (R&D System, USA), rabbit polyclonal anti-ICAM-1 (85–110 kDa) at 1:500, anti-eNOS (140 kDa) at 1:200, anti-phospho-eNOS (peNOS Ser 1177, 140 kDa) at 1:500, anti-TGF-βRII (70 kDa) at 1:500, goat polyclonal antibody anti-endoglin (90–95 kDa) at 1:500, obtained from Santa Cruz Biotechnology, Inc. (USA). Equal loading of proteins onto the gel was confirmed by immunodetection of GAPDH antibody at 1:10000 (Sigma-Aldrich, USA). As secondary antibodies, HRP-conjugated rabbit anti-goat IgG at 1:5000 (Pierce Biotechnology, USA) for endoglin and VCAM-1, HRP-linked F(ab′)2 fragment donkey anti-rabbit antibody (GE Healthcare, Czech Republic) at dilution 1:2000 (for P-selectin, eNOS, TGF-βRII and peNOS) and 1:1000 (for ICAM-1), and HRP-conjugated goat anti-mouse IgG at 1:20000 (Sigma-Aldrich, USA) for GAPDH were used. Chemiluminescent process and quantification of immunoreactive bands on the exposed films were carried out as described previously [23].
Statistical analysis
All values in the graphs are presented as mean ± SEM. Statistical significance in the differences between groups was assessed by t test using GraphPad Prism 6.03 software (GraphPad Software, Inc., USA). P values of 0.05 or less were considered statistically significant.
Results
Biochemical analysis of cholesterol levels in mice
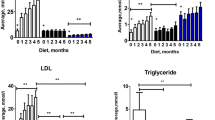
Biochemical analysis of total cholesterol levels and triglyceride (TAG) levels in blood on chow or cholesterol diet was performed (Fig. 1). On chow diet, significantly higher levels of total cholesterol (2.76 ± 0.04 vs. 1.8 ± 0.07 mmol/l, P < 0.001) were demonstrated in C3H mice when compared to B6 mice. Similarly, after cholesterol diet, there were significantly higher levels of total cholesterol (2.40 ± 0.09 mmol/l vs. 2.10 ± 0.06, P < 0.05) in C3H mice when compared to B6 mice. Cholesterol levels were significantly lower after cholesterol diet in C3H mice when compared to C3H mice on chow diet (2.40 ± 0.09 vs. 2.76 ± 0.04 mmol/l, P < 0.01). On the contrary, cholesterol levels were significantly higher after cholesterol diet in B6 mice when compared to B6 mice on chow diet (2.11 ± 0.06 vs. 1.82 ± 0.07 mmol/l, P < 0.01). Performing the comparison of TAG levels revealed no significant changes either between both strains on a relevant diet or between diets in a relevant strain.
Comparison of total cholesterol levels and triglyceride levels in B6 and C3H mice on chow diet and on cholesterol diet. Inter-strain differences are marked with full line. Dotted line shows differences in cholesterol levels within each strain. Values are mean ± SEM, n = 8. ***P < 0.001, **P < 0.01, *P < 0.05
Lower expression of cell adhesion molecules and higher eNOS in C3H mice when compared to B6 mice
Western blot analysis was performed to evaluate the differences in the expression of P-selectin, VCAM-1, ICAM-1, endoglin and eNOS at either baseline (chow diet) and after cholesterol diet between C3H and B6 mouse strains. On chow diet, P-selectin, VCAM-1 and ICAM-1 protein levels were significantly lower in C3H mice, by 37, 60 and 27 % (Fig. 2a, c, e, respectively). Western blot analysis in mice on cholesterol diet revealed a lower expression of P-selectin by 7 %, VCAM-1 by 51 % and ICAM-1 by 6 % (Fig. 2b, d, f, respectively). Analysis of eNOS and endoglin showed that baseline eNOS expression was lower by 39 % and baseline endoglin expression was lower by 53 % in C3H mice when compared to B6 mice (Fig. 2g, i, respectively). On the other hand, the expression of eNOS was higher in C3H mice (by 18 %) in comparison with B6 mice on cholesterol diet (Fig. 2h). There was no significant difference between both mouse strains in endoglin expression on cholesterol diet (Fig. 2j).
