Abstract
Histopathological studies have suggested that early revascularization for acute myocardial infarction (MI) limits the size, transmural extent, and homogeneity of myocardial necrosis. However, the long-term effect of early revascularization on infarct tissue characteristics is largely unknown. Cardiovascular magnetic resonance (CMR) imaging with contrast enhancement (CE) allows non-invasive examination of infarct tissue characteristics and left ventricular (LV) dimensions and function in one examination. A total of 69 patients, referred for cardiac evaluation for various clinical reasons, were examined with CE-CMR >1 month (median 6, range 1–213) post-acute MI. We compared patients with (n = 33) versus without (n = 36) successful early revascularization for acute MI. Cine-CMR measurements included the LV end-diastolic and end-systolic volumes (ESV), LV ejection fraction (LVEF, %), and wall motion score index (WMSI). CE images were analyzed for core, peri, and total infarct size (%), and for the number of transmural segments. In our population, patients with successful early revascularization had better LVEFs (46 ± 16 vs. 34 ± 14%; P < 0.01), superior WMSIs (0.53, range 0.00–2.29 vs. 1.42, range 0.00–2.59; P < 0.01), and smaller ESVs (121 ± 70 vs. 166 ± 82; P = 0.02). However, there was no difference in core (9 ± 6 vs. 11 ± 6%), peri (9 ± 4 vs. 10 ± 4%), and total infarct size (18 ± 9 vs. 21 ± 9%; P > 0.05 for all comparisons); only transmural extent (P = 0.07) and infarct age (P = 0.06) tended to be larger in patients without early revascularization. CMR wall motion abnormalities are significantly better after revascularization; these differences are particularly marked later after infarction. The difference in scar size is more subtle and does not reach significance in this study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the setting of an acute myocardial infarction (MI), early revascularization within 6–12 h by means of primary percutaneous coronary interventions (PCI) reduces the infarct size and improves clinical outcome [1–4]. Postmortem studies on infarcted hearts previously showed that necrosis was uniform after permanent occlusion of the culprit coronary artery, whereas some myocardium survived in the necrotic region following early revascularization [5]. While early revascularization may alter infarct morphology by salvage of myocardial cells [6–8], a significant proportion of patients still develops transmural myocardial necrosis [9].
Cardiovascular magnetic resonance (CMR) imaging in combination with the contrast enhancement (CE) technique permits detailed non-invasive cardiac assessment of survivors of MI. This technique has the unique capability to study myocardial tissue characteristics (size, heterogeneity, and transmurality of necrosis) as well as the geometry and function of the left ventricle [10]. However, to the best of our knowledge, CE-CMR has not yet been used to assess potential differences in tissue characteristics between patients with versus without early revascularization for acute MI.
Therefore, in a consecutive series of patients with prior MI in whom CE-CMR was performed for clinical reasons, we compared data from patients with versus without early revascularization for acute MI. Based on the findings of previous histopathological studies, we hypothesized that in patients with early revascularization, infarct areas may be smaller, less homogeneous, and less transmural on CE-CMR than in patients without early revascularization.
Methods
Patient population
We conducted this study between October 2007 and January 2010 at Thoraxcentrum Twente in a consecutive series of patients with prior MI who were referred for cardiac evaluation for various clinical reasons [e.g., viability and/or left ventricular (LV) function, residual ischemia, and selection for implantable cardioverter defibrillator therapy]. Patients were included in the study if (1) the MI occurred at least 1 month prior to CMR (according to the definition of a healed MI) [11], (2) complete CE-CMR data were obtained, and (3) a positive infarct pattern of CE was found. To reduce the chance of having impaired function of the LV that was not mainly due to the MI, patients with CMP (i.e., idiopathic dilated, hyperthrophic) or relevant concomitant disease (e.g., myocarditis, tachycardiomyopathy) were excluded from this study.
