Abstract
Fungal communities may have different response to fertilization under different conditions. Therefore, it is necessary to reveal the effects of fertilizations on fungal communities considering temporal heterogeneity at different crop stages. To address this, soil samples were collected across eight typical plant growth stages of a wheat-rice rotation system under four fertilization regimes: no nitrogen fertilizer (NNF), chemical fertilizer (CF), organic-inorganic mixed fertilizer (OIMF), and organic fertilizer (OF). Soil temperature and moisture that co-vary with plant growth stages were the strongest predictors of fungal community compositions; meanwhile, fertilization regimes also played important roles. Shannon index and the relative abundance of Ascomycota were consistently increased when compared OF treatment with CF and OIMF treatments, while CF treatment had a higher relative abundance of Zygomycota when compared with NNF and OF treatments. For the functional guilds, application of urea-nitrogen fertilizers (CF and OIMF treatments) significantly decreased the relative abundance of saprotrophs and symbiotrophs, while soil treated with OF had less relative abundance of pathotrophs when compared to inorganic fertilizers. Our study provided a detailed picture of how fungal community composition responded to fertilization regimes across different plant growth stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fungi are one of the most important decomposers in soil due to their ability to degrade complex organic matters and due to high biomass, and therefore, the shift in fungal community compositions can also influence the dynamics of carbon (C) and nitrogen (N) cycles (McGuire et al. 2010). However, compared to bacteria, there are fewer researches concerning fungal ecology and function.
Due to anthropogenic disturbance, excess N enters into ecosystems, which can impact ecosystem processes and microbial communities (Allison et al. 2007). Fungi are important player in C and N cycling, and changes in N availability caused by fertilizations can feed back to influence fungal community compositions in arable soils (Koranda et al. 2014). Making use of the rapid development of high-throughput sequencing, the entire fungal community can now be characterized at quite an accurate level compared with single gene survey. However, characterization is limited by the difficulty of parsing fungal phylogenetic taxa into ecologically meaningful categories, and researchers generally discuss changes in the abundance and compositions of fungal taxa and referred to their trophic strategies. Specific composition of fungi community is generally organized into modes by their trophic traits; these modes are saprotroph, symbiotroph, and pathotroph (Nguyen et al. 2016). Most species of soil fungi are saprotrophs, which obtain C from degradation of organic matters (Cuadros-Orellana et al. 2013). Symbiotrophs (symbionts) receive nutrients from host cell assimilation products and supply mineral nutrients to host cells in exchange (Lin et al. 2012). Pathotrophs (pathogens) receive nutrients by harming host cells. These three trophic traits often have different response to fertilizations. For example, it is often reported that saprotrophic fungi were stimulated by the addition of N in arable soils (Rousk and Bååth 2011; Sun et al. 2016), whereas a meta-analysis found a decrease in mycorrhizal abundance by 15% due to N fertilization (Treseder 2004). In addition, fungi of different trophic strategies appear to have different response to organic and inorganic N fertilizations. Application of chemical fertilizers has been shown to increase the proportion of pathogenic fungi (Cwalina-Ambroziak et al. 2010; Paungfoo-Lonhienne et al. 2015), whereas application of organic fertilizers could enhance the suppression of pathogens (Ghorbani et al. 2008; Zinati 2005). Additionally, the application of organic fertilization can provide C and N sources that stimulate the abundance and diversity of saprotrophic fungi (Boddy et al. 2007; Song et al. 2015). Trophic strategies can be shared by many different species and are not conserved within taxa. Characterization of the response of fungal community composition to fertilizations by taxa is problematic due to some fungal taxa have two or three trophic strategies (e.g., Basidiomycota and Ascomycota) (Jones et al. 2009). Grouping of taxa into functional guilds could reveal niche differentiation and specific ecosystem functions of fungal community. For the reason that different functional guilds transform soil nutrients in different ways, we could understand the prevalence of different functional guilds under different fertilization regimes (Sterkenburg et al. 2015).
Wheat and rice rotation agricultural systems are one of the highest yielding production systems in the world. Due to difference in water management between wheat and rice seasons, this ecosystem frequently alternates between oxic and anoxic soil conditions. This makes it an attractive model for analyzing microbial ecology and biogeochemical processes (Noll et al. 2005). As described above, application of different fertilizers could influence the compositions of soil fungal communities. However, researches based on a single time point only provide a snapshot of potential responses of fungal community compositions to fertilizations (Xue et al. 2018). To our best knowledge, little information is available regarding the time course of changes in fungal communities and functional groups under different fertilization practices. We therefore lack a detailed picture of how the compositions of fungal communities respond to fertilization regimes in different plant growth stages, and this limits our understanding of the roles of fungi in agricultural ecosystem functioning.