Differences in expression of P-selectin (a, b), VCAM-1 (c, d), ICAM-1 (e, f), eNOS (g, h) and endoglin (i, j) between C3H and B6 mice at baseline (chow diet) and on cholesterol diet, respectively. Values are expressed as mean ± SEM of seven values. ***P < 0.001, **P < 0.01, *P < 0.05 vs. control. Top densitometric analysis (control = 100 %); bottom representative immunoblots (three representatives out of seven samples for each group). Equal loading of protein was confirmed by GAPDH
Endoglin/TGF-βRII and eNOS are upregulated after cholesterol diet in C3H but not in B6 mouse aorta
To evaluate the changes of eNOS and endoglin expression and related proteins in each strain after cholesterol diet, we analyzed expression of endoglin, TGF-βRII, eNOS and peNOS in aorta, by comparison of diets within the relevant strain. This analysis demonstrated that cholesterol diet significantly increased the expression of endoglin (by 97 %), TGF-βRII (by 50 %), eNOS (by 21 %) and peNOS (by 122 %) in C3H mice when compared to chow diet (Fig. 3a, c, e, g, respectively). On the other hand, in B6 mice, cholesterol diet did not cause any significant changes in expression of these proteins (Fig. 3b, d, f, h, respectively).
Effects of cholesterol diet on expressions of endoglin (a, b), TGF-βRII (c, d), eNOS (e, f) and peNOS (g, h) in C3H and B6 mice. Values are expressed as mean ± SEM of seven values. ***P < 0.001, **P < 0.01, *P < 0.05 vs. control. Top densitometric analysis (control = 100 %); bottom representative immunoblots (three representatives out of seven samples for each group). Equal loading of protein was confirmed by GAPDH
Discussion
Rodents, including mice, are resistant to development of atherosclerosis. In the two most studied strains, it was demonstrated that C57BL/6J mice are the most susceptible and C3H/HeJ mice the most resistant to the development of atherosclerosis [1–3]. In response to an atherogenic diet, B6 mice develop fatty streaks in the proximal aorta, whereas C3H mice are totally resistant to fatty streak formation [24]. Moreover, atherogenic diet causes an induction of inflammatory and oxidative stress genes in the liver of B6 mice, but not in C3H mice [25]. However, and more importantly, different behaviors of endothelium to various stimuli were suggested to play a significant role in different predispositions to atherosclerosis [7, 26] in B6 and C3H mice. Shi et al. [7, 26] demonstrated that minimally modified LDL induced marked production of MCP-1, M-CSF, VCAM-1 and HMOX1 in endothelial cells isolated from thoracic aorta of B6 and B6 apoE−/− mice, but not from C3H and C3H apoE−/− mice. In addition, they also demonstrated that not only endothelial cells are crucial for the different predispositions to atherosclerosis in these mice, but also other vessel wall cells, including macrophages (foam cells formation) [6] and smooth muscle cells [5].
Cell adhesion molecules are pro-inflammatory and pro-atherogenic proteins that represent a hallmark of endothelial dysfunction and atherosclerosis. P-selectin, VCAM-1 and ICAM-1 were demonstrated to be involved in the initiation and progression of atherosclerosis [8, 27, 28].
Previous studies showed that endoglin is able to regulate and affect eNOS expression and activity [17, 18, 29], suggesting its important role in the protection of endothelium against development and/or manifestation of endothelial dysfunction. Moreover, our latest results showed that endoglin expression was increased together with eNOS simultaneously with reduced size of atherosclerotic plaques after atorvastatin treatment in hypercholesterolemic mice [15, 20, 21]. Therefore, it was proposed that endoglin might represent an anti-atherogenic protein that is able to improve function of endothelium via expression of eNOS [15].
Thus, in this study, we wanted to evaluate the aortic expression of pro-atherogenic P-selectin, VCAM-1, ICAM-1 and anti-atherogenic endoglin and eNOS in the basal conditions and after cholesterol diet in B6 and C3H mice.
