Abstract
Urinary liver-type fatty acid-binding protein (L-FABP) reflects the degree of stress in proximal tubules of the kidney. We examined the level of L-FABP in type-2 diabetes mellitus (T2DM) patients with chronic kidney disease (CKD) stage G1 and G2, and its relationship with cardiac markers and electrocardiographic (ECG) abnormalities. T2DM patients whose estimated glomerular filtration rate (eGFR) was ≥60 mL/min/1.73 m2 were recruited [n = 276 (165 males), mean age 64 years]. The median level of urinary L-FABP was 6.6 μg/gCr. Urinary L-FABP showed significant correlation with urinary albumin-to-creatinine ratio (ACR) (r = 0.51, p < 0.0001). Median (25th–75th percentile) eGFR was 82 (72–95) mL/min/1.73 m2. We divided patients into four subgroups (group 1, L-FABP ≤8.4 μg/gCr and ACR ≤30 mg/gCr; group 2, L-FABP ≤8.4 μg/gCr and ACR >30 mg/gCr; group 3, L-FABP >8.4 μg/gCr and ACR ≤30 mg/gCr; group 4, L-FABP >8.4 μg/gCr and ACR >30 mg/gCr). Compared with group 1, group 4 was significantly higher in systolic blood pressure, and eGFR using standardized serum cystatin C, high-sensitivity troponin T, and N-terminal pro-brain natriuretic peptide (NT-proBNP). Group 4 had significantly higher level of NT-proBNP than group 3. Groups 2, 3 and 4 showed more ECG abnormalities than group 1. These findings suggest that simultaneous measurement of urinary L-FABP and ACR should be useful to assess cardiovascular damage reflecting on the elevation of cardiac markers and ECG abnormalities in T2DM with CKD G1 and G2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic nephropathy is one of the major causes of chronic kidney disease (CKD), which has attracted attention as a new national disease [1–3]. Remission can be obtained by carrying out active treatment for early nephropathy, so the importance of the early diagnosis of diabetic nephropathy is increasing [4, 5]. The most sensitive marker of glomerular injury due to diabetic nephropathy is urinary albumin-to-creatinine ratio (ACR) [6, 7]. In addition to glomerular injury, tubulointerstitial injury has been noted to have important roles in the progression of CKD [8]. Type-2 diabetes mellitus (T2DM) and CKD are known to be important risk factors for cardiovascular diseases (CVDs) [9–11]. ACR has also been reported to be a predictive factor for CVD in diabetes and non-diabetes patients [12, 13]. The liver-type fatty acid-binding protein (L-FABP) gene encodes a 14-kDa protein that is expressed abundantly in hepatocytes and in the proximal tubular cells of the kidney [14]. Urinary L-FABP reflects the oxidative stress cause of the progression of renal disease, and is secreted specifically from proximal tubules [14]. The usefulness of urinary L-FABP in the early diagnosis of acute kidney injury has been reported, and a high urinary L-FABP level reflects the progression of diabetic nephropathy [15–19]. Patients with diabetes have been shown to have a higher urinary L-FABP level than healthy controls, and their L-FABP level was correlated to ACR, suggesting that urinary L-FABP level reflects early tubulointestinal damage in nephropathy associated with T2DM [20]. Indeed, high urinary L-FABP levels are associated with progression to end-stage renal disease (ESRD) or induction of hemodialysis in T2DM [21]. Furthermore, urinary L-FABP measurements have been suggested to be useful for identifying high-risk patients for future cardiovascular events after acute coronary syndrome [22, 23]. Araki et al. [24] recently reported that urinary L-FABP may be a predictive marker for renal and cardiovascular prognosis in T2DM patients without advanced nephropathy. However, there is limited information about urinary L-FABP levels associated with cardiovascular risk in early-stage nephropathy in T2DM. In the present study, we evaluated the utility of urinary L-FABP as well as ACR to assess both glomerular and tubulointestinal damage in T2DM with CKD stage G1 and G2. We aimed to clarify the role of urinary L-FABP in cardio-renal interaction by checking its association with other cardiac markers and electrocardiographic (ECG) abnormalities.