In our study, we investigated the effects of fertilization regime on the compositions of fungal community of soils under long-term fertilization so as to compare our previous studies about the compositions of the soil bacterial and ammonia-oxidizers’ communities in the same sites. Fertilization had stronger effect on the compositions of ammonia-oxidizing bacterial community than plant growth stages, whereas it was opposite for the compositions of the whole bacterial community (Wang et al. 2016, 2017). To analyze the response of fungal trophic strategies to fertilizations, we grouped fungal taxa using a database of fungal functional guilds in the present study (Nguyen et al. 2016). We hypothesized that (1) plant growth stages also play a critical role in driving the variation of the compositions of the whole fungal community; (2) different functional groups of fungi are likely to respond differently to plant growth stages and fertilizations; and (3) different types of N fertilizations have distinct impacts on fungal diversity and composition.
Materials and methods
Experimental design, soil samples collection, and determination of soil properties
Soil samples were collected from a long-term experimental field site which was established in 2005 (Wang et al. 2016). The paddy soil is situated in Changshu, Jiangsu Province, China ((31°18′ N, 120° 37′ E, 6 m asl). The site has a humid subtropical monsoon climate with an average annual rainfall of ≈ 1063 mm (Wang et al. 2016).
This study concerned the following four fertilization regimes with three replicated plots for each treatment: (1) no N fertilizer treatment (NNF) with 750 kg ha−1 superphosphate (12% P2O5) and 183 kg ha−1 potassium chloride (60% K2O) inputs; (2) chemical fertilizer treatment (CF) with application of 391 kg ha−1 urea (46% N), 750 kg ha−1 superphosphate, and 183 kg ha−1 potassium chloride; (3) organic fertilizer treatment (OF) with 4500 kg ha−1 pure organic fertilizer (26.4% organic C, 2.5% total N, 1.6% P2O5, and 1.3% K2O, made of composted rice straw and pig manure by Tianniang Ltd. of Changshu, China); and (4) organic-inorganic mixed fertilizer treatment (OIMF) with application of 1500 kg ha−1 organic-inorganic mixed fertilizer (11.0% organic C, 12.0% total N, 4.1% P2O5 and 4.1% K2O). One thousand five hundred kilograms of OIMF was pelleted with 625 kg organic fertilizers, 357 kg urea, 428 kg superphosphate, and 89 kg potassium chloride, which was made by Tianniang Ltd. (Changshu, China). An extra application of 28.5 kg ha−1 superphosphate and 48.5 kg ha−1 potassium chloride was also applied in OIMF treatment to reach the equal quantities of N, P2O5, and K2O as CF plots.
Same quantity of fertilizers was applied twice as basal fertilizer on both October 25, 2012, for wheat and on June 5, 2013, for rice crops.
Soil samples were collected with four cores (5 cm diameter × 20 cm deep) on each of 8 typical crops growth stages in 2013: wheat tillering stage in March 1 (Mar), wheat jointing stage in April 1 (Apr), wheat heading stage in May 1 (May), wheat ripening stage in June 4 (Jun), rice tillering stage in July 7 (Jul), rice jointing stage in August 16 (Aug), rice heading stage in September 15 (Sep), and rice ripening stage in October 31 (Oct). Soil samples were placed in plastic bags and kept with drikold for transportation. To remove the plant materials, roots, and stones, all the 96 soil samples (4 fertilizations × 8 stages × 3 plots) were sieved.
Data of soil available phosphorus, available potassium, available N (exchangeable NH4+ and NO3−), soil organic matter, soil total N, soil pH and EC, and soil moisture can be found in Supporting Information Table S1. These data were derived from our previous study (Wang et al. 2016).
DNA extraction and fungal ITS gene high-throughput sequencing
Soil DNA was extracted using MoBio Power Soil™ DNA Isolation Kits (MoBio Laboratories, Carlsbad, CA, USA) according to the manufacturer’s instructions, which has been described previously (Wang et al. 2016). The PCR reaction of fungal ITS1 region was performed using 5 μL of Q5 Reaction Buffer (5×), 5 μL of Q5 GC High Enhancer (5×), 1 μL (10 μM) of ITS1F primer (5′- CTTGGTCATTTAGAGGAAGTAA -3′) (Gardes and Bruns 1993), 1 μL (10 μM) of ITS2 primer (5′- GCTGCGTTCTTCATCGATGC -3′) (Baldwin 1992), 2 μL of dNTP (2.5 mM), 1 μL of DNA template (20 ng μl−1), 0.25 μL of Q5 Polymerase (5 U μl−1), and 9.75 μL of ddH2O for a final volume of 25 μL. The PCR ran with a hot start for 5 min at 98 °C, 27 cycles of 98 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s with a final extension for 5 min at 72 °C. The Illumina sequencing adapter ligated reverse primer contained a 6-bp barcode specific for sample identification (Caporaso et al. 2012). After amplification, the triplicate PCR products were pooled and purified using the PCR cleanup Kit (Axygen Biosciences, Union City, CA, USA). Sequencing was performed on a single lane of Illumina MiSeq platform at Personal Biotechnology Co., Ltd. (Shanghai, China).
