Abstract
Hyperuricemia is common in chronic kidney disease (CKD), but data regarding the relationship between serum uric acid levels and the long-term outcomes of CKD patients have been limited. The present study evaluated the associations between baseline serum uric acid levels with mortality and end-stage renal disease (ESRD). The subjects of this study were 551 stage 2–4 CKD patients. Cox proportional hazards models were used to evaluate the relationship between serum uric acid tertiles and all-cause mortality, cardiovascular disease (CVD) mortality, 50 % reduction in estimated glomerular filtration rate (eGFR), and development of ESRD, initially without adjustment, and then after adjusting for several groups of covariates. The mean age of the study subjects was 58.5 years, 59.3 % were men, and 10.0 % had diabetes. The mean eGFR was 42.02 ± 18.52 ml/min/1.73 m2. In all subjects, the mean serum uric acid level was 6.57 ± 1.35 mg/dl, and 52.2 % of study subjects were on hypouricemic therapy (allopurinol; 48.3 %) at baseline. Thirty-one patients (6.1 %) died during a follow-up period of approximately 6 years. There was no significant association between serum uric acid level and all-cause mortality, CVD mortality, development of ESRD and 50 % reduction in eGFR in the unadjusted Cox models. In the adjusted models, hyperuricemia was found to be associated with all-cause mortality and CVD mortality after adjustment with CVD risk factors, kidney disease factors, and allopurinol, but not associated with development of ESRD and 50 % reduction in eGFR. The results of this study showed that hyperuricemia but not serum uric acid levels were associated with all-cause mortality, CVD mortality after adjustments with CVD risk factors, kidney disease factors, and allopurinol in stage 2–4 CKD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperuricemia is common in chronic kidney disease (CKD), and it is thought to develop secondary to a decrease in glomerular filtration rate (GFR) or hyperinsulinemia in the metabolic syndrome [1]. It is generally accepted that hyperuricemia is prevalent in patients who have several risk factors for cardiovascular disease (CVD) [2], and elevated serum uric acid levels have been found to predict the development of CVD and CKD in the general population [3, 4]. Moreover, hyperuricemia is an adverse prognostic factor and is associated with increased mortality [5]. However, neither the Framingham Heart Study [6] nor the Atherosclerosis Risk in Communities (ARIC) Study [7] found any such associations, and a recent analysis of the ARIC database demonstrated that although higher serum uric acid concentrations were associated with increased mortality in the non-CKD population even after adjustment for metabolic syndrome, the presence of CKD weakened the association [5]. The inconsistency of the data was confirmed by a recently published study of a large historical cohort of a national insurance provider that documented a stronger association between serum uric acid concentrations and cardiovascular morbidity in patients with severely decreased GFR [8]. Thus, the question of whether uric acid has a pathogenic role in the onset and progression of CKD and CVD remains unanswered.
Several observational studies have investigated whether an elevated serum uric acid level is an independent risk factor for the development and progression of CKD, but the results were inconclusive and conflicting [9]. A previous study reported that 20–60 % of subjects with gout and hyperuricemia developed renal impairment that was accompanied by histological lesions of glomerulosclerosis, interstitial fibrosis, and arteriosclerosis, and often by focal deposition of urate crystals in the outer medulla [10]. Elevated serum uric acid levels have been reported to predict the development of renal insufficiency in individuals with normal renal function [11]. Interestingly, the serum uric acid level at the time of a renal biopsy in IgA nephropathy patients is an independent predictor of renal disease progression, although a causal relationship cannot be established by such studies [12, 13].
A Japanese study of 6,403 individuals with normal renal function assessed the significance of hyperuricemia in relation to the early detection of renal dysfunction and found that the baseline serum uric acid levels were significantly correlated to the degree of the increases in serum creatinine (Cr) levels over a 2-year follow-up period [14]. More specifically, after adjusting for various confounding factors, serum uric acid levels >8.0 mg/dl were found to be associated with greater risk (2.9 times greater in men and 10.4 times greater in women) of developing an increase in serum Cr level. In a subsequent study, the same research group investigated the significance of hyperuricemia as a risk factor for end-stage renal disease (ESRD) in 48,177 people over 20 years of age in the general population [15]. The results showed that hyperuricemia was an independent predictor of development of ESRD in women over a 7-year follow-up period, while in men the difference was not statistically significant. However, information on the treatment regimens in these cohort studies was not available. The aim of the present study was to evaluate the relationship between baseline serum uric acid levels and mortality and development of ESRD in a screened cohort of Japanese CKD patients.