The clinical charts, electrocardiograms (ECG), and angiographies of included MI patients were carefully examined to classify patients into two groups: patients with versus without successful early revascularization. The early revascularization group consisted of patients who underwent successful (vessel patency and/or resolution of ST-segment elevation and relief of symptoms) early revascularization by means of PCI (in the revascularization era) within 12 h after the start of symptoms [12]. Patients without early revascularization fulfilled at least one of these criteria: (1) (late) presentation of MI >12 h after the onset of symptoms, (2) conservative treatment, or (3) a failed revascularization procedure (narrowed culprit artery).
CMR data acquisition
CMR examination was performed on a 1.5-T whole body scanner (Achieva Scan, Philips Medical System, Best, The Netherlands) using commercially available cardiac CMR software. For signal reception a five-element cardiac synergy coil was used. ECG triggering was performed with a vector-ECG setup. Subjects were examined in the supine position. Morphological images in the multislice cardiac short axis, four-chamber long axis, three-chamber, and two-chamber long axis, and LV outflow tract views were acquired by using fast field echo cine images (slice thickness 8.0 mm, repetition time 3.4 ms; echo time 1.7 ms; flip angle 60°; matrix 256 × 256). Papillary muscles were regarded as part of the ventricular cavity. Myocardial scar was assessed on CE multislice short- axis, long-axis, and four-chamber views, obtained 10 min after intravenous bolus injection of 0.2 mmol gadolinium/kg body weight (Schering AG, Berlin, Germany). A three-dimensional turbo field echo-inversion recovery T1-weighted sequence was used with the following parameters: repetition time 4.0 ms; echo time 1.3 ms; flip angle 15°; inversion time individually optimized to null myocardial signal (usually between 180 and 250 ms); matrix 157; and slice thickness 10 mm.
CMR data analysis and definitions
CMR data were analyzed on a workstation using dedicated software for cardiac analysis (Philips MR workspace, Release 2.5.3.0 2007-12-03; Philips, The Netherlands).
LV geometry and function
Left ventricular end-diastolic and end-systolic volumes (EDV and ESV; ml), left ventricular ejection fraction (LVEF; %), and end-diastolic wall mass (EDWM; g) were calculated from contiguous short-axis loops by segmentation of endocardial and epicardial borders on each frame. Papillary muscles were regarded as part of the ventricular cavity. End-diastolic wall thickness (EDWT; mm) at the infarcted wall area was measured quantitatively at the center of the infarct region [13].
The LV wall regions were further divided into 17 segments according to a standardized myocardial segmentation model [14]. Wall motion of all 17 separate segments was assigned the following scores: normal wall motion was 0, hypokinesia 1, severe hypokinesia 2, akinesia 3, and dyskinesia 4. The wall motion score index (WMSI) was calculated by dividing the sum of scores in each segment by the total number of segments (17 segments). WMSI of 0 was considered as normal, 0–1 as moderate, 1–2 as poor, and >2 as bad.
Infarct tissue characteristics
The infarcted myocardium was defined as the zone of hyper-enhancement on the CE images, in contrast with the dark-gray signal of the normal myocardium. Infarct size was quantified by a semi-automatic thresholding technique with the full width at half-maximum approach as previously validated to maximize accuracy and reproducibility [15–17]. After outlining the myocardial segment containing the region with high signal intensity, the maximum signal intensity region was determined. Scar was divided into an infarct core zone and a heterogeneous zone (i.e., peri-infarct zone). The infarct core was then defined as myocardium with a signal intensity ≥50% of the maximal signal intensity. The heterogeneous zone was defined as myocardium with a signal intensity between ≥35 and <50% of the maximal signal intensity. Total scar was defined as the sum of infarct core plus heterogeneous zone [18]. By use of planimetry, the extent of CE was first determined on contiguous short-axis images, then summed up to a volume, and finally expressed as a percentage of the total myocardial volume.