Western blot analysis revealed that basal levels of P-selectin, VCAM-1 and ICAM-1, were lower in C3H mice when compared to B6 mice. Moreover, even after cholesterol diet, the expression of P-selectin, VCAM-1 and ICAM-1 was lower in aorta of C3H mice in comparison with B6 mice. On the contrary, total cholesterol levels in blood were higher in C3H mice when compared to B6 on both chow diet and after cholesterol diet. We might propose, that it is unlikely that small changes in levels of cholesterol (cholesterol levels in range 2–3 mmol/l) can affect vascular wall in mice in this study. Indeed, high levels of total cholesterol about 30–40 mmol/l are at least partially responsible for changes in protein expression in the vessels and development of atherosclerotic plaques as was shown in mouse models of experimental atherosclerosis [30, 31]. In addition, previous paper demonstrated that cholesterol levels are not responsible for the different predispositions to atherosclerosis in B6 and C3H mice even on an apoE-deficient background [31]. Thus, the lower expression of crucial pro-inflammatory cell adhesion molecules in C3H mice suggests that aorta in these mice is not prone to the development of inflammation and endothelial dysfunction as it seems to be in B6 mice. This is also in line with previously mentioned work by Shi et al. [5–7]. However, it is of interest to point out that Shi et al. performed studies with cultured endothelial cells and smooth muscle cells treated with minimally modified LDL ex vivo. On the contrary, our results show different expression of P-selectin, VCAM-1 and ICAM-1 in aorta in vivo.
Further analysis showed a lower expression of eNOS in C3H mice in basal conditions, but a significantly higher expression after cholesterol diet when compared to B6 mice. Thus, C3H mice presented a lower expression of pro-atherogenic adhesion molecules and a higher expression of anti-atherogenic eNOS in aorta, suggesting that blood vessel characteristics are responsible for different predispositions to atherosclerosis in these mice.
Since eNOS expression was lower in basal conditions and higher after cholesterol diet in C3H mice when compared to B6 mice, it was of interest to evaluate a possible background for this eNOS increase. Therefore, we decided to study the expression of markers that potentially regulate eNOS expression in each strain after cholesterol diet.
As mentioned above, levels of eNOS are strongly related to the amount of endoglin, both in vivo and in vitro [17, 18]. Toporsian et al. [17] found that partial or total loss of endoglin in murine endothelial cells is associated with 50 % decrease in eNOS levels. It was also shown that endoglin interacts with TGF-β1 only when it is associated with TGF-βRII [16]. Therefore, it seems that the interaction between endoglin and TGF-βRII is likely important for TGF-β1 signaling in vivo [13]. Endoglin expression did not significantly differ between strains on cholesterol diet. On the other hand, Western blot analysis of aorta from each strain revealed that the expressions of endoglin, eNOS, phosphorylated eNOS (Ser1177, active form) and TGF-βRII were strongly upregulated in C3H mice after cholesterol diet, but not in B6 mice. Indeed, phosphorylation of eNOS in Ser1177 was shown to increase the enzyme activity and production of NO thus providing endothelial protection [32]. Therefore, we might suggest that the upregulation of endoglin/TGF-βRII results in an increased eNOS expression/activity and endothelial protection only in C3H mice, but not in B6 mice. Therefore, we propose that this might also contribute to the weaker susceptibility of C3H mice to development of inflammation, endothelial dysfunction and possibly atherosclerosis. As mentioned above, it is unlikely that mild (but significant) changes of total cholesterol could be involved in the specific reaction of endothelium in these mice. Indeed, significantly higher levels of cholesterol in B6 mice did not result in any changes in the expression of studied proteins. However, it is of interest to mention that an upregulation of endoglin/TGF-βRII/eNOS in cholesterol fed C3H mice was detected and cholesterol fed C3H mice had lower levels of cholesterol when compared to C3H mice on chow diet. In any case, the exact cause for the increased expression of anti-atherogenic proteins in C3H mice remains to be elucidated.
It must be emphasized that our study was not performed to determine whether these mice are differentially predisposed to the development of atherosclerosis, which was clearly demonstrated previously [4, 7, 26]. We aimed to show some other possible mechanisms that may contribute to this susceptibility in in vivo context for the first time.