Materials and methods
Subjects
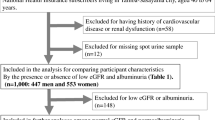
T2DM patients [n = 276 (165 males); age, 64 (58–71) years; HbA1c, 7.2 (6.2–7.6) %; estimated glomerular filtration rate (eGFR), ≥60 mL/min/1.73 m2] in the outpatient clinic of Fujita Health University Hospital were recruited for this study (Table 1). Insulin treatment was achieved in 30.4 % of subjects. Dyslipidemia was defined as the patients with high serum level of low-density lipoprotein cholesterol (above or equal to 120 mg/dL for the patients without prevalent CVD, and above or equal to 100 mg/dL for the patients with CVD), high level of serum triglyceride (above or equal to 150 mg/dL), low level of high density lipoprotein cholesterol (<40 mg/dL), and the users of lipid-lowering medication, mainly HMG-CoA reductase inhibitors. According to this definition, 73.9 % of our patients had dyslipidemia. Hypertension, defined as use of anti-hypertensive medication or had average measured blood pressure >140/90 mmHg, was diagnosed in 44.9 % of patients. The profile of anti-hypertensive medication was: angiotensin-converting enzyme inhibitor/angiotensin-receptor blocker (39.5 %), calcium-channel blocker (23.6 %), diuretics (9.1 %), and β-blocker (4.7 %). We include the patients with prior myocardial infarction or chronic heart failure to evaluate the utility under clinical setting. There were only 10 % cases in each group, and did not affect overall result itself. This descriptive study was approved by the Review Board for Epidemiology and Clinical Studies of Fujita Health University (Aichi, Japan). It was therefore undertaken in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from each subject.
Clinical parameters of serum and ambulatory spot urine
Serum and ambulatory spot urine samples were collected upon the routine visits of patients to our hospital. Urinary L-FABP was measured with a sandwich enzyme-linked immunosorbent assay kit following the manufacturer’s protocol (CMIC Co. Ltd., Tokyo, Japan) in an early morning spot urine sample. Urinary albumin was measured with an immunoturbidimetric assay (Denka Seiken Co. Ltd, Tokyo, Japan).
Serum levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) and high-sensitivity troponin T (hsTnT) were measured using an electrochemiluminescence immunoassay based on the Cobas E601 system (Roche Diagnostics, Tokyo, Japan). Serum levels of cystatin C were measured using a particle-enhanced nephelometric immunoassay-based BN-II system (Siemens Healthcare Diagnostics, Tokyo, Japan). Routine blood chemistries were measured using an Olympus AU5232 Automatic Analyzer (Olympus, Tokyo, Japan). The eGFR was calculated from the following formula [25]:
The eGFR using standardized serum cystatin C (eGFR-cys C) was calculated from the following formula [26]:
-
Male: eGFR-cys C = (104 × serum cys C concentration−1.019 × 0.996age) − 8.
-
Female: eGFR-cys C = (104 × serum cys C concentration−1.019 × 0.996age × 0.929) − 8.
The value of HbA1c is equivalent to the internationally used HbA1c (%) [HbA1c (NGSP)] defined by the National Glycohemoglobin Standardization Program (NGSP). It is expressed by adding 0.4 % to HbA1c (JDS) (%) as defined by the Japan Diabetes Society (JDS) [27]. Electrocardiography was carried out with a FDX-4520 system (Fukuda Denshi Co., Ltd, Tokyo, Japan). ECG abnormalities were defined as left ventricular hypertrophy, ST–T deviation, negative T or complete left bundle branch block.
Statistical analyses
All data analyses were undertaken using SPSS, ver11.0 (SPSS, Chicago, IL, USA). Continuous data are median ± SD, and skewed variables are medians and interquartile ranges. Continuous variables were analyzed using the unpaired Student’s t test and analysis of variance (ANOVA) followed by multiple comparisons. If data were not distributed normally, the Mann–Whitney U test and Kruskal–Wallis test were used. Categorical variables were compared by the χ 2 test. Adjustments for multiple comparisons (Bonferroni correction) were performed. p < 0.05 was considered significant.