Patients and methods
Subjects
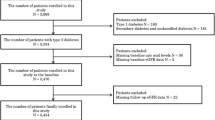
This is a retrospective cohort study in CKD patients. The study cohort has been described in detail previously [16]. The 1,115 participants were entered into this study during the period between September 2005 and October 2006. The entry criteria included being a CKD patient whose age was between 20 and 80 years old and giving written informed consent to enter this study. The exclusion criteria were: type 1 diabetes mellitus, insulin-dependent type 2 diabetes, glomerulonephritis secondary to an autoimmune disease, obstructive uropathy, renal artery stenosis, proteinuria greater than 3.5 g/day, systolic blood pressure (BP) greater than 180 mmHg, and prior kidney transplantation. Since 82 patients were lost to follow-up in less than 6 months, 286 patients had more than 20 % missing data, and 236 patients had stage 1 or stage 5 CKD, the final study population consisted of 511 stage 2–4 CKD patients. The causes of the CKD were chronic glomerulonephritis in 203 patients (39.7 %), nephrosclerosis in 283 patients (55.4 %), diabetic nephropathy in 19 patients (3.7 %), and polycystic kidney disease in six patients (1.2 %). Estimated GFR (eGFR) was estimated by using the modified MDRD equation for Japanese patients as previously described, and the subjects were stratified according to the stage of their CKD [17]. Only stage 2–4 CKD patients were entered into the study.
Our study cohort did not include stage 5 CKD patients. The Institutional Research Ethics Committee approved the study protocol (No. 2184). This study was conducted in compliance with the Declaration of Helsinki.
Measurements
The baseline variables included demographic features (age and sex), medical history variables (smoking status, diabetes mellitus, hypertension, and CVD), body mass index (BMI), systolic and diastolic BP, laboratory data, including hemoglobin concentration and serum levels of Cr, uric acid, albumin, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglyceride, glucose, and C-reactive protein (CRP), and urinary protein. Medical records were checked to collect data on prescription of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), hypouricemic agents (allopurinol), and diuretics (furosemide and thiazide). Hypertension was recorded as present when systolic BP was >140 mmHg and/or diastolic BP > 90 mmHg, or an antihypertensive agent had been prescribed. Diabetes mellitus was recorded as present when the fasting blood glucose level >126 mg/dl and/or HbA1c level >6.5 % (JDS), or a glucose-lowering agent had been prescribed. CVD was recorded as present when there was a clinical diagnosis of heart failure, acute or chronic ischemic heart disease, cerebrovascular disease, or peripheral artery disease. BMI was calculated based on the baseline measurements and using the formula: weight in kilograms divided by height in meters squared. The systolic and diastolic BP and laboratory data of each subject measured 3 months before enrollment and measured immediately after enrollment of the CKD cohort were averaged and analyzed as previously described [16].
Outcomes
We followed-up the subjects until January 2012 and assessed four outcomes: all-cause mortality, CVD mortality, 50 % reduction in eGFR, and development of ESRD, which was defined as requirement for dialysis or transplantation. Survival status and causes of death through December 31, 2011, were ascertained by a review of death certificates.
Statistical analysis
The distribution and normality of the variables of interest were evaluated by using box plots and histograms. Summary statistics according to tertiles of baseline serum uric acid levels are presented as percentages for categorical data, as the mean ± standard deviation for approximately normally distributed continuous variables, and as the median (interquartile range) for skewed continuous variables. Differences between the uric acid tertiles were statistically tested for significance by using the Chi-square test for categorical variables, ANOVA for approximately normally distributed continuous variables, and the Kruskal–Wallis test for skewed continuous variables.
Cox proportional hazards models were used to evaluate the relationships between uric acid tertiles and all-cause mortality, CVD mortality, 50 % reduction in eGFR, and development of ESRD, initially without adjustment, and then after adjusting for several groups of covariates. Covariates were selected for inclusion in the model if the p value was <0.1 in the univariate analysis (Table 1). Model 1 adjusted for randomization assignments to gender and smoking status. Model 2 adjusted for CVD risk factors including history of CVD, systolic BP, HDL cholesterol, triglyceride, hemoglobin and CRP (log) in addition to the variables in model 1. Model 3 adjusted for variables in model 2 as well as for kidney disease factors, i.e., eGFR, proteinuria (log), etiology of kidney disease and use of diuretics and model 4 adjusted for model 3 covariates + allopurinol use.