Bioinformatics and statistical analysis
The obtained sequences were processed following the Quantitative Insights into Microbial Ecology (QIIME) pipeline (Caporaso et al. 2010). Barcodes and the standard primer set sequences were excluded. Low-quality sequences (quality score below 25, read length below 200 bp) were removed from the dataset. Chimera sequences were identified and removed using UCHIME (Edgar et al. 2011). The number of sequences was resampled with 36,255 sequences per sample. Sequences were clustered and assigned to operational taxonomic units (OTUs) using the implementation of QIIME according to a threshold of 97% pairwise identity. For taxonomic assignment of the OTUs, the representative sequences were identified using the UNITE database (Abarenkov et al. 2010) with higher than 80% similarity, and the non-fungal OTUs were discarded.
Fungal taxa were assigned a putative functional guild if they matched a genus that belonged to a single functional group using the FUNGuild v1.0 (Nguyen et al. 2016), the current database for assigning fungal functional guilds. FUNGuild assigns function based on matches at the genus and species level along with a confidence level (Kivlin and Hawkes 2016; Nguyen et al. 2016). All 2221 OTUs were assigned to 36 different guilds. We removed 1073 OTUs that were assigned to “Unassigned” and used the rest of 1148 OTUs for further analysis. The details of the fungal functional guilds can be found in Supporting Information Table S2.
The richness and Shannon indexes were calculated to estimate α-diversity of each sample using R software (Team 2014). Multivariate ANOVAs based on the Shannon and richness indexes were performed to determine the effects of stage and fertilization regimes on the fungal diversities, followed by Tukey’s HSD as a post hoc test using SPSS 18.0. Permutational multivariate analyses were performed to determine the effects of plant growth stage, fertilization regimes, and their interactions on fungal compositions and functional guilds based on the OTU relative abundance and functional database of 36 guilds, respectively. Non-metric multidimensional scaling (NMDS) analyses were performed to determine the fungal β-diversity and functional guilds, which were based on the “bray” distance of OTU relative abundance and functional database after Hellinger transformation, respectively. Mantel tests were used to analyze the correlations between the Euclidean distance of soil properties and OTU compositions and functional guilds. Permutational multivariate analyses, NMDS analyses, and Mantel test were performed using “vegan” package in R software (Team 2014). One way ANOVAs with Duncan’s tests using SPSS 18.0 were performed to compare the relative abundance of saprotroph, symbiont, and pathogen across eight plant growth stages or under four fertilization regimes, respectively.
We performed linear discriminant effect size (LEfSe) analyses to identify significant differences in fungal taxa between fertilization regimes based on the combined data across all times points. In LEfSe analysis, the Kruskal-Wallis sum-rank test was used to detect the features which were significantly different between assigned classes, and then the effect size of each differentially abundant taxon was estimated using linear discriminant analysis (Segata et al. 2011). The default threshold value (2.0) was tried in LEfSe analysis but contained too much redundant information; therefore, 3.0 was used as threshold value to reduce the redundant information and make the results brief in our present study.
Sequencing data were deposited in the NCBI Sequence Read Archive (SRA) database under the accession number SRX2987082.
Results
Fungal α-diversity
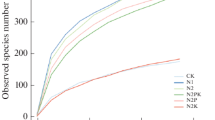
As shown in Table 1, the Shannon index of soil fungi was significantly influenced (P < 0.001) by different plant growth stages and different fertilization regimes. As shown in Fig. 1a, the Shannon index ranged from 4.2 to 5.0 across the eight stages. The rice stages (Jul–Oct), as compared with the wheat stages (Mar–Jun), had a significantly higher Shannon index (Tukey’s HSD, P < 0.01). NNF had the lowest Shannon index, and when CF treatment was compared with OF treatment, its Shannon index was significantly (Tukey’s HSD, P < 0.05) decreased (Fig. 1c). The richness index was also significantly changed (P < 0.001) by plant growth stages (Table 1). As shown in Fig. 1b, the later growth stages (May and Jun for wheat, and Sep and Oct for rice) of wheat and rice have significantly (Tukey’s HSD, P = 0.011) higher average richness than that of the early growth stages (Mar and Apr for wheat, and Jul and Aug for rice), while different fertilization treatments had no significant effect on fungal richness (Table 1, Fig. 1d).
