Abstract
The aim of the present study was to assess whether elevated B-type natriuretic peptide (BNP) levels, as an objective marker of heart failure, is a predictor of subsequent thromboembolic events in patients with atrial fibrillation (AF) during oral anticoagulant therapy. This was a post hoc analysis of a single-center, prospective, observational study. Consecutive patients with AF (261 patients, 74 ± 9 years old, 153 paroxysmal AF) treated with warfarin were included for the analysis. BNP level at baseline examination was measured to assess the relationship of this parameter with subsequent thromboembolic events. BNP levels at the time of entry were 161 ± 188 (5–1,500, median 105) pg/ml. During an average follow-up time of 762 ± 220 (median 742) days, nine (1.8%/year) thromboembolic events occurred. Receiver operating characteristic curve showed that an optimal cut-off value for BNP to predict thromboembolic events was 218 pg/ml. There were six thromboembolic events observed among patients with a baseline BNP levels ≥200 pg/ml (n = 73) as compared to three such events in those with baseline BNP levels <200 pg/ml (n = 188). Kaplan–Meier curves for BNP level showed that elevated BNP level (≥200 pg/ml) was significantly associated with thromboembolic events (p < 0.01). Cox-proportional hazard analysis also revealed that a high BNP level (≥200 pg/ml) was a significant predictor of subsequent thromboembolic events (hazard ratio 5.32, p = 0.018). Elevated BNP levels (≥200 pg/ml) could be a useful marker of subsequent thromboembolic events in patients with AF during oral anticoagulant therapy. However, the number of patients and events in this study was small and drawing a definite conclusion was not possible with this small sample size. Therefore, further larger-scale, multicenter studies are needed to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is associated with hemostatic abnormality and increased risk of thromboembolic events [1, 2]. Congestive heart failure (CHF) is also recognized as an established risk factor for stroke [3, 4]. For the evaluation of the severity of CHF, NYHA functional classification is an established criterion and is widely used in daily practice. However, there are certain challenges in AF patients, since symptoms of heart failure, such as dyspnea and palpitation, may be similar to those of AF.
On the other hand, B-type natriuretic peptide (BNP), which derives from both atrial and ventricular cardiomyocytes, was recognized as the single most accurate predictor of the presence and absence of heart failure [5]. A conventional BNP cut-off value of 100 pg/ml is often used for the diagnosis of heart failure in patients who visit the emergency department with acute dyspnea [5]. However, BNP levels are reported to be increased in AF patients [6–8], and thus a higher cut-off value may be appropriate. Knudsen et al. [8], using the database from the Breathing Not Properly Multinational study, reported that the optimal BNP cut-off value to detect CHF in patients with AF was 262 pg/ml with receiver operating characteristic (ROC) curve analysis and recommended that a cut-off value of 200 pg/ml was appropriate in daily practice in an urgent situation.
Shimizu et al. [9] reported that plasma BNP was high in patients with episodes of thromboembolisms in nonvalvular AF. Shibazaki et al. [10] reported that plasma BNP was elevated in the acute phase of stroke and declined in a month in patients with AF. Therefore, BNP may be a promising potent biomarker for differentiating high-risk AF from low-risk AF. However, there have been no reports on whether BNP could be used for prediction of thromboembolic complications before the onset of such events in patients with AF. The aim of the present study was to elucidate (1) whether BNP could be a predictor of subsequent thromboembolic events in AF patients and, if so, (2) to decide the proper BNP cut-off value.
