Abstract
Penicillium bilaiae is a P-solubilizing fungus that is used as an inoculant to promote plant growth or P uptake in various crops. Bacillus simplex has also been shown to have positive effects on different crops. However, the interaction between these inoculants and their effect on plant growth and acquisition of nutrients other than P are poorly understood. In two pot trials at different levels of inorganic P fertilization, we investigated the effect of single and combined inoculation with Penicillium bilaiae and Bacillus simplex on the growth and nutrient status of winter wheat (Triticum aestivum L.), as well as inoculant persistence and colonization. Quantitative PCR analyses revealed that both inoculants colonized and persisted on seeds and roots. The microbial inoculants increased the P concentration in root biomass at all P levels and concentrations of Mg, Mn and S in shoot biomass in low-P soil, without however having any significant effect on plant growth. Furthermore, unlike the single inoculations, the combined inoculation increased P uptake in low-P soil. These results indicate that use of the studied microbial inoculants has the potential to improve the nutritional status of winter wheat in low-P soil, especially when applied as a consortium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crop production is often limited by low plant availability of essential macro- and micronutrients. The sufficient supply of all 14 essential macro and micronutrients is required for optimal crop yield, health and quality as these are needed for a broad variety of biochemical and physiological functions. Plants require macronutrients, including nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg) and sulphur (S), in large amounts, whereas they require smaller amounts of micronutrients such as boron (B), manganese (Mn), zinc (Zn) and copper (Cu) (White and Brown 2010). Plants take up these nutrients from the soil through the roots in the form of soluble ions. Availability and uptake of nutrients depend on numerous soil factors such as pH, moisture, temperature, texture and microbial activity (Baligar and Fageria 2015).
Phosphorus is often growth-limiting for plants, despite being abundant in many soils in both inorganic and organic forms. Plants take up P from the soil solution in the form of orthophosphate, and several processes determine its concentration, including precipitation-dissolution, adsorption-desorption and biological immobilization-mineralisation (Frossard et al. 2000). The concentration of orthophosphate ions in soil solution seldom exceeds 10 μM (Hinsinger 2001), and its replenishment is often insufficient to fulfil crop demands. Many farmers therefore regularly apply soluble P fertilizer to increase P availability. However, due to the high reactivity of P in soil, only 15 to 25% of the applied P is typically recovered by crops in the year of application (Smil 2000). Furthermore, the fossil sources of P fertilizers are non-renewable (Scholz and Wellmer 2018), and their exploitation is associated with economic and environmental costs. It is therefore important to optimize P fertilizer-use efficiency and uptake by crops (White and Brown 2010).
The use of microbial inoculants (MI) on crops represents a promising technology for improving the sustainability of agricultural systems and has received increasing attention in recent years (Calvo et al. 2014). When applied to a soil-plant system, MI may increase nutrient availability in the rhizosphere, modify root growth and morphology, and increase plant performance and crop yields (Vessey 2003; Adesemoye and Kloepper 2009). In particular, some MI, known as biofertilizer, can improve the availability and plant uptake of essential nutrients such as P, Fe and N (Sharma et al. 2003; Yadav et al. 2011; Pii et al. 2015). Some MI are also known for their capacity to acidify their surroundings through the release of organic acids (Sharma et al. 2003), which can promote the acquisition of micronutrients with restricted mobility in soil, such as Zn and Cu. The availability of nutrients could also be improved in a more indirect way by microbial stimulation of root growth, leading to a larger area for accessing nutrients (White and Broadley 2009). However, the effects of MI on micronutrients have still not been fully investigated.
Fungal strains of the species Penicillium bilaiae have demonstrated an ability to solubilize P (Kucey 1983; Asea et al. 1988; Wakelin et al. 2004; Takeda and Knight 2006), and promote plant growth or P uptake for various crops and conditions (Vessey and Heisinger 2001; Ram et al. 2015). These effects have been attributed to the release of organic acids, which can acidify specific areas in the rhizosphere or function as a chelator of cationic partners of the phosphate anion (Kucey 1988). Furthermore, P. bilaiae may stimulate root growth (Downey and van Kessel 1990; Gulden and Vessey 2000; Vessey and Heisinger 2001; Gómez-Muñoz et al. 2017), which can indirectly improve plant P acquisition and uptake from the soil.
Bacteria of the species Bacillus simplex have been shown to have positive effects on the plant and root growth of different crops (Erturk et al. 2010, 2012; Hassen and Labuschagne 2010). The effect possibly stems from the ability of some B. simplex strains to produce auxin (Erturk et al. 2010; Schwartz et al. 2013), which subsequently modifies root growth and development (Erturk et al. 2010; Gutiérrez-Luna et al. 2010). Recently, strains of B. simplex isolated from P. bilaiae hyphae in soil have been found to stimulate P. bilaiae growth and P solubilization under laboratory conditions (Ghodsalavi 2016), but their effects on plant nutrient uptake and growth after inoculation in soil, individually or as part of a consortium with P. bilaiae, have not yet been tested.
Despite their promise, the beneficial effects of MI under laboratory conditions are not always consistent when applied to soil systems (Karamanos et al. 2010). Important barriers to the success of inoculants are their ability to survive and colonize the plants roots, and their capacity to compete with the indigenous microbial community (Richardson 2001; Herrmann and Lesueur 2013). The application of different MI species or strains together in consortia has been proposed as a strategy to increase the fitness of the inoculants when confronted with the complex and highly competitive native soil community. Furthermore, the benefits of MI appear to vary according to P availability in soil, being smaller in soils with either high or too low soil P availability (Gómez-Muñoz et al. 2018). Further research is therefore required to understand the conditions under which MI can improve the nutritional status of the crop as regards P and other essential macro- and micronutrients.
The aim of the current study was to quantify the effects of single and combined seed inoculation of P. bilaiae and B. simplex on shoot growth, root growth and nutrient uptake by winter wheat (Triticum aestivum L.) at different levels of mineral P fertilization. In addition, the study determined the colonization and persistence of P. bilaiae and B. simplex on the seed and roots of the inoculated winter wheat plants. The main hypotheses were (1) combined inoculation with P. bilaiae and B. simplex increases root length and P uptake of winter wheat and leads to greater plant and root growth in comparison to single inoculation, (2) combined inoculation with P. bilaiae and B. simplex increases the uptake and content of essential micro- and macronutrients other than P in the plant, and (3) the plant response to inoculation is dependent on P availability in the soil.