Fungal Shannon index (a) and richness (b) across eight plant growth stages. Shannon index (c) and richness (d) under four fertilization regimes. Significant difference is indicated with different letters (ANOVA followed by Tukey HSD test, P < 0.05). In the box plot, the band inside the box is the median; outlier is plotted as individual point. NNF no N fertilizer, CF chemical fertilizer, OIMF organic-inorganic mixed fertilizer, OF organic fertilizer. Mar, wheat tillering stage; Apr, wheat jointing stage; May, wheat heading stage; Jun, wheat ripening stage; Jul, rice tillering stage; Aug, rice jointing stage; Sep, rice heading stage; Oct, rice ripening stage
Fungal community compositions
The most abundant fungal phyla in the soil samples were Ascomycota and Basidiomycota, which accounted for 68.9 and 19.9% on an average, respectively. As shown in Fig. S1, the wheat stages (Mar–Jun) had a higher relative abundance of Ascomycota than the rice stages (Jul–Oct). The relative abundance of Basidiomycota increased with the growth of wheat (Supporting Information Fig. S1) but fluctuated with the growth of rice. The other five phyla of Zygomycota, Rozellomycota, Glomeromycota, Chytridiomycota, and Neocallimastigomycota accounted for 4.2, 1.6, 1.3, 1.2, and 0.05% of the relative abundance, respectively.
Different fertilization regimes had different fungal compositions. Compared with the NNF treatment, N fertilizations inputs increased the relative abundance of the Zygomycota phylum (Supporting Information Fig. S1) and Incertaesedis and Tremellomycetes orders (Fig. 2a). Compared with the CF treatment, partial substitution of organic fertilizer for chemical fertilizer decreased the abundance of the Sordariomycetes order (Fig. 2b). Soils treated with organic fertilizer, when compared with the application of urea-N fertilizers (CF and OIMF), had a significantly higher abundance of the Ascomycota phylum (Supporting Information Fig. S1) and the Pezizomycates order (Fig. 2c, d) across all plant growth stages, whereas the abundance of the Zygomycota phylum and the Tremellomycetes order decreased after OF treatment. In addition, the abundance of Basidiomycota was significantly decreased by the OF treatment when compared with the OIMF treatment (Fig. 2c; Supporting Information Fig. S1); however, this decrease was not observed when compared with CF treatment.
Cladogram indicating the phylogenetic distribution of fungal lineages. LDA coupled with effect size measurements identifies the different abundant taxa between fertilizer regimes: NNF versus CF treatments (a), CF versus OIMF treatments (b), OF versus OIMF treatments (c), and CF verses OF treatments (d). Lineages with LDA values higher than 3.0 are displayed. Significantly (P < 0.05) abundant taxa are represented in the color of red or green. Circles represent phylogenetic levels from domain to genus from the inside outwards. NNF no N fertilizer, CF chemical fertilizer, OIMF organic-inorganic mixed fertilizer, OF organic fertilizer
As revealed by permutation test (Table 1), fungal community compositions were significantly influenced by plant growth stages (P = 0.001). NMDS analysis also revealed that fungal compositions were apparently dispersed by eight plant growth stages, and that temporally separated stages had more differences in fungal community compositions than more adjacent stages (Fig. 3a). Although the effect of fertilization regimes was less than that of plant growth stages, the effect on fungal community composition was also significant (Table 1). As displayed in Fig. 3b, in the second and third dimensions of NMDS, different fertilization regimes had distinctive community compositions, with OF treatment being more apart from the other three treatments.
Fungal community compositions as analyzed by non-metric multidimensional scaling analysis (NMDS) based on OTU relative abundance. Plots of axes 1 and 2 are colored with eight sampling stages (a) and axes 2 and 3 are colored with four fertilization regimes (b). The stress was 0.136, the non-metric fit R2 value was 0.981, and the linear fit R2 value was 0.915. NNF no N fertilizer, CF chemical fertilizer, OIMF organic-inorganic mixed fertilizer, OF organic fertilizer. Mar, wheat tillering stage; Apr, wheat jointing stage; May, wheat heading stage; Jun, wheat ripening stage; Jul, rice tillering stage; Aug, rice jointing stage; Sep, rice heading stage; Oct, rice ripening stage
Mantel analysis revealed that soil moisture and temperature were the strongest drivers of fungal community composition, which could explain 20.7 and 16.2% for the variation, respectively. In addition, soil available potassium and available phosphorus contents also significantly influenced fungal community composition (Table 2). For the wheat stages, soil available potassium content significantly affected fungal community compositions and functional guilds, soil moisture also played a significant role in affecting fungal functional guilds. For the rice stages, soil temperature had a significant effect on fungal community compositions (Table 2).
Fungal functional guilds
In all 96 samples, sequences from the high-throughput sequencing were clustered into 2221 OTUs. Most of the OTUs, 50.1% were grouped as saprotrophs. Pathotrophs and symbiotrophs were the next most common functional guilds accounting for 5.3 and for 4.1% of the OTUs, respectively. As shown in Fig. 4, 59.2% of Ascomycota, 22.6% of Basidiomycota, 98.8% of Zygomycota, and 59.5% of Chytridiomycota phyla were assigned as saprotrophs, respectively, whereas 6.3% of Ascomycota, 4.2% of Basidiomycota, and 9.9% of Chytridiomycota phyla were assigned as pathotrophs, respectively. The percentage assigned as symbiotrophs was 1.2% of Ascomycota, 9.9% of Basidiomycota, and 100% of Glomeromycota phyla, respectively.