Methods
The study design and main results have recently been published [11]. Briefly, this was a post hoc analysis of a single-center, prospective, observational study. Patients eligible for our study were those with AF treated with warfarin for at least 4 weeks in Yatsushiro General Hospital from January 2006 to April 2007. Initially, 301 patients with AF were screened and 269 patients were included. In the present analysis, six patients with hemodialysis and two patients in whom BNP levels were not measured at the time of entry were excluded. Finally, 261 patients (74 ± 9 years old, 114 female, 153 paroxysmal AF) were analyzed. BNP levels were measured at the time of enrollment with optimized medical treatments (Table 2). Target prothrombin time-international normalized ratio (PT-INR) was set at 1.5–3.0 according to the Japanese Circulation Society Guideline [12] and it was measured every 1–2 months throughout the follow-up periods. Optimal PT-INR control was achieved in 86% of the patients at the time of entry and 82% at the end of the study. They were followed prospectively with an average follow-up time of 762 ± 220 (median 742) days. The end points were thromboembolic events including ischemic strokes, transient ischemic attacks, and peripheral embolisms. CHF was defined as having NYHA class ≥II symptoms and/or congestive signs requiring loop diuretics at the time of entry. Hypertension was defined as either systolic blood pressure exceeding 140 mmHg or diastolic blood pressure exceeding 90 mmHg, or taking antihypertensive medications. Diabetes mellitus was defined as either fasting plasma glucose ≥126 mg/dl, casual glucose level ≥200 mg/dl, HbA1c ≥ 6.1%, receiving oral hypoglycemic medications, or using insulin. Ischemic stroke was defined as sudden onset of neurological symptoms lasting more than 24 h and subsequently confirmed by computed tomography (CT). Transient ischemic attack was defined as complete recovery of ischemic neurological symptoms within 24 h without apparent CT findings. Left ventricular ejection fraction was calculated using M mode echocardiography if wall motion was normal and Simpson’s method was applied if local wall motion abnormalities were found. eGFR was calculated according to the Japanese modified version [13] of Modification of Diet in Renal Disease Study equation [14]. All assays were performed in the laboratory of our institution. BNP was measured by automated enzyme immunoassay analyzer, AIA-360 (TOSOH, Tokyo, Japan, detection limits, 5 pg/ml).
Statistical analysis
Data are presented as the mean ± SD or percentages, as appropriate. Event frequencies were compared using the Chi-square test. Other comparisons between two groups of data were made with unpaired Student’s t test or Mann–Whitney test, as appropriate. The optimal BNP cut-off level for the detection of thromboembolic events was evaluated by ROC curve. Logistic regression models were applied to assess the determinants of high BNP levels. The outcomes were displayed with a Kaplan–Meier event-free curve and compared with the use of log-rank tests. The prognostic values of BNP and other clinical variables were analyzed using Cox proportional hazard models. A p level of <0.05 was accepted as statistically significant. The statistical software package SPSS (version 14.0, SPSS Inc., Chicago, IL, USA) and StatView (StatView Version 5.0, Berkeley, USA) were used for analyses.
The study protocol was approved by the institutional ethics committee and informed written consent was obtained from all the patients included in this study.
Results
There were nine thromboembolic events (1.8%/year): seven ischemic strokes, one transient ischemic attack, and one peripheral embolism. ROC curve analysis (Fig. 1) revealed that the optimum cut-off point, identified as the point closest to the upper left corner, yielded BNP level of 218 pg/ml to detect subsequent thromboembolic events. Based on this finding, we defined the cut-off value of BNP as 200 pg/ml.
Table 1 shows baseline characteristics of the patients with high (≥200 pg/ml) BNP levels. Patients with high (≥200 pg/ml) BNP levels were older than those with low (<200 pg/ml) BNP levels. Ejection fraction was smaller in patients with high BNP levels. The presence of CHF, chronic AF, and structural heart disease were more common in patients with high BNP levels. Cardiac medications, including angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor antagonists (ARB), β blockers, and diuretics were more frequently used in patients with high BNP levels. PT-INR was not different between these groups.
Table 2 shows determinants of high (≥200 pg/ml) D-dimer levels by logistic regression analysis. Univariate analysis showed that age, ejection fraction, eGFR, presence of chronic AF, CHF, and structural heart disease were the significant determinants of high BNP levels. Multivariate analysis including these six parameters and gender revealed that age, presence of chronic AF, and CHF were independent determinants of high BNP levels.