Scar tissue characteristics were further quantified according to location by use of a 17 segmental model [14]. Each segment was scored as follows: a scar score of 0 was considered as normal, 1 as 1–25% scar, 2 as 26–50% scar, 3 as 51–75% scar, and 4 as 76–100% scar of the segmental area.
The segmental scar score was calculated by dividing the sum of the individual segmental scores by the total number of observed segments. The transmural extent of myocardial scar was defined as the number of segments with a scar score 3 or 4. In addition, a segmental regional scar score was calculated in order to relate scar size to the territories of the three major coronary arteries as previously described in detail [14].
Statistical analysis
Continuous variables had a normal distribution and were expressed as mean ± standard deviation (SD). Categorical data were expressed as frequencies and percentages. To compare patients with versus without early revascularization, Student’s t test and Mann-Whitney U test were used to compare continuous variables, and chi-square and Fisher's exact test were used to compare categorical variables. P values <0.05 were considered statistically significant. A post hoc analysis was accomplished in order to assess the difference in infarct size characteristics that could be detected with the available sample size, presented as detectable alternatives. Linear regression was performed to correct for confounding factors.
Results
Study patients
A total of 69 patients (59 ± 11 years old; 56 men) at a median of 6 (range 1–213) months after acute MI were examined in this study. Almost half of the patients (n = 33, 48%) had undergone successful early revascularization by means of PCI. The group of patients without early revascularization (n = 36, 52%) consisted of patients with subclinical MI (n = 12), late clinical presentation of MI (n = 16), or failed revascularization therapy (n = 8). All patients with early successful revascularization demonstrated ST elevation on the ECG. In patients without early revascularization, 11 patients demonstrated ST elevation, and 25 patients had q waves on the ECG. Demographics and baseline characteristics did not differ between groups, except for the prevalence of diabetes mellitus (9 vs. 26%; P = 0.04) and diuretic usage (33 vs. 58%; P = 0.02), which both presented more often in patients without early revascularization. Infarct age did not significantly differ between subgroups (see also Table 1).
CMR results
CE-CMR was performed for viability and/or LV function (n = 29), selection of implantable cardioverter defibrillator therapy (n = 26), residual ischemia (n = 5), or other reasons (n = 9). No significant difference (P = 0.56) between both subgroups according to clinical indication for CMR was found.
LV geometry and function
In the early revascularization group, LVEF was significantly higher (46 ± 16 vs. 34 ± 14%; P = <0.01), WMSI was superior [0.53 (range 0.00–2.29) vs. 1.42 (range 0.00–2.59); P ≤ 0.01], ESV was significantly smaller (121 ± 70 vs. 166 ± 82; P = 0.02), and EDWT at the infarct center was larger (5.99 ± 2.98 vs. 4.40 ± 2.27). In addition, in patients with early revascularization, a trend towards a lower LV EDV (210 ± 61 vs. 24073; P = 0.07) was found. All cine CMR data are presented in Table 2. Because of the almost significant (P = 0.06) difference in infarct age between both groups, we performed a linear regression analysis to see if the difference in LV function (i.e., LVEF and WMSI) remained significant after correction for infarct age. This resulted in a non-significant difference in LVEF of 6.5% (P = 0.13) and a significant difference in WMSI of 0.38 (P = 0.04) between revascularized and non-revascularized patients in favor of the group with early successful revascularization.
Infarct tissue characteristics
There was no significant difference in the size of infarct core (9 ± 6 vs. 11 ± 6%; P = 0.17; detectable alternative 4), peri-infarct zone (9 ± 4 vs. 10 ± 4%; P = 0.34; detectable alternative 3), or total infarct area (18 ± 9 vs. 21 ± 9%; P = 0.18; detectable alternative 6); additionally, regional scar scores did not differ between groups. Only the transmural extent of infarction tended to be greater in patients without early revascularization (2.18 ± 1.94 vs. 3.08 ± 2.08; P = 0.07). All CE-CMR data are presented in Table 3 (Fig. 1).