Materials and methods
Experimental setup
Two pot experiments were set up to investigate the effect of single and combined inoculation of P. bilaiae and B. simplex on (1) the growth and nutrient status of winter wheat under different P application levels and (2) the colonization and persistence of the microbial inoculants on the seeds and roots of winter wheat. The Penicillium bilaiae strain DBS5 and the Bacillus simplex strains 313 and 371 isolated from P. bilaiae hyphae were used in these experiments.
A sandy loam soil from the Long-Term Nutrient Depletion Trial in Denmark (55° 40′ N, 12° 17′ E) was collected from the plough layer (0–25 cm), air-dried, and sieved to obtain a fraction ≤ 4 mm. The soil contained 164 g kg−1 clay, 173 g kg−1 silt, 333 g kg−1 fine sand, 312 g kg−1 coarse sand, 1.70 g kg−1 total N and 17 g kg−1 of soil organic matter. The soil was collected from a plot that has been fertilized with animal slurry (120 kg NH4-N ha−1y−1) annually since 1996 and also with mineral P fertilizer (20 kg P ha−1y−1) since 2010. The soil pH (water) was 5.9, and the available Olsen-P (extraction with sodium bicarbonate solution) was 17 mg P kg−1. A detailed history of the soil and management practices can be found in van der Bom et al. (2018).
Plant growth study (experiment 1)
Soil and pot preparation
For the first experiment, 24 treatments with 6 replicates were set up comprising 6 levels of triple superphosphate (TSP) fertilizer: 0, 15, 30, 60, 90 and 120 mg P kg soil−1 (0P, 15P, 30P, 60P, 90P and 120P, respectively) and 4 levels of inoculation: (1) non-inoculated control (C), (2) single inoculation with P. bilaiae (PB), (3) single inoculation with B. simplex (BS) and (4) combined inoculation with P. bilaiae and B. simplex (PB + BS). For each pot, 3 kg (dry weight) of soil mixture, consisting of 2.25 kg soil and 0.75 kg acid-washed sand, was thoroughly mixed in a plastic bag. The water-holding capacity (WHC) of the soil-sand mixture was 26% (w/w). Nutrients were added in a liquid solution to each bag at the following levels (per kg of soil mixture): 150 mg N, 113 mg K, 15 mg Mg, 40 mg S, 30 mg Ca, 0.45 mg Mn, 0.3 mg Zn, 0.15 mg Cu, 0.01 mg Mo, 0.22 mg B and 0.3 mg Fe (added as NH4NO3, KCl, MgSO4, CaCl2, MnSO4, ZnSO4, CuSO4, Na2MoO4, H3BO3 and C10H12FeN2NaO8). After 4 days of drying, TSP fertilizer containing 18% P was added in amounts equivalent to 0, 15, 30, 60, 90, 120 mg P kg soil−1. The dry soil was thoroughly mixed and packed in 28-cm high PVC pots with an inner diameter of 10 cm, and closed with a plastic stopper with holes in the bottom of the pot. The pots were packed to reach a bulk density of 1.4 g cm−3.
Seed treatments
For the P. bilaiae treatment, winter wheat (Triticum aestivum L.) var. Benchmark seeds (weight 50 ± 3 mg) were coated with the dry spores of P. bilaiae at a dose of 2 × 105 spores seed−1. Briefly, 12.5 mg of P. bilaiae spores and 300 μL of a carrier solution consisting of 66.54% sterile water, 0.10% K2HPO4, 0.02% KH2PO4, 21.67% maltodextrin and 11.67% maltose-monohydrate were added to 250 wheat seeds in a sterile plastic container and shaken for 15 min in a turn-over shaker. For the B. simplex treatment, winter wheat seeds were inoculated with a liquid solution of B. simplex at a dose of 8 × 103 spores seed-1. Briefly, 275 μL of carrier solution were added to 37.5 μL of a solution of B. simplex strains 313 and 371, carefully mixed and added to 250 wheat seeds in a sterile plastic container, and shaken for 15 min in a turn-over shaker. For the combined treatment, the inoculation doses used were the same as for the single treatments: 2 × 105 spores seed-1 for P. bilaiae and 8 × 103 spores seed-1 for B. simplex. The inoculation was performed by combining the procedures described above for the individual strains. For the non-inoculated control treatment, 313 μL of carrier solution were added to 250 wheat seeds and shaken in the same manner as above.
After seed treatment, colony-forming units per seed (CFU seed-1) were determined by recovering the seed organisms using deionized water with 0.1% Tween80, followed by dilution series and plating on tryptone yeast agar with 10 mg L−1 nystatin (for treatments with B. simplex) and potato dextrose agar with 10 mg L−1 of streptomycin and penicillin (for treatments with P. bilaiae). The determined values were as follows: P. bilaiae treatment − 1 × 105 CFU seed−1; B. simplex treatment – 4.9 × 104 CFU seed−1; combined treatment – 9.6 × 104P. bilaiae CFU seed−1 + 5.5 × 104B. simplex CFU seed−1.
Growth conditions
Four seeds of wheat were sown in each pot. The surface of each pot was covered with 50 g of plastic beads to reduce water evaporation from the soil surface. After emergence, the number of plants was reduced to three per pot. The pots were watered approximately every second day by weighing and watering up to 50% WHC at the start of the experiment, rising gradually to 80% WHC throughout the experimental period. The conditions in the growth chamber were set as follows: daylight period 16 h, temperature 10/15 °C (day/night), relative air humidity 65/72% (day/night) and photosynthetically active radiation 300/0 μmol m−2 s−1 (day/night).