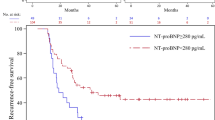
There were six thromboembolic events observed among patients with high (≥200 pg/ml) BNP levels as compared to three such events in those with low (<200 pg/ml) BNP levels. Kaplan–Meier curves for BNP showed that high BNP levels were associated with thromboembolic events (p < 0.01, Fig. 2). Univariate Cox proportional hazard analysis showed that high BNP levels (≥200 pg/ml), natural log transformation of BNP (continuous variable), chronic AF, CHF, and history of stroke were the significant predictors of thromboembolic events (Table 3). Since there were only nine thromboembolic events in this study, multivariate analysis was difficult; however, when we adjusted for age and gender, high BNP (≥200 pg/ml) was also a significant predictor of subsequent thromboembolic events (p = 0.032, hazard ratio 4.66, confidence interval 1.14–19.0).
Discussion
This post hoc analysis demonstrated that in patients with AF during anticoagulant therapy, high BNP levels defined as ≥200 pg/ml predicted subsequent thromboembolic events.
A marked reduction in BNP levels after medications, such as diuretics, ACEI/ARB were reported [15]. BNP levels in our study population were lower than those of Knudsen’s report (median BNP value: 202 vs. 421 pg/ml) because it was measured in stable condition with optimal medical treatments. ROC analysis (not shown) revealed that the optimal BNP cut-off value to detect CHF symptoms in our study was 136 pg/ml, which was also smaller than that of Knudsen’s [8] report (262 pg/ml). Regardless of the BNP cut-off value to detect CHF, in other words, whether patients had CHF symptoms, our study provided some evidence that BNP levels ≥200 pg/ml predicted subsequent thromboembolic events in patients with AF when the patient received optimal medical therapies, including anticoagulant therapy and heart failure therapy. On the other hand, CHF was an established risk factor for stroke in AF patients and was included as a risk factor for stroke in several clinical trials and risk stratification schemes [16–18]. Although multivariate analysis could not be performed, the presence of CHF seemed to be a stronger determinant than that of high (≥200 pg/ml) BNP in our study (Table 3). In fact, there were eight thromboembolic events observed among patients with CHF as compared to only one event in those without CHF. However, it is sometimes difficult to diagnose CHF symptoms in patients with AF because exertional dyspnea or palpitation due to heart failure and those due to AF may be in some cases hard to discriminate. In addition, patients with CHF, especially older patients, may occasionally not complain of symptoms because they limit their daily activities on purpose. Moreover, there may be some asymptomatic CHF patients who can only be found by measuring BNP levels. Therefore, both BNP measurements and detecting CHF symptoms and signs are important and should be used both for the risk stratification of stroke in patients with AF. Whether thromboembolic events in patients with AF can be prevented by treating CHF remains to be elucidated.
Study limitations
This study had several limitations. First, target PT-INR levels in this study were set slightly below the recommended guideline of Western countries [1, 2, 16] because of racial differences in the optimal anticoagulation intensity [19]. Japanese Circulation Society Guidelines recommended target PT-INR should be 1.6–2.6 when the patients were older than 70 years old [12]. We have basically titrated our anticoagulation regimen based on this guideline, but it may not be applicable to Western populations. Second, it is speculated that BNP levels might increase at the time of events [10], but it was measured only at the entry periods. In addition, biological variations of BNP levels in patients with CHF might affect the results [20]. Third, more than 90% of the study population received at least one cardiac medication, which might affect the results. However, BNP levels before medication were not known and the treatment option was entirely dependent on attending cardiologists who were not always blinded in regards to BNP levels. Finally, and most importantly, the number of patients and events in this study was small and drawing definite conclusion was not possible with this small sample size; therefore further larger-scale, multicenter studies are needed to confirm these findings.
Conclusions
Elevated BNP levels (≥200 pg/ml) could be a useful marker of subsequent thromboembolic events in patients with AF during oral anticoagulant therapy.