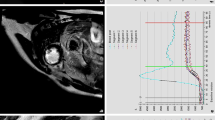
CE-CMR results in a patient with and a patient without early revascularization. a, b CE-CMR imaging short axis and four-chamber view in an early revascularized patient; presence of transmural CE is observed anteroseptally and apically. c, d CE-CMR imaging short axis view and four-chamber view in a patient without early revascularization; presence of transmural CE is observed anteroseptally and apically
Discussion
To the best of our knowledge, this is the first study to evaluate infarct tissue characteristics in patients with versus without successful early revascularization for acute MI using CE-CMR. The main findings of the present study in patients with versus without successful early revascularization for acute MI were (1) that in this population infarct tissue characteristics did not differ significantly between groups (only transmural infarction tended to be smaller after early revascularization), while (2) in the latter group LV function (LVEF, WMSI) was significantly worse, and the myocardium at infarct site and LV dimensions were less preserved (i.e., more LV remodelling).
Both study groups showed no significant difference in baseline characteristics except for a higher prevalence of diabetics and a higher diuretic usage in the group of patients without early revascularization. This may actually be expected as diabetics are known to have more silent MI (50% in our population) without the chance to perform an early revascularization therapy [19], and the use of diuretics may imply severe heart failure, and thus reflect a poor clinical condition caused by LV dysfunction, which was significantly more represented in patients without early revascularization.
Revascularization and infarct tissue characteristics
Previous histopathological postmortem studies assessing hearts 0–9 days after acute MI found that permanent occlusion of the culprit coronary artery resulted in a uniform transmural necrosis in the infarcted area, while after early revascularization heterogeneously infarcted tissue was observed [5, 7]. Previous clinical studies using various non-invasive imaging modalities to evaluate the impact of early revascularization on infarct tissue characteristics reported conflicting results. Compared to our present data, these studies were performed much earlier after an acute MI. Using scintigraphy 10–14 days after the index MI, Schomig et al. [20] found no significant relation between time to revascularization and infarct size. However, Schomig et al. [21] also found that following randomly assigned invasive or conservative treatment of patients with acute MI more than 12 h after symptom onset, invasive treatment still reduced infarct size as determined by scintigraphy. More recently, Francone et al. [1] assessed the relation between CE-CMR-determined infarct size versus time-to-revascularization 3 ± 2 days after the index MI. They found that revascularization ≤90 min after the onset of symptoms was associated with a smaller infarct size and a larger area of salvaged myocardium, whereas revascularization >360 min after symptom onset was associated with a larger infarct size and a very limited myocardial salvage. In a chronic ischemic heart disease population, Heidary et al. [22] also recently found no difference in infarct tissue characteristics between patients with medical management only versus patients with previous revascularizations.
Nevertheless, while in our specific study population no significant difference in core, peri and total infarct size was found, total infarct size was 14% smaller in revascularized patients compared to non-revascularized patients (18 vs. 21%), which may actually be of clinical importance. In addition, there was a strong but non-significant trend towards less transmural extent of infarct tissue in favor of early revascularized patients. This is in agreement with the fact that successful early revascularization reduces in particular the transmural expansion of necrosis from the endocardial to the epicardial myocardium.
Conversely, while ACE-inhibitor therapy was initially allocated for higher risk subgroups patients (i.e., large anterior MI) [23], our findings indirectly support recent guidelines that recommend a broad application of ACE inhibitors post-MI, not only restricted to high-risk patients [12].
The results in our study population cannot be extrapolated to an “all comers” population. First of all, patients were only selected if there was a clinical indication for performing CE-CMR imaging and if the MI had occurred at least 1 month prior to CMR. In addition, our study population represents a series of long-term survivors of MI. The median infarct age in our study population was 6 months (range 1–213), whereas previous (histopathological and clinical) studies investigated differences in infarct tissue characteristics no more than half a month after the index MI. In addition, previous histopathological studies examined patients with fatal outcomes after very recent MI only. Differences in study design and the inherent selection bias may affect the likelihood of detecting differences in infarct size. This may explain differences between the results of the present study and previous histopathological studies (in patients with lethal course of the disease) and clinical studies in other types of patient populations.