Results
The clinical characteristics of study subjects are shown in Table 1. Previous myocardial infarction, stroke, and heart failure were present in 18 (6.5 %), 29 (10.5 %), and 4 (1.4 %) patients, respectively. The median (25th–75th percentile) urinary L-FABP level was 6.6 μg/gCr (3.8–13.3) (Fig. 1). The prevalence of increased urinary L-FABP (>8.4 μg/gCr) according to the manufacturer’s data was 42.0 %. Patients with a high urinary L-FABP level (>8.4 μg/gCr) were older; had higher systolic blood pressure (SBP) (p = 0.02); had lower level of eGFR-cysC (p = 0.02); had higher levels of hsTnT (p = 0.007), NT-proBNP (p < 0.0001), and ACR (p < 0.0001) (Table 2). Urinary L-FABP levels showed a significant correlation with ACR (r = 0.51, p < 0.0001) (Fig. 2). Urinary L-FABP levels were positively associated with hsTnT (r = 0.223, p < 0.001) and NT-proBNP (r = 0.244, p < 0.001), as ACR were related to hsTnT (r = 0.124, p = 0.042) and NT-proBNP (r = 0.122, p = 0.047).
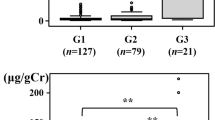
We divided patients into four subgroups according to urinary levels of L-FABP and ACR: group 1, urinary L-FABP ≤8.4 μg/gCr and ACR ≤30 mg/gCr; group 2, urinary L-FABP ≤8.4 μg/gCr and ACR >30 mg/gCr; group 3, urinary L-FABP >8.4 μg/gCr and ACR ≤30 mg/gCr; group 4, urinary L-FABP >8.4 μg/gCr and ACR >30 mg/gCr. The percentage of the number of groups 1, 2, 3 and 4 was 44, 15, 17, and 25 %, respectively (Table 3). Both urinary L-FABP and ACR in group 4 were significantly higher than those in other groups. There was no significant difference between groups 1 2, and 3. Compared with group 1, group 4 was significantly higher co-existence of hypertension (p < 0.01); had higher SBP (p < 0.01); had lower levels of eGFR-cysC (p < 0.05); and higher levels of hsTnT (p < 0.01), NT-proBNP (p < 0.01). Group 4 had a significantly higher co-existence of hypertension (p < 0.01) as well as significantly higher level of NT-proBNP (p < 0.05) than group 3 (Table 3). Groups 2, 3 and 4 had a higher prevalence of ECG abnormalities (p < 0.05) than group 1 (Fig. 3).
Categorical distribution of electrocardiography (ECG) abnormalities. Group 1, urinary liver-type fatty acid-binding protein (L-FABP) ≤8.4 μg/gCr and urinary albumin-to-creatinine ratio (ACR) ≤30 mg/gCr; group 2, urinary L-FABP ≤8.4 μg/gCr and ACR >30 mg/gCr; group 3, urinary L-FABP >8.4 μg/gCr and ACR ≤30 mg/gCr; group 4, urinary L-FABP >8.4 μg/gCr and ACR >30 mg/gCr. Data were compared by the χ 2 test. Adjustments for multiple comparisons (Bonferroni correction) were performed and p < 0.05 was considered significant
Discussion
Diabetic nephropathy can result in ESRD, which is a life-threatening disorder with many complications (including CVDs) [3]. To prevent ESRD in diabetes patients, early detection of nephropathy is necessary. ACR is well known to reflect glomerular damage in diabetic nephropathy, and to predict the development of further chronic renal impairment in these patients [6]. Although ACR is considered to be the best predictor of the progression of nephropathy in diabetes, estimation of renal tubular function can provide an early indication of renal impairment [4]. Urinary L-FABP is specifically secreted from proximal tubules, and is a more sensitive marker than urinary protein for predicting the progression of CKD [14–17]. In the present study, we measured the urinary L-FABP level as well as ACR in T2DM patients with an eGFR ≥60 mL/min/1.73 m2 (CKD stage G1 and G2). We found that 42.0 % of patients showed a higher urinary L-FABP level than the upper limit of the normal population (8.4 μg/gCr). Patients with high L-FABP levels were older and had higher SBP and ACR than subjects with low L-FABP levels. Urinary L-FABP was significantly correlated to ACR, suggesting the progression of glomerular and tubular injury in T2DM with CKD stage G1 and G2. To better define the clinical characteristics of urinary L-FABP from ACR, we divided patients into four subgroups according to urinary levels of L-FABP and ACR. There was no difference between group 1 (low L-FABP, low ACR) and group 2 (low L-FABP, high ACR). In addition, there was no difference between groups 2 and 3 (high L-FABP, low ACR). However, both urinary L-FABP and ACR in group 4 (high L-FABP, high ACR) were significantly higher than those in other groups, and group 4 showed a significantly higher distribution of hypertension, higher SBP, and lower eGFR-cysC levels than group 1. These results suggest that simultaneous measurement of urinary L-FABP and ACR could elicit more information than single measurement in T2DM with CKD stage G1 and G2. eGFR-cysC has been recently found to be more sensitive, specific and accurate marker to predict renal function than eGFR (calculated by serum creatinine) [26], so patients with high levels of urinary L-FABP and ACR could progress more rapidly to advanced-stage CKD.