References
Hart RG, Pearce LA, Aguilar MI (2007) Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 146:857–867
Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE (2003) Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 290:2685–2692
Loh E, Sutton MS, Wun CC, Rouleau JL, Flaker GC, Gottlieb SS, Lamas GA, Moyé LA, Goldhaber SZ, Pfeffer MA (1997) Ventricular dysfunction and the risk of stroke after myocardial infarction. N Engl J Med 336:251–257
Lip GYH, Gibbs CR (1999) Does heart failure confer a hypercoagulable state? Virchow’s triad revisited. J Am Coll Cardiol 33:1424–1426
Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA, Breathing Not Properly Multinational Study Investigators (2002) Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 347:161–167
Silvet H, Young-Xu Y, Walleigh D, Ravid S (2003) Brain natriuretic peptide is elevated in outpatients with atrial fibrillation. Am J Cardiol 92:1124–1127
Kim BJ, Hwang SJ, Sung KC, Kim BS, Kang JH, Lee MH, Park JR (2007) Assessment of factors affecting plasma BNP levels in patients with chronic atrial fibrillation and preserved left ventricular systolic function. Int J Cardiol 118:145–150
Knudsen CW, Omland T, Clopton P, Westheim A, Wu AH, Duc P, McCord J, Nowak RM, Hollander JE, Storrow AB, Abraham WT, McCullough PA, Maisel A (2005) Impact of atrial fibrillation on the diagnostic performance of B-type natriuretic peptide concentration in dyspneic patients: an analysis from the breathing not properly multinational study. J Am Coll Cardiol 46:838–844
Shimizu H, Murakami Y, Inoue S, Ohta Y, Nakamura K, Katoh H, Sakne T, Takahashi N, Ohata S, Sugamori T, Ishibashi Y, Shimada T (2002) High plasma brain natriuretic polypeptide level as a marker of risk for thromboembolism in patients with nonvalvular atrial fibrillation. Stroke 33:1005–1010
Shibazaki K, Kimura K, Okada Y, Iguchi Y, Terasawa Y, Aoki J (2009) Heart failure may be associated with the onset of ischemic stroke with atrial fibrillation: a brain natriuretic peptide study. J Neurol Sci 281:55–57
Sadanaga T, Sadanaga M, Ogawa S (2010) Evidence that D-dimer levels predict subsequent thromboembolic and cardiovascular events in patients with atrial fibrillation during oral anticoagulant therapy. J Am Coll Cardiol 55:2225–2231
Ogawa S, Aizawa F, Atarashi H, Inoue H, Okumura K, Kamakura S, Kumagai K, Koretsune Y, Sugi K, Mitamura H, Yasaka M, Yamashita T (2008) Guidelines for pharmacotherapy of atrial fibrillation (JCS 2008) (in Japanese). Circ J 72(Suppl):1581–1638
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A (2009) Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, for the Modification of Diet in Renal Disease Study Group (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130:461–470
Doust J, Lehman R, Glasziou P (2006) The role of BNP testing in heart failure. Am Fam Physician 74:1893–1898
Stroke Prevention in Atrial Fibrillation Investigators (1996) Adjusted-dose warfarin versus low-intensity, fixed-dose warfarin plus aspirin for high-risk patients with atrial fibrillation: stroke prevention in atrial fibrillation III randomised clinical trial. Lancet 348:633–638
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ (2001) Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285:2864–2870
Stroke Risk in Atrial Fibrillation Working Group (2008) Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke 39:1901–1910
Yamaguchi T, for Japanese Nonvalvular Atrial Fibrillation-Embolism Secondary Prevention Cooperative Study Group (2000) Optimal intensity of warfarin therapy for secondary prevention of stroke in patients with nonvalvular atrial fibrillation: a multicenter, prospective, randomized trial. Stroke 31:817–821
Wu AH, Smith A, Wieczorek S, Mather JF, Duncan B, White CM, McGill C, Katten D, Heller G (2003) Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol 92:628–631
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadanaga, T., Kohsaka, S., Mitamura, H. et al. Elevated B-type natriuretic peptide level as a marker of subsequent thromboembolic events in patients with atrial fibrillation. Heart Vessels 26, 530–535 (2011). https://doi.org/10.1007/s00380-010-0084-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-010-0084-2