Sensitivity analysis
Since allopurinol lowers the serum uric acid level, individuals treated with allopurinol may have been misclassified into the low uric acid group despite having previously been exposed to high uric acid levels for extended periods. The presence of hyperuricemia was recorded if they were taking allopurinol or if their serum uric acid levels were >7 mg/dl according to the guideline of the Japanese Society of Gout and Nucleic Acid Metabolism [18]. We repeated the analyses after classifying into two groups according to whether they had hyperuricemia. Cox proportional hazards models were used to evaluate the relationship between the presence of hyperuricemia and all-cause mortality and CVD mortality, initially without adjustment, and subsequently adjusting for the following covariates selected on the basis of p < 0.1 in univariate analysis. Model 1 adjusted for gender and smoking status. Model 2 adjusted for history of CVD, HDL cholesterol, triglyceride, hemoglobin and CRP (log) in addition to the variables in model 1. Model 3 adjusted for variables in model 2 as well as for kidney disease factors, i.e., eGFR, proteinuria (log), etiology of kidney disease, and allopurinol use.
Results
Baseline characteristics according to serum uric acid tertiles
The mean age of the study cohort was 58.5 years and 59.3 % of the subjects were men; 10.0 % of the subjects had diabetes (Table 1). The mean eGFR of the entire cohort was 42.02 ± 18.52 ml/min/1.73 m2. The mean uric acid level at baseline was 6.57 ± 1.35 mg/dl, and 52.2 % of the subjects were on hypouricemic therapy (allopurinol; 48.3 %, benzbromarone; 3.9 %). There was no significant difference in age between the groups of subjects according to tertile of their baseline serum uric acid level (Table 1). The p value stands for comparison between all three tertiles of serum uric acid. Subjects in the highest quartile of uric acid were more likely to be male, a current smoker, to have experienced a previous CVD, and have a lower eGFR, heavier proteinuria, lower serum HDL cholesterol level, and lower hemoglobin concentration, and higher serum CRP level, and higher serum triglyceride level (Table 1). The prevalence of allopurinol use was highest in the highest uric acid tertile. As shown in Fig. 1, serum uric acid levels at the time of entry were gradually increased according to the progression of CKD stages (p < 0.0001).
Uric acid and all-cause mortality and CVD mortality
Thirty-one patients (6.1 %) died during a median follow-up period of approximately 6 years. The causes of death were myocardial infarction in 11 patients, congestive heart failure in eight patients, pneumonia in four patients, sepsis in three patients, and unknown origin in five patients. The crude all-cause mortality rates in uric acid tertile 1, tertile 2, and tertile 3 were 5.6, 5.1, and 7.5 %, respectively (Fig. 2). There were no associations between the uric acid tertiles and all-cause mortality in the unadjusted Cox model. This relationship was not significant after adjustment for demographic factors and clinical parameters (Table 2). Nineteen patients (3.7 %) died of CVD during a median follow-up period of approximately 6 years. The crude CVD mortality rates in uric acid tertile 1, tertile 2, and tertile 3 were 3.1, 3.4, and 4.6 %, respectively (Fig. 2). There were no associations between the uric acid tertiles and CVD mortality in unadjusted Cox model. This relationship was not significant after adjustment for demographic factors and clinical parameters (Table 2). In an attempt to clarify the correlation between uric acid and long-term outcomes in CKD, we re-examined the data after excluding the patients taking allopurinol. There were no significant correlations between serum uric acid levels and all-cause mortality (p = 0.6938) and CVD mortality (p = 0.8188) after adjustment for demographic factors and clinical parameters.
Uric acid and ESRD and 50 % decline in eGFR
Ninety-nine subjects (19.4 %) developed ESRD during a median follow-up period of approximately 6 years. The crude ESRD rates in uric acid tertile 1, tertile 2, and tertile 3 were 16.0, 16.6, and 25.3 %, respectively (Fig. 2). There was a significant association between the uric acid tertiles and ESRD in the unadjusted Cox model (p = 0.0247). After adjustment for demographic and clinical factors and allopurinol use, there was no association between serum uric acid level and the development of ESRD (Table 2). There was no association between the uric acid tertiles and 50 % reduction in eGFR in the unadjusted and adjusted Cox models. We also re-examined the data after excluding the patients taking allopurinol. There were no significant correlations between serum uric acid levels and ESRD (p = 0.7034) and 50 % reduction in eGFR (p = 0.4683) after adjustment for demographic factors and clinical parameters.