CVDs and renal dysfunctions frequently coexist [28], and ACR is known to have prognostic value for CVDs in the non-diabetes and diabetes population [12, 13]. Urinary L-FABP has been reported to be elevated in patients with acute coronary syndrome and could be a predictive marker for vascular diseases including both CVD and renal impairment [23, 24]. We found that high urinary L-FABP level and ACR were associated with high levels of NT-proBNP and hsTnT. NT-proBNP reflects left ventricular overload and is an independent risk factor for cardiovascular events in healthy subjects and diabetes patients [29, 30]. TnT is a specific marker used to detect the cardiac damage (especially myocardial infarction) and structural heart diseases (e.g., left ventricular hypertrophy) involved in the progression of chronic heart failure and CKD and, combined with brain natriuretic peptide (BNP), may have prognostic value [31–35]. We have also reported that hsTnT is a useful marker to predict cardiovascular damage in T2DM [36]. In this study, we found significant increase of hsTnT levels in the patients with the past history of CVD (p = 0.037), but not NT-proBNP (p = 0.215). As we exclude very high-risk groups who had both CVD and renal impairment (eGFR below 60), it seems likely that the impact of the history of CVD might decrease in this study. In addition, we did not find the elevation of L-FABP or ACR in the subjects with past history of CVD, though both L-FABP and ACR were correlated with hsTnT and NT-proBNP. These results suggested us that the elevation of L-FABP and ACR does not completely depend on cardiac damage due to past CVD events, but reflect multifactorial renal impairment which could result in cardiovascular damage in T2DM. When we divided patients according to levels of urinary L-FABP and ACR, group 4 (high L-FABP, high ACR) showed significantly higher NT-proBNP levels than group 1 (low L-FABP, low ACR) and group 3 (high L-FABP, low ACR), though the prevalence of a history of CVD was identical in each group. In addition, the hsTnT level in group 4 was higher than in group 1. We found that the elevation of the L-FABP level was associated with ECG abnormalities in the patients with elevated urinary L-FABP and/or ACR (group 2, 3 and 4). Taken together, these findings suggested that measurement of both urinary L-FABP and ACR could provide better predictive value for CVD than ACR alone in T2DM patients with CKD stages G1 and G2. Further prospective study would be required to confirm clinical utility of combined measurement of urinary L-FABP and ACR for the prediction of future CVD in T2DM with CKD stages G1 and G2.
CVD frequently coexists with renal dysfunction, and this is referred to as the cardio-renal syndrome. Despite the growing recognition of this syndrome, the precise mechanism is not totally clarified. Urinary L-FABP binds FFA produced by proteinuria, ischemia, oxidative stress, and toxic insults [18]. Therefore, urinary L-FABP excretion could reflect, at least in part, systemic oxidative stress, which further deteriorates systemic atherosclerosis. In addition, in vitro studies suggest that L-FABP is an indirect antioxidant protein essential for sequestering FFA [37], and L-FABP could have an important role in preventing age- or diet-induced obesity [38]. Therefore, increase of oxidized FFA, which could increase urinary L-FABP, might contribute to obesity-induced CVD. Further investigations would be required to clarify the role of L-FABP in cardio-renal syndrome.
There were several limitations in this study. Firstly, it was a cross-sectional study. Cardiovascular events should be observed by a prospective study to evaluate the value of urinary L-FABP as a surrogate marker for these events. Secondly, this study was done at one center. Thirdly, the treatments for T2DM, hypertension, and dyslipidemia could affect the results because the physician in charge selected the treatments.
In conclusion, simultaneous measurement of urinary L-FABP and ACR should be useful to detect cardiovascular damage reflecting on cardiac markers and ECG abnormalities in T2DM with CKD G1 and G2.