Similar to fungal community composition, plant growth stages and fertilization regimes also had significant influence on the fungal functional guilds (Table 1). As with fungal community composition, the impact of plant growth stages was greater than the impact of fertilization regime (Table 1, Fig. 5). Wheat growth stages (Mar–Jun) had a higher abundance of saprotrophs but a lower abundance of symbiotrophs when compared to rice growth stages (Jun–Oct) (Fig. 6a, b). In terms of pathotrophs, the early growth stages (Mar and Apr, Jul and Aug) of wheat and rice had higher abundance than the later growth stages (May and Jun, Sep and Oct) (Fig. 6c). With regard to the influence of different fertilization regimes on the functional guilds, application of urea-N fertilizers (CF and OIMF treatments) significantly (P < 0.05) decreased the abundance of saprotrophs and symbiotrophs when compared with the other two N fertilization regimes (Fig. 6d, e). Application of OF significantly decreased the relative abundance of pathotrophs as compared with other fertilization regimes (Fig. 6f). Compared with the CF treatment, OIMF and OF treatments decreased the relative abundance of plant pathogens by 78.8 and 68.3% on an average, respectively (Supporting Information Fig. S2B).
Fungal community compositions as analyzed by non-metric multidimensional scaling analysis (NMDS) based on functional guilds. Plots of axes 1 and 2 are colored with eight sampling stages (a) and axes 2 and 3 are colored with four fertilization regimes (b). The stress was 0.198, the non-metric fit R2 value was 0.96, and the linear fit R2 value was 0.848. NNF no N fertilizer, CF chemical fertilizer, OIMF organic-inorganic mixed fertilizer, OF organic fertilizer. Mar, wheat tillering stage; Apr, wheat jointing stage; May, wheat heading stage; Jun, wheat ripening stage; Jul, rice tillering stage; Aug, rice jointing stage; Sep, rice heading stage; Oct, rice ripening stage
Relative abundance of fungal guild of saprotrophs (a), symbiotrophs (b), and pathotrophs (c) across eight plant growth stages. Relative abundance of fungal guild of saprotrophs (d), symbiotrophs (e), and pathotrophs (f) under four fertilization regimes. Significant difference is indicated with different letters (ANOVA followed by Tukey HSD test, P < 0.05). In the box plot, the band inside the box is the median; outlier is plotted as individual point. NNF no N fertilizer, CF chemical fertilizer, OIMF organic-inorganic mixed fertilizer, OF organic fertilizer. Mar, wheat tillering stage; Apr, wheat jointing stage; May, wheat heading stage; Jun, wheat ripening stage; Jul, rice tillering stage; Aug, rice jointing stage; Sep, rice heading stage; Oct, rice ripening stage
Mantel tests demonstrated that soil moisture and temperature were the factors having the greatest influence on fungal functional guilds (Table 2). Additionally, soil exchangeable ammonium and available potassium contents also significantly influenced the functional guilds.
Discussion
Plant growth stages determined the variation of fungal communities
The plant growth stage played the most important role in affecting the compositions of bacterial (Wang et al. 2016) and fungal communities in the studied soils, which supported our first hypothesis, whereas the fertilization regimes had higher effects than the plant growth stage in affecting the compositions and abundance of ammonia-oxidizing bacterial communities than different plant growth stages (Wang et al. 2017) probably because the fertilization affected soil N availability (e Silva et al. 2013; Kowalchuk and Stephen 2001). However, for the whole bacterial and fungal communities, employed by 8-year fertilization regimes were less powerful than the plant growth stages in the wheat-rice rotation system, which has been practiced for thousands of years in the experimental area. It should be mentioned that soil fungal community compositions and diversity were also varied in different years (Pickles et al. 2010), thus addressing seasonal changes should also consider the month × year interactions (Mundra et al. 2015). Therefore, a longer term fertilization regime across different years is needed to observe the fertilization effect for the further study.
Higher Shannon indexes demonstrated that soils under rice rotation had higher α-diversities than that of wheat rotation. Rice plots were flooded with 5 cm of standing water from July 10 to September 30, which caused a deficiency of soil oxygen (Lee et al. 2009), with the exception of the uppermost 2 mm and in the shallow zone around the roots of rice, where an oxygen gradient is established (Noll et al. 2005; Revsbech et al. 1999). Therefore, we speculate that rice growth stages soil provided more heterogeneous niches with oxygen gradients, and different fungal compositions occupied more different niches, as rice soils had more numbers of fungal taxa or lineages than those of wheat soils. In addition, the differences in the eight growth stages of wheat and rice, including different quality and quantity of plant-derived labile C, litter, and root exudates, may also affect the diversity and richness of fungi (Broeckling et al. 2008; Thormann 2006). This could explain our result of higher fungal richness in the heading stage when the plants had their most vigorous growth and also in the ripening stage when plants produce the highest amount of litter (Yoshida 1981).