Sensitivity analysis
We repeated the analyses after classifying the subjects into two groups according to whether they had hyperuricemia, which was assumed to be present if they were taking allopurinol or if their serum uric acid level was >7 mg/dl. Sixty-two percent (n = 316) of the subjects had hyperuricemia at baseline. The crude hyperuricemia rates were shown in Fig. 3. Hyperuricemia was not associated with all-cause mortality and CVD mortality in the unadjusted Cox models (Table 3). However, hyperuricemia was associated with all-cause mortality and CVD mortality in the adjusted Cox model 2 and 3 that was adjusted for covariates such as gender, smoking status, history of CVD, HDL cholesterol, triglyceride, hemoglobin, CRP (log), eGFR, proteinuria (log), etiology of kidney disease and use of allopurinol (Table 3). Hyperuricemia was not associated with 50 % reduction in eGFR in the unadjusted and adjusted Cox models.
Analysis by gender
Since the proportion of male subjects was 59.3 % in the study population, gender may have affected the results of the present study. According to additional statistical analysis, the death due to CVD was significantly higher in men than women (p = 0.049), while there were no significant gender differences in all-cause mortality (p = 0.9129), ESRD (p = 0.5866), and 50 % reduction in eGFR (p = 0.1708).
Discussion
After the widespread use of allopurinol, hyperuricemia was found to be associated with all-cause mortality or CVD mortality during the 6-year follow-up period of our cohort of predominantly non-diabetic patients with stage 2–4 CKD after adjustments with demographic or clinical factors. However, no significant relationships between hyperuricemia and renal outcome, including progression to ESRD and 50 % reduction in eGFR, were observed in adjusted Cox models. For sensitivity analysis, hyperuricemia was defined if the patients were taking allopurinol or if their serum uric acid level was >7 mg/dl, suggesting that the use of allopurinol may be associated with CKD progression.
Data in regard to the relationship between serum uric acid levels and mortality in the early stages of CKD prior to reaching ESRD have been limited. A previous study that investigated the impact of nontraditional CVD risk factors on CVD outcomes in 1,678 patients with eGFR <60 ml/min/1.73 m2 showed that the serum uric acid level at baseline was not an independent predictor of a composite outcome of myocardial infarction, stroke, and all-cause mortality [19]. Two recent studies investigated the relationship between serum uric acid levels and mortality in CKD subjects [19, 20]. Madero et al. [20] reported that hyperuricemia appeared to be an independent risk factor for all-cause mortality and CVD mortality in the MDRD trial, which was conducted on 840 predominantly non-diabetic stage 3–4 CKD patients, and Liu et al. [21] reported finding that hyperuricemia was a risk factor for all-cause mortality and cardiovascular events in stage 3–5 CKD. The relationship between mortality and serum uric acid levels at baseline observed in our study was not consistent with the relationship reported by these previous studies.
Despite multiple epidemiological and prospective studies, the role of uric acid in the progression of CKD and development of ESRD remains controversial. In a study of 6,400 individuals with normal kidney function at baseline, serum uric acid levels >8.0 mg/dl were found to be associated with a 2.9-fold and tenfold increase in risk of developing CKD (defined as creatinine >1.2 mg-dl in women and 1.4 mg/dl in men) within 2 years in men and women, respectively [14]. A recent study of 13,338 individuals with normal kidney function based on estimated GFR evaluated the relationship between the serum uric acid level at baseline and incident kidney disease (defined as a GFR decrease ≥15 ml/min/1.73 m2 with final GFR <60 ml/min/1.73 m2). During a follow-up period of 8.5 years, each 1-mg/dl increment in serum uric acid level at baseline was found to be associated with an approximately 10 % increase in risk of incident kidney disease in multivariable adjusted models [19]. The fact that no significant association between serum uric acid level at baseline and ESRD or 50 % reduction in eGFR was observed in our study was consistent with the relationship reported by two previous studies [20, 21].