During the process of infarct healing, which is generally considered to occur within 1 month after the MI, infarct tissue characteristics may change as a result of replacement of necrotic tissue by scar tissue [11]. Further gradual changes of infarct tissue may occur after this process of infarct healing (i.e., >1 month after MI) in response to residual myocardial ischemia, increased wall stress, arterial hypertension, or medical therapy [24, 25].
Recent data from electro-anatomic mapping in long-term survivors of MI (13 ± 9 years) suggested that infarct tissue characteristics are different in patients with versus without revascularization during the index MI [24], but these data were obtained in a patient population with documented episodes of sustained monomorphic ventricular tachycardia, which represents another significant selection bias.
Revascularization and LV remodeling
Infarct size has been regarded as the primary determinant of LV remodeling and is associated with an adverse LV function [26–28]. A scintigraphic study previously investigated the impact of early revascularization on LV remodeling in patients 1 month after index MI; this study showed a smaller infarct size, smaller LVEDV and LVESV, and a higher LVEF following early revascularization [29].
In our present study, LV function (i.e., LVEF and WMSI) was also significantly better after successful early revascularization, and LV dimensions and wall thickness in the infarct area tended to be more preserved, whereas patients without early revascularization showed a greater extent of remodeling late after the index MI. However, between both groups we found no difference in infarct tissue characteristics as determined with CE-CMR.
Our findings (based on available CE-CMR data of a specific patient population referred for cardiac evaluation for various clinical reasons) may suggest that the process of remodeling >1 month post-MI—especially in patients without early revascularization—is greatly determined by mechanisms that go beyond the extent of infarct size. Potential mechanisms involved may be pressure and volume overload hypertrophy, compensatory neurohormonal mechanisms, and genetic mechanisms such as adapted gene expression in the setting of heart failure [30, 31]. The results of our regression analysis suggest that infarct age should be taken into account when investigating LV (dys)function in patients with previous MI.
Our data underline the importance of investigating the various mechanisms that are potentially involved in the process of LV remodeling, both in basic research and in clinical studies.
Limitations
As in most previous CE-CMR studies, the sample size was relatively small. Our study population represents long-term survivors of MI. Because of our study design, we cannot exclude a certain bias towards patients with a more favorable or fatal clinical course following MI. However, notably, other studies on infarct tissue characteristics that were histopathological studies had other significant selection biases, as they only examined patients following fatal MI. A prospective study design therefore would be ideal to investigate differences in infarct tissue characteristics in patients with versus without successful early revascularization for acute MI using CE-CMR.
Conclusion
CMR wall motion abnormalities are significantly better after revascularization; these differences are particularly marked later after infarction. The difference in scar size is more subtle and does not reach significance in this study.