References
Meguid El Nahas A, Bello AK (2005) Chronic kidney disease: the global challenge. Lancet 365:331–340
Barsoum RS (2006) Chronic kidney disease in the developing world. N Engl J Med 354:997–999
Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG, Chronic Kidney Disease Prognosis Consortium (2012) Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 380:1662–1673
Kalansooriya A, Jennings P, Haddad F, Holbrook I, Whiting PH (2007) Urinary enzyme measurements as early indicators of renal insult in type 2 diabetes. Br J Biomed Sci 64:153–156
Unsal A, Koc Y, Basturk T, Akgun AO, Sakaci T, Ahbap E (2012) Risk factors for progression of renal disease in patient with diabetic nephropathy. Eur Rev Med Pharmacol Sci 16:878–883
Cohen-Bucay A, Viswanathan G (2012) Urinary markers of glomerular injury in diabetic nephropathy. Int J Nephro : 146987. doi: 10.1155/2012/146987
Saito T, Mochizuki T, Uchida K, Tsuchiya K, Nitta K (2013) Metabolic syndrome and risk of progression of chronic kidney disease: a single-center cohort study in Japan. Heart Vessels 28:323–329
Tang SC, Lai KN (2012) The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol Dial Transplant 27:3049–3056
Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M (1998) Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229–234
Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108:2154–2169
Kaneko H, Yajima J, Oikawa Y, Tanaka S, Fukamachi D, Suzuki S, Sagara K, Otsuka T, Matsuno S, Kano H, Uejima T, Koike A, Nagashima K, Kirigaya H, Sawada H, Aizawa T, Yamashita T (2013) Long-term incidence and prognostic factors of the progression of new coronary lesions in Japanese coronary artery disease patients after percutaneous coronary intervention. Heart Vessels. doi:10.1007/s00380-013-0382-6
Araki S, Haneda M, Koya D, Kashiwagi A, Uzu T, Kikkawa R (2008) Clinical impact of reducing microalbuminuria in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 82(Suppl 1):S54–S58
Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT (2010) Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375:2073–2081
Veerkamp JH, Peeters RA, Maatman RG (1991) Structural and functional features of different types of cytoplasmic fatty acid-binding proteins. Biochim Biophys Acta 1081:1–24
Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hikawa A, Hirano N, Hirata Y, Goto A, Omata M (2004) Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med 143:23–30
Kamijo A, Sugaya T, Hikawa A, Yamanouchi M, Hirata Y, Ishimitsu T, Numabe A, Takagi M, Hayakawa H, Tabei F, Sugimoto T, Mise N, Kimura K (2005) Clinical evaluation of urinary excretion of liver-type fatty acid-binding protein as a marker for the monitoring of chronic kidney disease: a multicenter trial. J Lab Clin Med 145:125–133
Kamijo A, Sugaya T, Hikawa A, Yamanouchi M, Hirata Y, Ishimitsu T, Numabe A, Takagi M, Hayakawa H, Tabei F, Sugimoto T, Mise N, Omata M, Kimura K (2006) Urinary liver-type fatty acid binding protein as a useful biomarker in chronic kidney disease. Mol Cell Biochem 284:175–182
Kamijo-Ikemori A, Sugaya T, Kimura K (2006) Urinary fatty acid binding protein in renal disease. Clin Chim Acta 374:1–7
Katoh H, Nozue T, Kimura Y, Nakata S, Iwaki T, Kawano M, Kawashiri MA, Michishita I, Yamagishi M (2013) Elevation of urinary liver-type fatty acid-binding protein as predicting factor for occurrence of contrast-induced acute kidney injury and its reduction by hemodiafiltration with blood suction from right atrium. Heart Vessels. doi:10.1007/s00380-013-0347-9
von Eynatten M, Baumann M, Heemann U, Zdunek D, Hess G, Nawroth PP, Bierhaus A, Humpert PM (2010) Urinary L-FABP and anaemia: distinct roles of urinary markers in type 2 diabetes. Eur J Clin Invest 40:95–102
Kamijo-Ikemori A, Sugaya T, Yasuda T, Kawata T, Ota A, Tatsunami S, Kaise R, Ishimitsu T, Tanaka Y, Kimura K (2011) Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 34:691–696
Fukuda Y, Miura S, Zhang B, Iwata A, Kawamura A, Nishikawa H, Shirai K, Saku K (2009) Significance of urinary liver-fatty acid-binding protein in cardiac catheterization in patients with coronary artery disease. Intern Med 48:1731–1737