Conclusion
We propose that lower expressions of P-selectin, VCAM-1 and ICAM-1 and a higher expression of eNOS in vivo in aorta of C3H mice might represent another potential mechanism how C3H mice are less susceptible to atherosclerosis when compared to B6 mice. In addition, endoglin-related signaling also seems to be involved in an upregulation of eNOS only in C3H mice, which however requires further investigation. Thus, we propose that aorta of C3H mice is less susceptible to the expression of pro-inflammatory and endothelial dysfunction markers when compared to B6 mice, regardless of cholesterol levels in blood and before any signs of atherosclerotic process.
References
Paigen B, Ishida BY, Verstuyft J, Winters RB, Albee D (1990) Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis 10:316–323
Paigen B, Mitchell D, Reue K, Morrow A, Lusis AJ, LeBoeuf RC (1987) Ath-1, a gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Proc Natl Acad Sci USA 84:3763–3767
Paigen B, Albee D, Holmes PA, Mitchell D (1987) Genetic analysis of murine strains C57BL/6J and C3H/HeJ to confirm the map position of Ath-1, a gene determining atherosclerosis susceptibility. Biochem Genet 25:501–511
Ishida BY, Blanche PJ, Nichols AV, Yashar M, Paigen B (1991) Effects of atherogenic diet consumption on lipoproteins in mouse strains C57BL/6 and C3H. J Lipid Res 32:559–568
Miyoshi T, Tian J, Matsumoto AH, Shi W (2006) Differential response of vascular smooth muscle cells to oxidized LDL in mouse strains with different atherosclerosis susceptibility. Atherosclerosis 189:99–105
Shi W, Pei H, Fischer JJ, James JC, Angle JF, Matsumoto AH, Helm GA, Sarembock IJ (2004) Neointimal formation in two apolipoprotein E-deficient mouse strains with different atherosclerosis susceptibility. J Lipid Res 45:2008–2014
Shi W, Wang NJ, Shih DM, Sun VZ, Wang X, Lusis AJ (2000) Determinants of atherosclerosis susceptibility in the C3H and C57BL/6 mouse model: evidence for involvement of endothelial cells but not blood cells or cholesterol metabolism. Circ Res 86:1078–1084
Galkina E, Ley K (2007) Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol 27:2292–2301
Ley K, Huo Y (2001) VCAM-1 is critical in atherosclerosis. J Clin Invest 107:1209–1210
Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, Ley K (1999) Direct demonstration of P-selectin- and VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circ Res 84:1237–1244
Walpola PL, Gotlieb AI, Cybulsky MI, Langille BL (1995) Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler Thromb Vasc Biol 15:2–10
Ma X, Labinaz M, Goldstein J, Miller H, Keon WJ, Letarte M, O’Brien E (2000) Endoglin is overexpressed after arterial injury and is required for transforming growth factor-beta-induced inhibition of smooth muscle cell migration. Arterioscler Thromb Vasc Biol 20:2546–2552
Lopez-Novoa JM, Bernabeu C (2010) The physiological role of endoglin in the cardiovascular system. Am J Physiol Heart Circ Physiol 299:H959–H974
Ikemoto T, Hojo Y, Kondo H, Takahashi N, Hirose M, Nishimura Y, Katsuki T, Shimada K, Kario K (2011) Plasma endoglin as a marker to predict cardiovascular events in patients with chronic coronary artery diseases. Heart Vessels 27:344–351
Nachtigal P, Zemankova Vecerova L, Rathouska J, Strasky Z (2012) The role of endoglin in atherosclerosis. Atherosclerosis 224:4–11
Lastres P, Letamendia A, Zhang H, Rius C, Almendro N, Raab U, Lopez LA, Langa C, Fabra A, Letarte M, Bernabeu C (1996) Endoglin modulates cellular responses to TGF-beta 1. J Cell Biol 133:1109–1121
Toporsian M, Gros R, Kabir MG, Vera S, Govindaraju K, Eidelman DH, Husain M, Letarte M (2005) A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ Res 96:684–692