Abbreviations
- MI:
-
Myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- CMR:
-
Cardiovascular magnetic resonance
- CE:
-
Contrast enhancement
- ECG:
-
Electrocardiogram
- EDV:
-
End-diastolic volume
- ESV:
-
End-systolic volume
- LVEF:
-
Left ventricular ejection fraction
- EDWM:
-
End diastolic wall mass
- WMSI:
-
Wall motion score index
- FWHM:
-
Full width at half maximum
- LAD:
-
Left anterior descending
- RCA:
-
Right coronary artery
- LCX:
-
Left circumflex
- EDWT:
-
End diastolic wall thickness
References
Francone M, Bucciarelli-Ducci C, Carbone I, Canali E, Scardala R, Calabrese FA, Sardella G, Mancone M, Catalano C, Fedele F, Passariello R, Bogaert J, Agati L (2009) Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST-segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol 54(23):2145–2153
Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group (1994) Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet 343(8893):311–322
Collaborative Group (1993) Randomised trial of late thrombolysis in patients with suspected acute myocardial infarction. EMERAS (Estudio Multicentrico Estreptoquinasa Republicas de America del Sur). Lancet 342(8874):767–772
Feng Y, Shen C, Ma G, Wang J, Chen Z, Dai Q, Zhi H, Yang C, Fu Q, Shang G, Guan Y (2010) Prolonged pain to hospital time is associated with increased plasma advanced oxidation protein products and poor prognosis in patients with percutaneous coronary intervention for ST-elevation myocardial infarction. Heart Vessels 25(5):374–378
Matsuda M, Fujiwara H, Onodera T, Tanaka M, Wu DJ, Fujiwara T, Hamashima Y, Kawai C (1987) Quantitative analysis of infarct size, contraction band necrosis, and coagulation necrosis in human autopsied hearts with acute myocardial infarction after treatment with selective intracoronary thrombolysis. Circulation 76(5):981–989
Eitel I, Desch S, Sareban M, Fuernau G, Gutberlet M, Schuler G, Thiele H (2009) Prognostic significance and magnetic resonance imaging findings in aborted myocardial infarction after primary angioplasty. Am Heart J 158(5):806–813
Karagueuzian HS, Fenoglio JJ, Jr, Weiss MB, Wit AL (1979) Protracted ventricular tachycardia induced by premature stimulation of the canine heart after coronary artery occlusion and reperfusion. Circ Res 44(6):833–846
Michelson EL, Spear JF, Moore EN (1980) Electrophysiologic and anatomic correlates of sustained ventricular tachyarrhythmias in a model of chronic myocardial infarction. Am J Cardiol 45(3):583–590
O’Regan DP, Ahmed R, Neuwirth C, Tan Y, Durighel G, Hajnal JV, Nadra I, Corbett SJ, Cook SA (2009) Cardiac MRI of myocardial salvage at the peri-infarct border zones after primary coronary intervention. Am J Physiol Heart Circ Physiol 297(1):H340–H346
Friedrich MG (2008) Tissue characterization of acute myocardial infarction and myocarditis by cardiac magnetic resonance. JACC Cardiovasc Imaging 1(5):652–662
Thygesen K, Alpert JS, White HD (2007) Universal definition of myocardial infarction. Eur Heart J 28(20):2525–2538
Van de WF, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Aguirre FV, Al Attar N, Alegria E, Andereotti F, Benzer W, Breithardt O, Danchin N, Di Mario C, Dudek D, Gulba D, Halvorsen S, Kaufmann P, Kornowski R, Lip GY, Rutten F (2008) Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 29(23):2909–2945
Roes SD, Kelle S, Kaandorp TA, Kokocinski T, Poldermans D, Lamb HJ, Boersma E, van der Wall EE, Fleck E, de Roos A, Nagel E, Bax JJ (2007) Comparison of myocardial infarct size assessed with contrast-enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol 100(6):930–936
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105(4):539–542
Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R, Nasir K, Kraitchman DL, Lima JA (2004) Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol 44(12):2383–2389
Bondarenko O, Beek AM, Hofman MB, Kuhl HP, Twisk JW, van Dockum WG, Visser CA, van Rossum AC (2005) Standardizing the definition of hyperenhancement in the quantitative assessment of infarct size and myocardial viability using delayed contrast-enhanced CMR. J Cardiovasc Magn Reson 7(2):481–485