Plant measurements
On harvest day, 33 days after sowing, shoot tillers were counted for each plant. The plants were harvested by cutting the aboveground plant biomass just above the soil surface. Subsequently, the roots were gently washed under running water to remove all the soil, and kept in 30% ethanol at 5 °C prior to analysis. To determine root length, the roots were scanned using a scanner (Epson STD4800) and analysed using the WinRHIZO software (V5.0, Regent instruments, Quebec, Canada). Shoots and roots were dried in an oven for 48 h at 60 °C, and biomass dry matter was determined. To estimate the content of micro- and macronutrients, the dried root and shoot samples from treatments 0P, 15P, 30P and 90P were milled and subsequently digested with 2.5 ml 70% HNO3 and 1 ml 15% H2O2. Shoots were analysed for their content of P, B, Ca, Cu, K, Mg, Mn, S and Zn by ICP-OES (Agilent 5100, Agilent Technology, Australia), while roots were analysed for their content of P by a flow injection analyser (FIA star 5000, Foss Analytical, Denmark).
Soil measurements
On harvest day, soil samples for water-extractable P and pH analysis from 0P, 15P, 30P and 90P treatments were collected. Four evenly distributed samples from bulk soil were taken from each pot, pooled, air-dried and sieved through a 2-mm sieve. Because the pot volume was fully explored by the growing roots, it is assumed that the soil in these samples was significantly influenced by root activity. Soil pH was measured using a soil-water suspension of 5 g soil and 25 ml of Milli-Q water (pH meter Mettler-Toledo AG, Switzerland). To determine the plant available P pool affected by our treatments, water-extractable P was analysed by extracting with Milli-Q water at a soil (g) to water (ml) ratio of 1:60, shaking for 1 h and then filtering through a 2.5-μm filter (Whatman no.5) (van der Paauw 1971). The filtered extracts were stored at 4 °C until the concentration of inorganic P (orthophosphate) was measured by a flow injection analyser (FIA star 5000, Foss Analytical, Denmark).
Colonization and persistence study (experiment 2)
Soil and pot preparation
Experiment 2 was set up at the same time as Experiment 1, but involved 16 treatments with 5 replicates comprising 2 levels of TSP fertilizer (0 and 60 mg P kg soil−1), 4 levels of inoculation and 2 harvest times (1 and 3 weeks after germination). A soil mixture consisting of 476 g soil and 155 g acid-washed sand (dw) was thoroughly mixed in a plastic bag. Pots (PVC, 20 cm high and 5.5 cm in diameter) were cut in half vertically and held together with duct tape in order to be able to open the pots on harvest day. The soil was packed, and the pots were closed with a plastic stopper with holes at the bottom of the pot. The rest of the procedure, including seed treatment, was as described above for Experiment 1.
Growth conditions and harvest
One seed of winter wheat var. Benchmark was sown in each pot. Growth conditions were as described above for Experiment 1 except that the surface of each pot was covered by 20 g of plastic beads to reduce water evaporation from the soil and watering was performed to reach 70% WHC throughout the experimental period.
Half of the pots were harvested 1 week after germination, and the other half of the pots 3 weeks after germination. Upon harvest, the pots were opened and the whole contents removed. The roots were gently hand-shaken (ten light strokes on the table) to leave only the soil closely adhering to the roots. The shoots were cut off right above the seeds and the roots just under the seed. Samples for quantitative PCR (qPCR) analysis were taken from two different zones: (A) seed and (B) upper roots (top 0–3 cm below the seed). Roots more than 3 cm below the seed were not analysed. All the samples were freeze-dried and homogenized by grinding prior to DNA extraction.
DNA extraction and qPCR analysis
The entire seed (zone A) or 0.5 g samples of the root+rhizosphere soil (zone B) were recovered from each of the five replicates of the control treatment and of the inoculated plant systems. The samples were immediately transferred into Bio101 Lysing Matrix E tubes. Hence, sampling followed the general recommendations of Vestergaard et al. (2017) regarding characterization, replication, inclusion of negative controls and short or cold storage. From these samples, DNA was extracted using phenol-chloroform, as previously described by Nicolaisen et al. (2008).
Quantification of B. simplex 313, B. simplex 371 and P. bilaiae DBS5 was performed by qPCR. All reactions were run on the MX3000® cycler (Agilent Technologies, USA) in a 20-μl reaction volume (1× Brilliant II SYBR Green QPCR Master mix) (Agilent Technologies, USA) containing 1 mg ml−1 bovine serum albumin (New England Biolabs® Inc., UK), 0.4 μM primer (B. simplex) and 0.5 μM primer (P. bilaiae) (Table S1), and 2 μl DNA template. Thermal cycling conditions were as follows: initial activation of the DNA polymerase at 95 °C for 3 min, followed by 40 cycles of 95 °C for 20 s, 63/65 °C (Table S1) for 25 s and 72 °C for 60 s. Melting curve analysis was performed at the end of a run. Standard curves used for quantification were based on tenfold dilutions of cell cultures and spore suspensions for B. simplex and P. bilaiae, respectively. Efficiency ranged from 102 to 108%, and R2 values were > 0.98 for all standard curves independent of the primer set used. All samples were run with five biological replicates. Primer specificity was validated in silico prior to analyses, and for the qPCR assay, primer specificity was checked for all samples by the observation of a single melting peak on the dissociation curve and the presence of a unique band of expected length in a 1.5% agarose gel. Furthermore, the qPCR set up included no-template (DNA-free) controls, and the samples included negative controls (non-inoculated); hence, following general recommendations from Schöler et al. (2017).
Statistical analysis
All statistical analyses were performed using R version 3.4.2 (R Core Team 2017) and by use of the RStudio development environment (RStudio Team 2017). Statistical differences were tested by two-way ANOVA with interaction between the factors ‘inoculation treatment’ and ‘P application level’. Backward regression was applied for model selection. Post-hoc comparisons were performed on the final model according to Tukey’s HSD test (α = 0.05) using the agricolae package (de Mendiburu 2019). All statistical models were checked for normality of residuals and homogeneity of variance using diagnostic plots. Where normality assumptions were not met, data were transformed making use of the boxCox function of the car package (Fox et al. 2018). All differences at p < 0.05 were reported as significant.
Results
Plant growth and nutrient status
Clear effects of the incremental P applications on plant growth were observed (Table 1). Shoot biomass significantly increased when P was applied and continued to increase up to 90P, after which it levelled off. This growth was reflected in the number of tillers, which steadily increased with larger P applications. Although the response was more variable for root growth, root biomass also tended to be greater for the three largest P application rates, which was significant for 60P and 90P. Overall, the effects of the MI were only small, with differences limited to an increase in root biomass at the 15P level in response to combined inoculation. Total root length was not affected by either P application level or MI inoculation (Table 1).