Additionally, the plant growth stage is not a separate variable explaining the observed variations. Mantel test showed that soil temperature and moisture that co-vary with plant growth stage were the most important factors influencing the compositions of fungal communities. Soil temperature and/or moisture have been demonstrated to be the predominating environmental factors in influencing the activity, composition, and diversity of microbial communities in soil (Bell et al. 2008, 2009; Lauber et al. 2013; Lee et al. 2011). Indeed different fungal species have different temperature and moisture requirement for optimal growth, and this may explain the effect of these two environmental factors on the compositions of fungal communities (Barcenas-Moreno et al. 2009; Frey et al. 1999; Pietikäinen et al. 2005). It should be noted that soil temperature and moisture were nested with the type of crops (wheat and rice). Actually, soil temperature only significantly affected the fungal compositions in rice growth stages, and soil moisture only significantly affected fungal functional guilds in wheat growth stages (Table 2). Therefore, it is needed to be careful in considering the effects of soil temperature and moisture on fungal communities.
Different functional groups of fungi responded differently to plant growth stages, which supported our second hypothesis. Fungal pathogens were more abundant in the early stages of plant growth, indicating that crops might suffer a higher risk of the infection from soil-borne fungal diseases during the early than late growth stages (Ou 1985).
Fertilization regimes played an important role in the variation of fungal diversity and compositions
Our third hypothesis which predicted that different types of N fertilizations have distinct impacts on fungal diversity and compositions was also proved in the present study. Consistent with the previous studies (Cwalina-Ambroziak and Bowszys 2009; Kamaa et al. 2012; Mäder et al. 2002; Song et al. 2015), the organic input to soil increased soil fungal α-diversity (Shannon index) compared to the mineral fertilizer treatment, and probably this depended on the fact that organic fertilizers contain polymers such as polysaccharide, lignin, polyaromatics, etc., which are degraded and used as energy sources by fungi (Leifeld et al. 2002; Hanson et al. 2008); therefore, organic fertilizations improved the fungal α-diversity by stimulating and providing more niches for saprotrophs (Moll et al. 2015; Sun et al. 2016). Despite the N application can decrease the diversities of fungal communities and plants (Arnolds 1991; Allison et al. 2007; Borer et al. 2014; Suding et al. 2005), the α-diversity of whole fungal communities and the Shannon index were lowest in the NNF treatment. The reasons for these inconsistent results are not clear and probably climate and edaphic factors might contribute to this discrepancy. There was no difference of Shannon index between CF and OIMF treatment, which was consistent with a previous study based on paddy soil (Chen et al. 2016).
Most saprotrophic fungi are Ascomycota, and previous studies have demonstrated that the abundance of Ascomycota were flourished in response to organic matter additions to soil (Ma et al. 2013; Lentendu et al. 2014), which agree with the findings that soils treated with OF had higher abundance of Ascomycota phylum compared with CF and OIMF soils. However, FUNGuild revealed that the predominant species of the Zygomycota phylum (98.8%) and the Tremellomycetes order (42.7%) are saprotrophs, and their abundance was significantly increased by mineral N fertilizers when compared with no N and organic N inputs. Zygomycota are the first colonizers fungi of fresh substrate, utilize the readily available nutrients for a short time, and their growth can be suppressed by metabolic byproducts (Moore-Landecker 2008). Therefore, Zygomycota preferred CF and OIMF soils with higher contents of available N. FUNGuild showed that all the saprotrophic Tremellomycetes group, as foliar epiphytes (Nakase et al. 2004), and the higher abundance of Tremellomycetes in CF and OIMF than the other two treatments could be explained by the higher crop biomass of these treatments (Wang et al. 2016), which had higher foliar litters compared with NNF and OF treatments.
The relative abundance of symbiotic fungi, including arbuscular mycorrhizal and ectomycorrhizal fungi, was decreased by the application of inorganic N fertilizers. It should be noted that neither rice nor wheat should form symbiosis with ectomycorrhizal fungi, and probably ectomycorrhizal fungi live as saprotrophic lifestyle (Martin et al. 2008). Higher N availability in soils will lead to less dependence of plants on mycorrhizal fungi to take soil nutrients (Treseder 2004; Treseder et al. 2018). These symbiotic fungi received less root-derived C for their growth (Johnson et al. 2010; Sterkenburg et al. 2015; Wei et al. 2013). However, the degradation of organic fertilizers could stimulate the activity of mycorrhizal fungi by the release of some growth-stimulating substances and/or nutrients (Gavito and Olsson 2003; Gryndler et al. 2006; Ravnskov et al. 1999). The Glomeromycota phylum only included arbuscular mycorrhizal fungi (Fig. 4), and our results showed that OF soil had a higher abundance of Glomeromycota than the CF soil across the wheat-rice rotation (Supporting Information Fig. S1). Inconsistently, LEfSe analysis showed that there was no significant difference of Glomeromycota abundance between CF and OF (Fig. 2); however, when the threshold value of LEfSe analyses decreased from 3.0 to 2.0 (default value), the abundance of Glomeromycota was significantly higher in OF and OIMF than in CF (data not shown). Higher abundance of Glomeromycota was also found when N and P inputs to grassland soils decreased (Leff et al. 2015).