Beek AM, Bondarenko O, Afsharzada F, van Rossum AC (2009) Quantification of late gadolinium enhanced CMR in viability assessment in chronic ischemic heart disease: a comparison to functional outcome. J Cardiovasc Magn Reson 11(1):6
Roes SD, Borleffs C, van der Geest RJ, Westenberg JJM, Marsan NA, Kaandorp TAM, Reiber JHC, Zeppenfeld K, Lamb HJ, de Roos A (2009) Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging 2(3):183
Kannel WB (1985) Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am Heart J 110(5):1100–1107
Schomig A, Ndrepepa G, Mehilli J, Schwaiger M, Schuhlen H, Nekolla S, Pache J, Martinoff S, Bollwein H, Kastrati A (2003) Therapy-dependent influence of time-to-treatment interval on myocardial salvage in patients with acute myocardial infarction treated with coronary artery stenting or thrombolysis. Circulation 108(9):1084–1088
Schomig A, Mehilli J, Antoniucci D, Ndrepepa G, Markwardt C, Di PF, Nekolla SG, Schlotterbeck K, Schuhlen H, Pache J, Seyfarth M, Martinoff S, Benzer W, Schmitt C, Dirschinger J, Schwaiger M, Kastrati A (2005) Mechanical reperfusion in patients with acute myocardial infarction presenting more than 12 hours from symptom onset: a randomized controlled trial. JAMA 293(23):2865–2872
Heidary S, Patel H, Chung J, Yokota H, Gupta SN, Bennett MV, Katikireddy C, Nguyen P, Pauly JM, Terashima M, McConnell MV, Yang PC (2010) Quantitative tissue characterization of infarct core and border zone in patients with ischemic cardiomyopathy by magnetic resonance is associated with future cardiovascular events. J Am Coll Cardiol 55(24):2762–2768
ACE Inhibitor Myocardial Infarction Collaborative Group (1998) Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials. Circulation 97(22):2202–2212
Wijnmaalen AP, Schalij MJ, von der Thusen JH, Klautz RJ, Zeppenfeld K (2010) Early reperfusion during acute myocardial infarction affects ventricular tachycardia characteristics and the chronic electroanatomic and histological substrate. Circulation 121(17):1881–1883
Yokota T, Osanai T, Hanada K, Kushibiki M, Abe N, Oikawa K, Tomita H, Higuma T, Yokoyama J, Hanada H, Okumura K (2010) Effects of telmisartan on markers of ventricular remodeling in patients with acute myocardial infarction: comparison with enalapril. Heart Vessels 25(6):460–468
Mollema SA, Liem SS, Suffoletto MS, Bleeker GB, van der Hoeven BL, van de Veire NR, Boersma E, Holman ER, van der Wall EE, Schalij MJ, Gorcsan J III, Bax JJ (2007) Left ventricular dyssynchrony acutely after myocardial infarction predicts left ventricular remodeling. J Am Coll Cardiol 50(16):1532–1540
Chareonthaitawee P, Christian TF, Hirose K, Gibbons RJ, Rumberger JA (1995) Relation of initial infarct size to extent of left ventricular remodeling in the year after acute myocardial infarction. J Am Coll Cardiol 25(3):567–573
Tarantini G, Razzolini R, Cacciavillani L, Bilato C, Sarais C, Marra MP, Napodano M, Ramondo A, Iliceto S (2006) Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol 98(8):1033–1040
Hirayama A, Adachi T, Asada S, Mishima M, Nanto S, Kusuoka H, Yamamoto K, Matsumura Y, Hori M, Inoue M (1993) Late reperfusion for acute myocardial infarction limits the dilatation of left ventricle without the reduction of infarct size. Circulation 88(6):2565–2574
Pfeffer MA, Braunwald E (1990) Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81(4):1161–1172
Ramos LW, Murad N, Goto E, Antonio EL, Silva JA, Jr, Tucci PF, Carvalho AC (2009) Ischemia/reperfusion is an independent trigger for increasing myocardial content of mRNA B-type natriuretic peptide. Heart Vessels 24(6):454–459
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olimulder, M.A.G.M., Kraaier, K., Galjee, M.A. et al. Infarct tissue characteristics of patients with versus without early revascularization for acute myocardial infarction: a contrast-enhancement cardiovascular magnetic resonance imaging study. Heart Vessels 27, 250–257 (2012). https://doi.org/10.1007/s00380-011-0150-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-011-0150-4