Jerkic M, Rivas-Elena JV, Prieto M, Carron R, Sanz-Rodriguez F, Perez-Barriocanal F, Rodriguez-Barbero A, Bernabeu C, Lopez-Novoa JM (2004) Endoglin regulates nitric oxide-dependent vasodilatation. FASEB J 18:609–611
Vasquez R, Farias M, Vega JL, Martin RS, Vecchiola A, Casanello P, Sobrevia L (2007) d-glucose stimulation of l-arginine transport and nitric oxide synthesis results from activation of mitogen-activated protein kinases p42/44 and Smad2 requiring functional type II TGF-beta receptors in human umbilical vein endothelium. J Cell Physiol 212:626–632
Vecerova L, Strasky Z, Rathouska J, Slanarova M, Brcakova E, Micuda S, Nachtigal P (2012) Activation of TGF-beta receptors and Smad proteins by atorvastatin is related to reduced atherogenesis in ApoE/LDLR double knockout mice. J Atheroscler Thromb 19:115–126
Rathouska J, Vecerova L, Strasky Z, Slanarova M, Brcakova E, Mullerova Z, Andrys C, Micuda S, Nachtigal P (2011) Endoglin as a possible marker of atorvastatin treatment benefit in atherosclerosis. Pharmacol Res 64:53–59
Reeves PG, Nielsen FH, Fahey GC Jr (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951
Brcakova E, Fuksa L, Cermanova J, Kolouchova G, Hroch M, Hirsova P, Martinkova J, Staud F, Micuda S (2009) Alteration of methotrexate biliary and renal elimination during extrahepatic and intrahepatic cholestasis in rats. Biol Pharm Bull 32:1978–1985
Paigen B, Morrow A, Holmes PA, Mitchell D, Williams RA (1987) Quantitative assessment of atherosclerotic lesions in mice. Atherosclerosis 68:231–240
Liao F, Andalibi A, deBeer FC, Fogelman AM, Lusis AJ (1993) Genetic control of inflammatory gene induction and NF-kappa B-like transcription factor activation in response to an atherogenic diet in mice. J Clin Invest 91:2572–2579
Shi W, Haberland ME, Jien ML, Shih DM, Lusis AJ (2000) Endothelial responses to oxidized lipoproteins determine genetic susceptibility to atherosclerosis in mice. Circulation 102:75–81
Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS (2001) A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 107:1255–1262
Yano H, Horinaka S, Yagi H, Ishimitsu T (2013) Comparison of inflammatory response after implantation of sirolimus- and paclitaxel-eluting stents in patients on hemodialysis. Heart Vessels 28:308–315
Jerkic M, Rodriguez-Barbero A, Prieto M, Toporsian M, Pericacho M, Rivas-Elena JV, Obreo J, Wang A, Perez-Barriocanal F, Arevalo M, Bernabeu C, Letarte M, Lopez-Novoa JM (2006) Reduced angiogenic responses in adult Endoglin heterozygous mice. Cardiovasc Res 69:845–854
Dansky HM, Charlton SA, Sikes JL, Heath SC, Simantov R, Levin LF, Shu P, Moore KJ, Breslow JL, Smith JD (1999) Genetic background determines the extent of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 19:1960–1968
Grimsditch DC, Penfold S, Latcham J, Vidgeon-Hart M, Groot PH, Benson GM (2000) C3H apoE(−/−) mice have less atherosclerosis than C57BL apoE(−/−) mice despite having a more atherogenic serum lipid profile. Atherosclerosis 151:389–397
Forstermann U, Sessa WC (2012) Nitric oxide synthases: regulation and function. Eur Heart J 33:829–837 (pp 837a–837d)
Acknowledgments
This work was supported by grant from the Grant Agency of Charles University in Prague number 300911/C, Charles University in Prague project SVV/2014/260064. The publication is co-financed by the European Social Fund and the state budget of the Czech Republic, Project No. CZ.1.07/2.3.00/30.0061.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rathouska, J., Nemeckova, I., Zemankova, L. et al. Cell adhesion molecules and eNOS expression in aorta of normocholesterolemic mice with different predispositions to atherosclerosis. Heart Vessels 30, 241–248 (2015). https://doi.org/10.1007/s00380-014-0493-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-014-0493-8