Serum uric acid is eliminated principally by the kidneys, and while there is a compensatory increased removal by the gut in the setting of renal insufficiency, this is not completely effective, and serum uric acid increases as the GFR falls, with approximately half of the subjects becoming hyperuricemic by the time of dialysis initiation [22]. This makes it difficult to assess the role of uric acid in the progression of renal injury in subjects with CKD based on epidemiological studies. It is not surprising that serum uric acid has often not been found to predict CKD progression [20, 23]. Thus, elevated uric acid is associated with the development of CKD, but not with the progression of CKD. In addition, an elevated serum uric acid level has been associated with both the presence of intrarenal arteriolar lesions [24, 25] and with an increased risk for cardiovascular mortality in subjects with CKD [20, 26], consistent with the vascular effects observed in laboratory animals with hyperuricemia [27]. Haririan et al. have shown differential effects of hyperuricemia with different degrees of CKD in renal transplant recipients [28], suggesting that serum uric acid levels in CKD stages may affect renal outcomes and mortality.
There were several limitations in the present study. First, a single baseline serum uric acid measurement was used to predict events several years into the future. However, there is a precedent for doing so, and several previous studies have used this approach [19–21]. Second, although we used the averaged serum uric acid level 3 months before and after the enrollment, the uric acid level may have varied during the follow-up period. Also, since the treatment policy of hyperuricemia was not standardized, the effect of uric acid variation over time was ignored. However, averaged uric acid levels are more accurate than the single values that have often been used in published studies [19–21]. Third, our cohort included few diabetic patients. It is important to acknowledge that the results of the present study were primarily obtained in predominantly non-diabetic patients with stage 2–4 CKD. Measures of association between serum uric acid levels and CVD may be even greater in stage 2–4 populations in which diabetes is prevalent. However, the subjects of our study were non-diabetic, and they were not appreciably malnourished or acutely ill, which minimized the limitation of confounding by comorbid conditions, such as malnutrition, and preexisting CVD.
In conclusion, the results of this study showed that hyperuricemia but not serum uric acid levels at the time of entry was associated with all-cause mortality, CVD mortality after adjustments with CVD risk factors, kidney disease factors, and allopurinol in stage 2–4 CKD patients.
References
Saito T, Mochizuki T, Uchida K, Tsuchiya K, Nitta K (2013) Metabolic syndrome and risk of progression of chronic kidney disease: a single-center cohort study in Japan. Heart Vessels 28:323–329
Nishino M, Mori N, Yoshimura T, Nakamura D, Lee Y, Taniike M, Makino N, Kato H, Egami Y, Shutta R, Tanouchi J, Yamada Y (2013) Higher serum uric acid and lipoprotein (a) are correlated with coronary spasm. Heart Vessels. doi:10.1007/s00380-013-0346-x
Chonchol M, Shlipak MG, Katz R, Sarnak MJ, Newman AB, Siscovick DS, Kestenbaum B, Carney JK, Fried LF (2007) Relationship of uric acid with progression of kidney disease. Am J Kidney Dis 50:239–247
Feig DI, Kang DH, Johnson RJ (2008) Uric acid and cardiovascular risk. N Engl J Med 359:1811–1821
Navaneethan SD, Beddhu S (2009) Associations of serum uric acid with cardiovascular events and mortality in moderate chronic kidney disease. Nephrol Dial Transpl 24:1260–1266
Culleton BF, Larson MG, Kannel WB, Levy D (1999) Serum uric acid and risk for cardiovascular and death: the Framingham Heart Study. Ann Intern Med 131:7–13
Moriarity JT, Folsom AR, Iribarren C, Nieto FJ, Rosamond WD (2000) Serum uric acid and risk of coronary heart disease: Atherosclerosis Risk in Communities (ARIC) Study. Ann Epidemiol 10:136–143
Neri L, Rocca Rey LA, Lentine KL, Hinyard LJ, Pinsky B, Xiao H, Dukes J, Schnitzler MA (2011) Joint association of hyperuricemia and reduced GFR on cardiovascular morbidity: a historical cohort study based on laboratory and claims data from a national insurance provider. Am J Kidney Dis 58:398–408