The P concentration in shoot biomass ranged from 2.2 to 3.9 mg P g−1 and in root biomass from 1.0 to 2.2 mg P g−1. Increasing the P application level had a significant positive effect on P concentration in shoot biomass at all P levels (Fig. 1a), whereas the P concentration in roots increased significantly only at 90P when compared to the other three P levels (Fig. 1b). All three inoculant treatments significantly increased the P concentration in root biomass at all P application levels compared to the control (Fig. 1b). The increase was more pronounced at low P levels, with a 40–70% increase in P concentration at the 0P level and only a 25–30% increase at the 90P level. Furthermore, the inoculation induced a higher retention of P in root tissue as indicated by significantly lower shoot/root P ratio in inoculated treatments in comparison with non-inoculated control treatments (Table 2). In contrast, the inoculants did not have any effect on P concentration in the shoot biomass at any of the P application levels (Fig. 1a).
Phosphorus concentration in a shoot biomass (mg P g−1 dry shoot biomass), b root biomass (mg P g−1 dry root biomass) of winter wheat measured on harvest day at four different P application levels: 0, 15, 30 and 90 mg P kg soil−1 and four inoculation treatments: C = control, non-inoculated, PB = Penicillium bilaiae, BS = Bacillus simplex, PB + BS = combined inoculation with PB and BS. Different lower case letters indicate significant differences between P application levels and different capital letters indicate significant differences between the inoculation treatments (p < 0.05). Values presented are means with standard error bars (n = 6)
Total P uptake of winter wheat was significantly enhanced by P application level (Fig. 2). Single inoculation (P. bilaiae or B. simplex) did not have any effect on P uptake, whereas the combined inoculation increased P uptake at the 0P level by 25%. At higher P application levels, the inoculants did not affect P uptake significantly.
Total P uptake in shoots and roots (mg P pot−1) of winter wheat measured on harvest day at four different P application levels (mg P kg soil−1) and four different P application levels: 0, 15, 30 and 90 mg P kg soil−1 and four inoculation treatments: C = control, non-inoculated, PB = Penicillium bilaiae, BS = Bacillus simplex, PB + BS = combined inoculation with PB and BS. Different lower case letters indicate significant differences between P application levels and different capital letters indicate significant differences between the inoculation treatments (p < 0.05). Values presented are means with standard error bars (n = 6)
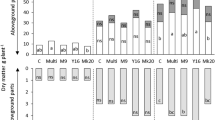
The concentrations of macronutrients (K, Ca) and micronutrients (B, Cu, Zn, Mn) in winter wheat shoots were significantly affected by the P application level. The concentration of B decreased with increasing P application and concentrations of Zn and Cu decreased at 90P, whereas the concentrations of Ca and K were lower at the 0P level compared to the other P levels (Fig. 3). The application of MI significantly affected shoot nutrient content, particularly at the 0P level (Fig. 3). At the 0P level, P. bilaiae increased the Mn concentration, while B. simplex increased the Mg, Mn and S concentrations and decreased the K concentration, even at higher P levels. Finally, the combined inoculation significantly increased the Mg, Mn and S concentrations at 0P level when compared to the control and decreased both the K concentration and the Cu concentration at all P levels. The P application level and inoculants had an interaction effect on the concentrations of Mg, Mn and S. The summary of statistical analysis on the effect of the inoculants compared to the control on different plant and soil parameters is given in Table S2.
Concentrations of B, Ca, Cu, Mg, K, Mn, S and Zn in winter wheat measured on harvest day at four different P application levels (mg P kg soil−1) and four inoculation treatments: C = control, non-inoculated, PB = Penicillium bilaiae, BS = Bacillus simplex, PB + BS = combined inoculation with PB and BS. Different lower case letters indicate significant differences between P application levels and different capital letters indicate significant differences between the inoculation treatments (p < 0.05). Dashed lines indicate deficiency thresholds for winter wheat (Reuter et al. 1997). Values presented are means with standard error bars (n = 6)
Soil pH and water-extractable P
Single inoculation with B. simplex and combined inoculation significantly decreased the soil pH at all four P application levels (Fig. 4a) compared with the control. Inoculation with P. bilaiae had no effect on soil pH at any P application level. The content of water-extractable P in soil significantly increased with increasing P application level (Fig. 4b). Inoculation with B. simplex significantly decreased the content of soil water-extractable P compared to P. bilaiae and combined inoculation treatments at all P levels, but not compared to the control.
a Soil pH measured at harvest time; b soil water-extractable phosphorus concentration measured at harvest time at four P application levels: 0, 15, 30 and 90 mg P kg soil−1 and four inoculation treatments: C = control, non-inoculated, PB = Penicillium bilaiae, BS = Bacillus simplex, PB + BS = combined inoculation with PB and BS. Different lower case letters indicate significant differences between P application levels and different capital letters indicate significant differences between the inoculation treatments (p < 0.05). Values presented are means with standard error bars (n = 6)
Colonization and persistence of microbial inoculants
Persistence of all inoculants in the soil system was determined by qPCR at the 0P and 60P application levels only. There was no difference in colonization or persistence of the inoculants between the P application levels (0P and 60P) (data not shown). Therefore, only the 0P data are presented. A background population was detected for all inoculants (Fig. 5). For B. simplex strain 313 and for P. bilaiae, an indigenous population below 103 cells or spore equivalents g−1 soil was determined on both the seed and roots (Fig. 5a–f). Slightly higher background quantities of up to 104 cells g−1 soil were found using the B. simplex strain 371 primer set (Fig. 5c, d). Furthermore, the indigenous population detected by the B. simplex 371 primers was stimulated by the presence of P. bilaiae both on the seed and on the root (Fig. 5c, d). For plants inoculated with B. simplex strains 313 and 371, the qPCR data did not indicate any proliferation or reduction in the population on the seed during the 3 weeks of incubation (Fig. 5a, c). However, quantification of P. bilaiae on seeds revealed a reduction in spore equivalents of the fungal inoculant at 3 weeks as compared to quantities detected after 1 week of plant growth (Fig. 5e). For the root samples, B. simplex strains 313 and 371 were clearly able to colonize the rhizosphere as the strains were found in significantly higher concentrations at week three compared to week one (Fig. 5b, d). For P. bilaiae, colonization of the rhizosphere was detected already after 1 week of inoculation, with a significantly higher abundance of P. bilaiae in pots receiving the fungal inoculant (Fig. 5f).