Matsumori R, Shimada K, Kiyanagi T, Hiki M, Fukao K, Hirose K, Ohsaka H, Miyazaki T, Kume A, Yamada A, Takagi A, Ohmura H, Miyauchi K, Daida H (2012) Clinical significance of the measurements of urinary liver-type fatty acid binding protein levels in patients with acute coronary syndrome. J Cardiol 60:168–173
Araki S, Haneda M, Koya D, Sugaya T, Isshiki K, Kume S, Kashiwagi A, Uzu T, Maegawa H (2013) Predictive effects of urinary liver-type Fatty Acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care 36:1248–1253
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR (2009) Revised equations for estimated GFR from serum creatinine in japan. Am J kidney Dis 53:982–992
Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S, Collaborators Developing the Japanese Equation for Estimated GFR (2013) GFR estimation using standardized serum cystatin C in Japan. Am J kidney Dis 61:197–203
Atsunori K, Masato K, Eiichi A, Yoshitomo O, Toshiaki H, Kume S, Kashiwagi A, Uzu T, Maegawa H (2012) International clinical harmonization of glycated hemoglobin in Japan: from Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Invest 3:39–40
Taub PR, Borden KC, Fard A, Maisel A (2012) Role of biomarkers in the diagnosis and prognosis of acute kidney injury in patients with cardiorenal syndrome. Expert Rev Cardiovasc Ther 10:657–667
Hildebrandt P, Richards AM (2008) Amino-terminal pro-B-type natriuretic peptide testing in patients with diabetes mellitus and with systemic hypertension. Am J Cardiol 2101:21–24
Hildebrandt P (2009) Natriuretic peptides: prediction of cardiovascular disease in the general population and high risk populations. Dis Markers 26:227–233
Freda BJ, Tang WH, Van Lente F, Peacock WF, Francis GS (2002) Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol 40:2065–2071
Ishii J, Nomura M, Nakamura Y, Naruse H, Mori Y, Ishikawa T, Ando T, Kurokawa H, Kondo T, Nagamura Y, Ezaki K, Hishida H (2002) Risk stratification using a combination of cardiac troponin T and brain natriuretic peptide in patients hospitalized for worsening chronic heart failure. Am J Cardiol 89:691–695
Ishii J, Cui W, Kitagawa F, Kuno T, Nakamura Y, Naruse H, Mori Y, Ishikawa T, Nagamura Y, Kondo T, Oshima H, Nomura M, Ezaki K, Hishida H (2003) Prognostic value of combination of cardiac troponin T and B-type natriuretic peptide after initiation of treatment in patients with chronic heart failure. Clin Chem 49:2020–2026
Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction (2007) Universal definition of myocardial infarction. J Am Coll Cardiol 50:2173–2195
Thygesen K, Mair J, Katus H, Plebani M, Venge P, Collinson P, Lindahl B, Giannitsis E, Hasin Y, Galvani M, Tubaro M, Alpert JS, Biasucci LM, Koenig W, Mueller C, Huber K, Hamm C, Jaffe AS, Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care (2010) Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J 31:2197–2204
Asano S, Suzuki A, Ishii J, Sekiguchi-Ueda S, Shibata M, Yoshino Y, Nakamura K, Akiyama Y, Kitagawa F, Sakuishi T, Fujita T, Itoh M (2012) Use of a new assay for high-sensitivity cardiac troponin t for stratifying the risk of cardiovascular disease in outpatients with type-2 diabetes. Diabetology Int 3:29–36
Smathers RL, Galligan JJ, Shearn CT, Fritz KS, Mercer K, Ronis M, Orlicky DJ, Davidson NO, Petersen DR (2013) Susceptibility of L-FABP-/- mice to oxidative stress in early-stage alcoholic liver. J Lipid Res 54:1335–1345
Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F (2010) Liver fatty acid-binding protein and obesity. J Nutr Biochem 21:1015–1032
Acknowledgments
We are indebted to Emiko Horii, Sayaka Nomura and Dr. Hiroyuki Naruse for technical assistance. This work was supported by JSPS KAKENHI (Grant Number 24590712).
Conflict of interest
None of the authors have any potential conflict of interest associated with this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeda, Y., Suzuki, A., Ishii, J. et al. Level of urinary liver-type fatty acid-binding protein is associated with cardiac markers and electrocardiographic abnormalities in type-2 diabetes with chronic kidney disease stage G1 and G2. Heart Vessels 30, 362–368 (2015). https://doi.org/10.1007/s00380-014-0489-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-014-0489-4