Previous studies reported a decrease of plant pathogenic fungal abundance after application of organic inputs to soil (Mokhtar and El-Mougy 2014). Bailey and Lazarovits (2003) suggested that the application of organic matters could release allelochemicals to reduce soil-borne diseases and Zinati (2005) suggested that compost amendments could suppress diseases by stimulating beneficial microorganisms acting through antibiosis, competition, and predation hyperparasitism or by inducing systemic resistance in the plant. On the contrary, high N fertilizer inputs can increase plant diseases as reviewed by Walters and Bingham (2007). Consistently, OF significantly decreased the relative abundance of plant and also animal pathogens, while CF had the highest relative abundance of pathogens (especially plant pathogens). Therefore, organic managements can potentially improve and cause a moderate degeneration of arable soil qualities, while application of urea-N fertilizers can be potentially harmful to agricultural ecosystem. However, our study was based on relative abundance and diversity of fungal communities and studies on fungal activities should be carried out to confirm the findings of this study.
Conclusion
As already observed for the bacterial communities (Wang et al. 2016), the present study showed that plant growth stages, co-varying with the changes of climate and crops growth, played a more important role in affecting the compositions of the fungal communities than fertilization regimes. This study also provides evidence for the effect of fertilizer on fungal community compositions. For instance, OF treatment increased the relative abundance of Ascomycota phylum but decreased that of Zygomycota phylum when compared with CF and OIMF treatments. In addition, different fertilizations changed the abundance of fungal taxa grouped by their trophic strategies. Probably the application of urea-N fertilizers can be harmful to agricultural ecosystems by increasing the relative abundance of pathotrophs and decreasing that of symbiotrophs. Compared to the studies based on individual time points, our study provides a more complete picture of the successional response of fungal communities to fertilization and plant growth stages.
References
Abarenkov K, Nilsson RH, Larsson KH, Alexander IJ, Eberhardt U, Erland S, Høiland K, Kjøller R, Larsson E, Pennanen T (2010) The UNITE database for molecular identification of fungi—recent updates and future perspectives. New Phytol 186:281–285
Allison SD, Hanson CA, Treseder KK (2007) Nitrogen fertilization reduces diversity and alters community structure of active fungi in boreal ecosystems. Soil Biol Biochem 39:1878–1887
Arnolds E (1991) Decline of ectomycorrhizal fungi in Europe. Agric Ecosyst Environ 35:209–244
Bailey K, Lazarovits G (2003) Suppressing soil-borne diseases with residue management and organic amendments. Soil Tillage Res 72:169–180
Baldwin BG (1992) Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: an example from the compositae. Mol Phylogenet Evol 1:3–16
Barcenas-Moreno G, Gomez-Brandon M, Rousk J, Bååth E (2009) Adaptation of soil microbial communities to temperature: comparison of fungi and bacteria in a laboratory experiment. Glob Chang Biol 15:2950–2957
Bell C, McIntyre N, Cox S, Tissue D, Zak J (2008) Soil microbial responses to temporal variations of moisture and temperature in a Chihuahuan Desert grassland. Microb Ecol 56:153–167
Bell CW, Acosta-Martinez V, McIntyre NE, Cox S, Tissue DT, Zak JC (2009) Linking microbial community structure and function to seasonal differences in soil moisture and temperature in a Chihuahuan desert grassland. Microb Ecol 58:827–842
Boddy L, Frankland J, Van West P (2007) Ecology of saprotrophic basidiomycetes vol 28. Academic Press, Amsterdam
Borer ET, Seabloom EW, Gruner DS, Harpole WS, Hillebrand H, Lind EM, Adler PB, Alberti J, Anderson TM, Bakker JD (2014) Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508:517–520
Broeckling CD, Broz AK, Bergelson J, Manter DK, Vivanco JM (2008) Root exudates regulate soil fungal community composition and diversity. Appl Environ Microbiol 74:738–744
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Caporaso JG, Lauber CL, Walters WA, Berglyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624
Chen C, Zhang J, Lu M, Qin C, Chen Y, Yang L, Huang Q, Wang J, Shen Z, Shen Q (2016) Microbial communities of an arable soil treated for 8 years with organic and inorganic fertilizers. Biol Fertil Soils 52:1–13