Colonization and persistence of BS 313 (graphs a, b), BS 371 (graphs c, d), PB DBS5 (graphs e, f) on the seed and top roots at P application level of 0 mg P kg soil−1 estimated via qPCR analysis 1 and 3 weeks after germination in the different treatments: C = control, non-inoculated soil, PB = Penicillium bilaiae, BS = Bacillus simplex, PB + BS = combined inoculation with PB and BS. Different capital letters indicate a significant difference between the inoculation treatments within each graph (p < 0.05). Values presented are means with standard error bars (n = 6)
Discussion
Inoculation with P. bilaiae
The success and efficiency of microbial inoculants in improving plant growth and nutrient uptake depend on their ability to survive and colonize the plant roots and compete with the indigenous microbial community (Zaidi et al. 2009). Colonization ability varies with crop and soil type. Previously, Gómez-Muñoz et al. (2017) detected P. bilaiae only on the seeds of maize after 27 days of a pot trial, whereas Efthymiou et al. (2018) showed the colonization potential of P. bilaiae in the wheat rhizosphere. In the current study, P. bilaiae also showed an ability to colonize the seed and roots of wheat and resulted in increased P concentrations in root biomass at all P levels. As P. bilaiae did not appear to acidify the soil significantly (according to (Fig. 4a)), this could potentially be due to the ability of P. bilaiae to increase root hair length (Gulden and Vessey 2000) as long root hairs enable higher soil exploration and thereby greater P uptake (Haling et al. 2010). Apart from P concentration in the root, P. bilaiae increased the concentration of Mn in shoot biomass, but only at the 0P level, indicating a potential to improve the nutrient status of winter wheat in low-P soil. However, the increased P concentration in root biomass and Mn concentration in shoot biomass did not translate into increased shoot or root biomass at this plant development stage. Gómez-Muñoz et al. (2017) found that seed inoculation with P. bilaiae increased concentrations of Mg, Fe, Mn, Zn, B and Cu in the root biomass of maize grown for 27 days without increasing shoot biomass. This may indicate a transient depiction of the nutrient concentrations at these specific growth stages that may be translocated and utilized by shoots at later stages. Some studies have not found any significant effects of P. bilaiae on plant growth (Wakelin et al. 2007; Efthymiou et al. 2018), while others have identified positive effects (Asea et al. 1988; Gómez-Muñoz et al. 2017). This indicates a varying effect depending not only on crop species but also on soil conditions such as pH and P status (Vessey and Heisinger 2001; Wakelin et al. 2007; Gómez-Muñoz et al. 2018). Several studies have described no effects of P. bilaiae in slightly acidic soil (Sánchez-Esteva et al. 2016; Efthymiou et al. 2018), as in the present study (pH 5–6), whereas positive effects on plant growth have been shown in alkaline soil (Sánchez-Esteva et al. 2016). Nevertheless, more studies are needed to unravel the full panel of environmental conditions needed for the optimal performance of P. bilaiae.
Inoculation with B. simplex
Inoculation with B. simplex resulted in increased shoot concentrations of Mg, Mn and S at the 0P level and increased P concentration in roots at all P levels. To our knowledge, B. simplex has so far only been shown to increase N concentrations in the shoot biomass of winter wheat (Barneix et al. 2005). Both B. simplex strains colonized the seed and even proliferated on roots with time. Several Bacillus strains, i.e. Bacillus amyloliquefaciens FZB42, B. amyloliquefaciens QST713 and B. subtilis, have previously been shown to promote plant growth and have the ability to colonize plant roots (Chen et al. 2007; Allard-Massicotte et al. 2016; Mendis et al. 2018). However, studies on B. simplex root colonization are limited (Hassen and Labuschagne 2010). The study by Hassen and Labuschagne (2010) further documented indole acetic acid production by B. simplex with a positive impact on plant and root growth. Mineralisation of organic P via excretion of phytate-degrading enzymes by Bacillus strains can also increase plant availability of soil P and stimulate plant growth as shown with B. amyloliquefaciens in the study of Idriss et al. (2002). Considering that almost 65% of the total P in this soil was organic P, a quarter of which were forms of inositol polyphosphate (phytate), P mobilization processes may have contributed to the observed increase in root P concentrations (van der Bom et al. 2019). However, the exact mode of action for the B. simplex strains presented here is not known. Despite the persistence and colonization of B. simplex strains 313 and 371 in the present study, positive effects on shoot or root growth of winter wheat were not found. This points to a different mode of action, with the B. simplex strains having a more profound effect on nutrient dynamics in the plant rather than enhancing plant growth. Whether the increased P concentration in roots would result in a long-term improvement in the plant growth parameter was, however, outside the scope of this study. As the strains applied in this study were originally isolated from fungal hyphae (Ghodsalavi 2016), the interactions with plant roots might have different characteristics because this site might not be the strains’ preferred niche. A more fungal-related ecology was supported by the finding that the indigenous population detected by the B. simplex 371 primers was stimulated by P. bilaiae inoculation on both the seed and root (Fig. 5c, d).