Kusano E (2011) Mechanism by which chronic kidney disease causes cardiovascular disease and the measures to manage this phenomenon. Clin Exp Nephrol 15:627–633
Berger L, Yu T (1975) Renal function in gout: an analysis of 524 gouty subjects including long-term follow-up studies. Am J Med 59:605–613
Beck L (1986) Requiem for gouty nephropathy. Kidney Int 30:280–287
Syrjanen J, Mustonen J, Pasternak A (2000) Hypertriglyceridemia and hyperuricemia are risk factors for progression of IgA nephropathy. Nephrol Dial Transplant 15:34–42
Ohno I, Hosoya T, Gomi H, Ichida K, Okabe H, Hikita M (2001) Serum uric acid and renal prognosis in IgA nephropathy. Nephron 87:333–339
Iseki K, Oshiro S, Tozawa M, Iseki C, Ikemiya Y, Takishita S (2001) Significance of hyperuricemia on the early detection of renal failure in a cohort of screened subjects. Hypertens Res 24:691–697
Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S (2004) Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 44:642–650
Yamashita T, Yoshida T, Ogawa T, Tsuchiya K, Nitta K (2011) Clinical outcomes in patients with chronic kidney disease: 1 5-year retrospective cohort study at a University Hospital in Japan. Clin Exp Nephrol 15:831–840
Imai E, Matsuo S, Makino H, Watanabe T, Akizawa T, Nitta K, Iimuro S, Ohashi Y, Hishida A (2010) Chronic Kidney Disease Japan Cohort study: baseline characteristics and factors associated with causative diseases and renal function. Clin Exp Nephrol 14:558–570
Yamanaka H, Japanese Society of Gout and Nucleic Acid Metabolism (2011) Japanese guideline for the management of hyperuricemia and gout: second edition. Nuceosides Nucleotides Nucleic Acids 30:1018–1029
Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS (2008) Uric acid and incident kidney disease in the community. J Am Soc Nephrol 19:1204–1211
Madero M, Sarnak MJ, Wang X, Greene T, Beck GJ, Kusek JW, Collins AJ, Levey AS, Menon V (2009) Uric acid and long-term outcomes in CKD. Am J Kidney Dis 53:796–803
Siu YP, Leung KT, Tong MK, Kwan TH (2006) Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47:51–59
Suliman ME, Johnson RJ, Garcia-Lopez E, Wureshi AR, Molinaei H, Carrero JJ, Heimburger O, Barany P, Axelsson J, Lindholm B, Steinvinkel P (2006) J-shaped mortality relationship for uric acid in CKD. Am J Kidney Dis 48:761–771
Sturm G, Kolleritis B, Neyer U, Ritz E, Kronenberg F (2008) Uric acid as a risk factor for progression of non-diabetic chronic kidney disease? The Mild to Moderate Kidney Disease (MMKD) Study. Exp Gerontol 43:347–352
Wu J, Chen X, Xie Y, Yamanaka N, Shi S, Wu D, Liu S, Cai G (2005) Characteristics and risk factors of intrarenal arterial lesions in patients with IgA nephropathy. Nephrol Dial Transplant 20:719–727
Akalin E, Ganeshan SV, Winston J, Muntner P (2008) Hyperuricemia is associated with the development of the composite outcomes of new cardiovascular events and chronic allograft nephropathy. Transplantation 86:652–658
Kanbay M, Yilmaz MI, Sonmez A, Solak Y, Saglam M, Cakir E, Unal HU, Arslan E, Verim S, Madero M, Caglar K, Oguz Y, McFann K, Johnson RJ (2012) Serum uric acid independently predicts cardiovascular events in advanced nephropathy. Am J Nephrol 36:324–331
Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ (2002) Hyperuricemia induces a primary renal arteriolopathy in rats by a blood-pressure-independent mechanism. Am J Physiol Renal Physiol 282:F991–F997
Haririan A, Nogueira JM, Zandi-Nejad K, Aiyer R, Hurley H, Cooper M, Klassen DK, Weir MR (2010) The independent association between serum uric acid and graft outcomes after kidney transplantation. Transplantation 89:573–579
Acknowledgments
This study was supported by a Grant-in-Aid from the Japan Promotion Society for Cardiovascular Diseases. We thank Ms. Etsuko Yamakoshi of Statcom Co., Ltd., Tokyo, Japan for statistically analyzing the clinical data.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyaoka, T., Mochizuki, T., Takei, T. et al. Serum uric acid levels and long-term outcomes in chronic kidney disease. Heart Vessels 29, 504–512 (2014). https://doi.org/10.1007/s00380-013-0396-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-013-0396-0