Combined inoculation of P. bilaiae and B. simplex
The combined beneficial effects of bacteria and arbuscular mycorrhizal fungi on plants have been studied, but knowledge on the interactions between bacteria and non-mycorrhizal fungi are still scarce (Artursson et al. 2006). In the present study, a positive effect of combined inoculation was found. Besides the same positive effects on P as well as on Mg, Mn and S concentrations exerted by B. simplex alone, combined inoculation also resulted in increased P uptake at the 0P level. This indicates that these MI, when applied as a consortium, may improve the uptake of P. Although P application level had no effect on colonization and persistence, the effect of the inoculants on plant nutrient uptake was generally best in soil with no P addition but moderately low P availability (0P) (Table S2), which is consistent with other studies on P uptake and yield response (Vessey and Heisinger 2001; Gómez-Muñoz et al. 2017; Schütz et al. 2018). The increase in the nutrient concentration of inoculated plants may be due to the inoculants’ ability to stimulate root hair growth, which may lead to higher soil exploration and improved access to nutrients (Gilroy and Jones 2000). The increased P concentration in the roots by B. simplex and combined inoculation may be due to the release of organic acids and a subsequent soil acidification effect (Hassen and Labuschagne 2010) because, in the present study, the combined inoculation resulted in the highest soil acidification. Another explanation could be the production of biologically active compounds by microbial inoculant. These could include phytohormones, antifungal compounds and enzymes, which can stimulate plant growth development, improve nutrient acquisition and provide resistance to various biotic and abiotic stressors (Egamberdieva et al. 2017). The MI increased root P concentrations in all instances including in the 90P treatment, which may be presumed not to be P limited. The increased root P content under these conditions suggests that indeed a mechanism other than P solubilization may be at work. Apart from homeostasis of phytohormones, P sequestration in the root-colonizing inoculants could offer an alternative explanation. Nevertheless, the increased nutrient content in winter wheat in the early growth stage did not result in increased plant growth. Whether this would happen during the later growth stage would require growing the plants to maturity.
Plant nutrition status
The increasing P application level influenced the concentrations of several nutrients in different ways. The concentrations of B, Cu, Zn and Mn decreased with increasing P level, which may be due to an indirect dilution effect following depressed concentration and increased biomass production or a direct effect due to decreased uptake (Murphy et al. 1981; Pedas et al. 2011). In contrast, the concentrations of P, K and Ca increased with increasing P application level. A sufficient P concentration in wheat shoots ranges from 3 to 5 mg g−1 during the vegetative stage of growth (Römhled 2012), indicating marginal deficiency in wheat shoots at 0P and 15P in the current study. Other micro- and macronutrient concentrations, except for Mg, were within the sufficient range (Reuter et al. 1997; White and Brown 2010). The Mg requirement for optimal growth is from 1.5 to 3.5 mg g−1 but the concentration of Mg at 0P was 1.33 mg g−1 in the non-inoculated control treatment. Inoculation with B. simplex and combined inoculation significantly increased the concentration above the deficiency limit to 1.51 mg g−1. This indicates the potential of MI to improve the quality of the crops as Mg is an important component of the chlorophyll molecule and plays a crucial role in protein synthesis (Hawkesford et al. 2012). However, the combined inoculation decreased the concentration of Cu, and inoculation with B. simplex and combined inoculation decreased the concentration of K in shoot biomass, especially at 15, 30 and 90P. However, as the concentrations of Cu and K were well above deficiency limits, no decrease in plant growth or quality is anticipated.
Conclusions
In the present study, the microbial inoculants P. bilaiae and B. simplex demonstrated their ability to persist and in some cases even proliferate on the seed and roots of winter wheat during the first 3 weeks of growth. Both inoculants increased the P concentration in root biomass at all P application levels and concentrations of Mg, Mn and S in low-P soil. However, no effects could be detected on plant growth. Furthermore, the combined inoculation with both inoculants increased P uptake in low-P soil, whereas the single inoculations did not. These results therefore indicate that the use of P. bilaiae and B. simplex as microbial inoculants in agriculture has the potential to improve plant nutritional status in low-P soil, especially when several strains are applied as a consortium. Nevertheless, more studies are needed to determine the full panel of conditions that influence these interactions and to improve understanding of the implications on final crop yield.
References
Adesemoye AO, Kloepper JW (2009) Plant-microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol 85:1–12. https://doi.org/10.1007/s00253-009-2196-0
Allard-Massicotte R, Tessier L, Lécuyer F, Lakshmanan V, Lucier J, Garneau D, Caudwell L, Vlamakis H, Bais HP, Beauregard PB (2016) Bacillus subtilis early colonization of arabidopsis thaliana roots involves multiple chemotaxis receptors. Am Soc Microbiol 7:1–10. https://doi.org/10.1128/mBio.01664-16.Invited
Artursson V, Finlay RD, Jansson JK (2006) Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ Microbiol 8:1–10. https://doi.org/10.1111/j.1462-2920.2005.00942.x
Asea PEA, Kucey RMN, Stewart JWB (1988) Inorganic phosphate solubilization by two Penicillium species in solution culture and soil. Soil Biol Biochem 20:459–464. https://doi.org/10.1016/0038-0717(88)90058-2
Baligar VC, Fageria NK (2015) Nutrient use efficiency: from basics to advances. In: Rakshit A, Singh HB, Sen A (Eds) Nutrient use efficiency : from basics to advances. Springer India, New Delhi, pp 125–161
Barneix AJ, Saubidet MI, Fatta N, Kade M (2005) Effect of rhizobacteria on growth and grain protein in wheat. Agron Sustain Dev 25:505–511. https://doi.org/10.1051/agro
Calvo P, Nelson L, Kloepper JW (2014) Agricultural uses of plant biostimulants. Plant Soil 383:3–41. https://doi.org/10.1007/s11104-014-2131-8
Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, Morgenstern B, Voss B, Hess WR, Reva O, Junge H, Voigt B, Jungblut PR, Vater J, Süssmuth R, Liesegang H, Strittmatter A, Gottschalk G, Borriss R (2007) Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium bacillus amyloliquefaciens FZB42. Mol Microb Ecol Rhizosph 2:883–898. https://doi.org/10.1002/9781118297674.ch83
de Mendiburu F (2019) Package “agricolae.” Stat Proced Agric Res
Downey J, van Kessel C (1990) Dual inoculation of Pisum sativum with Rhizobium leguminosarum and Penicillium bilaji. Biol Fertil Soils 10:194–196. https://doi.org/10.1007/BF00336135
Efthymiou A, Grønlund M, Müller-Stöver DS, Jakobsen I (2018) Augmentation of the phosphorus fertilizer value of biochar by inoculation of wheat with selected Penicillium strains. Soil Biol Biochem 116:139–147. https://doi.org/10.1016/j.soilbio.2017.10.006
Egamberdieva D, Wirth SJ, Alqarawi AA, Abd-Allah EF, Hashem A (2017) Phytohormones and beneficial microbes: essential components for plants to balance stress and fitness. Front Microbiol 8:1–14. https://doi.org/10.3389/fmicb.2017.02104
Erturk Y, Ercisli S, Cakmakci R (2012) Yield and growth response of strawberry to plant growth-promoting rhizobacteria inoculation. J Plant Nutr 35:817–826. https://doi.org/10.1080/01904167.2012.663437
Erturk Y, Ercisli S, Haznedar A, Cakmakci R (2010) Effects of plant growth promoting rhizobacteria (PGPR) on rooting and root growth of kiwifruit (Actinidia deliciosa) stem cuttings. Biol Res 43:91–98. https://doi.org/10.4067/S0716-97602010000100011
Fox J, Weisberg S, Price B (2018) Package ‘car’. Book Review: An R and S-PLUS Companion to Applied Regression 34:137–140. https://doi.org/10.1177/0049124105277200
Frossard E, Condron LM, Oberson A, Sinaj S, Fardeau JC (2000) Processes governing phosphorus availability in temperate soils. J Environ Qual 29:15. https://doi.org/10.2134/jeq2000.00472425002900010003x
Ghodsalavi B (2016) Hyphae colonizing bacteria associated with Penicillium bilaii PhD thesis. University of Copenhagen
Gilroy S, Jones DL (2000) Through form to function: root hair development and nutrient uptake. Trends Plant Sci 5:56–60. https://doi.org/10.1016/S1360-1385(99)01551-4
Gómez-Muñoz B, Jensen LS, de Neergaard A, Richardson AE, Magid J (2018) Effects of Penicillium bilaii on maize growth are mediated by available phosphorus. Plant Soil 431:159–173. https://doi.org/10.1007/s11104-018-3756-9
Gómez-Muñoz B, Pittroff SM, de Neergaard A, Jensen LS, Nicolaisen MH, Magid J (2017) Penicillium bilaii effects on maize growth and P uptake from soil and localized sewage sludge in a rhizobox experiment. Biol Fertil Soils 53:23–35. https://doi.org/10.1007/s00374-016-1149-x
Gulden RH, Vessey JK (2000) Inoculation increases root-hair production in field pea. Can J Plant Sci 80:801–804. https://doi.org/10.4141/P99-171
Gutiérrez-Luna FM, López-Bucio J, Altamirano-Hernández J, Valencia-Cantero E, De La Cruz HR, Macías-Rodríguez L (2010) Plant growth-promoting rhizobacteria modulate root-system architecture in Arabidopsis thaliana through volatile organic compound emission. Symbiosis 51:75–83. https://doi.org/10.1007/s13199-010-0066-2
Haling RE, Richardson AE, Culvenor RA, Lambers H, Simpson RJ (2010) Root morphology, root-hair development and rhizosheath formation on perennial grass seedlings is influenced by soil acidity. Plant Soil 335:457–468. https://doi.org/10.1007/s11104-010-0433-z
Hassen AI, Labuschagne N (2010) Root colonization and growth enhancement in wheat and tomato by rhizobacteria isolated from the rhizoplane of grasses. World J Microbiol Biotechnol 26:1837–1846. https://doi.org/10.1007/s11274-010-0365-z
Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Møller IS, White P (2012) Functions of macronutrients. In: Marschner’s mineral nutrition of higher plants: third edition. Elsevier Ltd, pp 135–189
Herrmann L, Lesueur D (2013) Challenges of formulation and quality of biofertilizers for successful inoculation. Appl Microbiol Biotechnol 97:8859–8873. https://doi.org/10.1007/s00253-013-5228-8
Hinsinger P (2001) Bioavailibility of soil inorganic P in the rhizosphere as effected by root-induced chemical changes: a review. Plant Soil 237:173–195. https://doi.org/10.1023/A:1013351617532
Idriss EE, Makarewicz O, Farouk A, Rosner K, Greiner R, Bochow H, Richter T, Borriss R (2002) Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology 148:2097–2109. https://doi.org/10.1099/00221287-148-7-2097
Karamanos RE, Flore NA, Harapiak JT (2010) Re-visiting use of fertilization of hard red spring wheat Penicillium bilaii with phosphorus fertilization of hard red spring wheat. Can J Plant Sci 90:265–277. https://doi.org/10.4141/CJPS09123
Kucey RMN (1983) Phosphate-solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Can J Soil Sci 63:671–678
Kucey RMN (1988) Plant growth-altering effects of Azospirillum brasilense and Bacillus C–11–25 on two wheat cultivars. J Appl Bacteriol 64:187–196. https://doi.org/10.1111/j.1365-2672.1988.tb03375.x
Mendis HC, Thomas VP, Schwientek P, Salamzade R, Chien JT, Waidyarathne P, Kloepper J, De La Fuente L (2018) Strain-specific quantification of root colonization by plant growth promoting rhizobacteria Bacillus firmus I-1582 and Bacillus amyloliquefaciens QST713 in non-sterile soil and field conditions. PLoS One 13:e0193119. https://doi.org/10.1371/journal.pone.0193119
Murphy LS, Ellis R Jr, Adriano DS (1981) Phosphorus-micronutrient interaction effects on crop production. J Plant Nutr 3:593–613
Nicolaisen MH, Bælum J, Jacobsen CS, Sørensen J (2008) Transcription dynamics of the functional tfdA gene during MCPA herbicide degradation by Cupriavidus necator AEO106 (pRO101) in agricultural soil. Environ Microbiol 10:571–579. https://doi.org/10.1111/j.1462-2920.2007.01476.x
Pedas P, Husted S, Skytte K, Schjoerring JK (2011) Elevated phosphorus impedes manganese acquisition by barley plants. Front Plant Sci 2:1–12. https://doi.org/10.3389/fpls.2011.00037
Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015) Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol Fertil Soils 51:403–415. https://doi.org/10.1007/s00374-015-0996-1
R Core Team (2017) R: a language and environment for statistical computing. R foundation for Statistical Computing, Vienna
Ram H, Malik SS, Dhaliwal SS, Kumar B, Singh Y (2015) Growth and productivity of wheat affected by phosphorus-solubilizing fungi and phosphorus levels. Plant Soil Environ 61:122–126. https://doi.org/10.17221/982/2014-PSE
Reuter DJ, Edwards DG, Wilhelm NS (1997) Temperate and tropical crops. In: Reuter DJ, Robinson JB (Eds) plant analysis an interpretation manual, second edi. CSIRO publishing, pp 81–284
Richardson AE (2001) Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust J Plant Physiol Aust J Plant Physiol 28:897–906. https://doi.org/10.1071/PP01093
Römhled V (2012) Diagnosis of deficiency and toxicity of nutrients. In: Marschner’s mineral nutrition of higher plants: third edition. Elsevier Inc., pp 299–314
RStudio Team (2017) RStudio: Integreated development for R. RStudio, Inc., Boston
Sánchez-Esteva S, Gómez-Muñoz B, Jensen LS, de Neergaard A, Magid J (2016) The effect of Penicillium bilaii on wheat growth and phosphorus uptake as affected by soil pH, soil P and application of sewage sludge. Chem Biol Technol Agric 3:1–11. https://doi.org/10.1186/s40538-016-0075-3
Schöler A, Jacquiod S, Vestergaard G, Schulz S, Schloter M (2017) Analysis of soil microbial communities based on amplicon sequencing of marker genes. Biol Fertil Soils 53:485–489. https://doi.org/10.1007/s00374-017-1205-1
Scholz RW, Wellmer FW (2018) Although there is no physical short-term scarcity of phosphorus, its resource efficiency should be improved. J Ind Ecol 23:1–6. https://doi.org/10.1111/jiec.12750
Schütz L, Gattinger A, Meier M, Müller A, Boller T, Mäder P, Mathimaran N (2018) Improving crop yield and nutrient use efficiency via biofertilization—a global meta-analysis. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.02204
Schwartz A, Ortiz I, Maymon M, Herbold C, Fujishige N, Vijanderan J, Villella W, Hanamoto K, Diener A, Sanders E, DeMason D, Hirsch A (2013) Bacillus simplex—a little known PGPB with anti-fungal activity—alters pea legume root architecture and nodule morphology when coinoculated with rhizobium leguminosarum bv. viciae. Agronomy 3:595–620. https://doi.org/10.3390/agronomy3040595
Sharma A, Johri BN, Sharma AK, Glick BR (2003) Plant growth-promoting bacterium Pseudomonas sp. strain GRP3 influences iron acquisition in mung bean (Vigna radiata L. Wilzeck). Soil Biol Biochem 35:887–894. https://doi.org/10.1016/S0038-0717(03)00119-6
Smil V (2000) Phosphorus in the environment : natural flows and human interferences. Annu Rev Energy Environ 25:53–88. https://doi.org/10.1146/annurev.energy.25.1.53
Takeda M, Knight JD (2006) Enhanced solubilization of rock phosphate by Penicillium bilaiae in pH-buffered solution culture. Can J Microbiol 52:1121–1129. https://doi.org/10.1139/w06-074
van der Bom F, Nunes I, Raymond NS, Hansen V, Bonnichsen L, Magid J, Nybroe O, Jensen LS (2018) Long-term fertilisation form, level and duration affect the diversity, structure and functioning of soil microbial communities in the field. Soil Biol Biochem 122:91–103. https://doi.org/10.1016/j.soilbio.2018.04.003
van der Bom FJT, McLaren TI, Doolette AL, Magid J, Frossard E, Oberson A, Jensen LS (2019) Influence of long-term phosphorus fertilisation history on the availability and chemical nature of soil phosphorus. Geoderma 355:113909. https://doi.org/10.1016/j.geoderma.2019.113909
van der Paauw F (1971) An effective water extraction method for the determination of plant-available soil phosphorus. Plant Soil 34:467–481. https://doi.org/10.1007/BF01372799
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586. https://doi.org/10.1023/A:1026037216893
Vessey JK, Heisinger KG (2001) Inoculation and phosphorus fertilisation on root and shoot parameters of field-grown pea. Can J Plant Sci 81:361–366. https://doi.org/10.4141/P00-083
Vestergaard G, Schulz S, Schöler A, Schloter M (2017) Making big data smart—how to use metagenomics to understand soil quality. Biol Fertil Soils 53:479–484. https://doi.org/10.1007/s00374-017-1191-3
Wakelin SA, Gupta VVSR, Harvey PR, Ryder MH (2007) The effect of Penicillium fungi on plant growth and phosphorus mobilization in neutral to alkaline soils from southern Australia. Can J Microbiol 53:106–115. https://doi.org/10.1139/w06-109
Wakelin SA, Warren RA, Harvey PR, Ryder MH (2004) Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol Fertil Soils 40:36–43. https://doi.org/10.1007/s00374-004-0750-6
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets - iron, zinc, copper, calcium, mangesium, selenium and iodine. New Phytol 182:49–84. https://doi.org/10.1111/j.1469-8137.2008.02738.x
White PJ, Brown PH (2010) Plant nutrition for sustainable development and global health. Ann Bot 105:1073–1080. https://doi.org/10.1093/aob/mcq085
Yadav J, Verma JP, Rajak VK, Tiwari KN (2011) Selection of effective indigenous rhizobium strain for seed inoculation of chickpea (Cicer aritenium L.) production. Bacteriol J 1(1):24–30
Zaidi A, Khan MS, Ahemad M, Oves M, Wani PA (2009) Recent advances in plant growth promotion by phosphate-solubilizing microbes. In: Khan MS (ed) Microbial strategies for crop improvement. Springer, Berlin, pp 23–50
Acknowledgments
This research was part of the Future Cropping project, funded by Innovation Fund Denmark (grant no. 5107-00002B). We would like to thank Lena Asta Byrgesen and Morten Læssøe Stephensen for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hansen, V., Bonnichsen, L., Nunes, I. et al. Seed inoculation with Penicillium bilaiae and Bacillus simplex affects the nutrient status of winter wheat. Biol Fertil Soils 56, 97–109 (2020). https://doi.org/10.1007/s00374-019-01401-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-019-01401-7