Cuadros-Orellana S, Leite LR, Smith A, Medeiros JD, Badotti F, Fonseca PL, Vaz AB, Góes-Neto A, Oliveira G (2013) Assessment of fungal diversity in the environment using metagenomics: a decade in review. Fungal Genom Biol 3:1–13
Cwalina-Ambroziak B, Bowszys T (2009) Changes in fungal communities in organically fertilized soil. Plant Soil Environ 55:1
Cwalina-Ambroziak B, Bowszys T, Wierzbowska J (2010) Fungi colonizing soil fertilized with composted sewage sludge and municipal waste. J Elem 15:39–51
e Silva MCP, Semenov AV, Schmitt H, van Elsas JD, Salles JF (2013) Microbe-mediated processes as indicators to establish the normal operating range of soil functioning. Soil Biol Biochem 57:995–1002
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Frey S, Elliott E, Paustian K (1999) Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol Biochem 31:573–585
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gavito ME, Olsson PA (2003) Allocation of plant carbon to foraging and storage in arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 45:181–187
Ghorbani R, Wilcockson S, Koocheki A, Leifert C (2008) Soil management for sustainable crop disease control: a review. Environ Chem Lett 6:149–162
Gryndler M, Larsen J, Hršelová H, Řezáčová V, Gryndlerová H, Kubát J (2006) Organic and mineral fertilization, respectively, increase and decrease the development of external mycelium of arbuscular mycorrhizal fungi in a long-term field experiment. Mycorrhiza 16:159–166
Hanson CA, Allison SD, Bradford MA, Wallenstein MD, Treseder KK (2008) Fungal taxa target different carbon sources in forest soil. Ecosystems 11:1157–1167
Johnson NC, Wilson GW, Bowker MA, Wilson JA, Miller RM (2010) Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc Natl Acad Sci U S A 107:2093–2098
Jones EBJ, Sakayaroj J, Suetrong S, Somrithipol S, Pang LK (2009) Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers 35:1–187
Kamaa MM, Mburu HN, Blanchart E, Chibole L, Chotte J-L, Kibunja CN, Lesueur D (2012) Effects of organic and inorganic applications on soil bacterial and fungal microbial communities diversity and impacts of earthworms on microbial diversity in the Kabete long-term trial, Kenya. In: Bationo A, Waswa B, Kihara J, Adolwa I, Vanlauwe B, Saidou K (eds) Lessons learned from long-term soil fertility management experiments in Africa. Springer, Dordrecht, pp 121–136
Kivlin SN, Hawkes CV (2016) Tree species, spatial heterogeneity, and seasonality drive soil fungal abundance, richness, and composition in neotropical rainforests. Environ Microbiol 18:4662–4673
Koranda M, Kaiser C, Fuchslueger L, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S, Richter A (2014) Fungal and bacterial utilization of organic substrates depends on substrate complexity and N availability. FEMS Microbiol Ecol 87:142–152
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529
Lauber CL, Ramirez KS, Aanderud Z, Lennon J, Fierer N (2013) Temporal variability in soil microbial communities across land-use types. ISME J 7:1641–1650
Lee K-W, Chen P-W, Lu C-A, Chen S, Ho T-HD, Yu S-M (2009) Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Sci Signal 2:ra61
Lee S-H, Kim C-G, Kang H (2011) Temporal dynamics of bacterial and fungal communities in a genetically modified (GM) rice ecosystem. Microb Ecol 61:646–659
Leff JW, Jones SE, Prober SM, Barberán A, Borer ET, Firn JL, Harpole WS, Hobbie SE, Hofmockel KS, Knops JM (2015) Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc Natl Acad Sci U S A 112:10967–10972
Leifeld J, Siebert S, Kögel-Knabner I (2002) Changes in the chemical composition of soil organic matter after application of compost. Eur J Soil Sci 53:299–309
Lentendu G, Wubet T, Chatzinotas A, Wilhelm C, Buscot F, Schlegel M (2014) Effects of long-term differential fertilization on eukaryotic microbial communities in an arable soil: a multiple barcoding approach. Mol Ecol 23:3341–3355
Lin X, Feng Y, Zhang H, Chen R, Wang J, Zhang J, Chu H (2012) Long-term balanced fertilization decreases arbuscular mycorrhizal fungal diversity in an arable soil in north China revealed by 454 pyrosequencing. Environ Sci Technol 46:5764–5771
Ma A, Zhuang X, Wu J, Cui M, Lv D, Liu C, Zhuang G (2013) Ascomycota members dominate fungal communities during straw residue decomposition in arable soil. PLoS One 8:e66146
Mäder P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697
Martin F, Aerts A, Ahrén D, Brun A, Danchin EGJ, Duchaussoy F, Gibon J, Kohler A, Lindquist E, Pereda V (2008) The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452:88–92
McGuire KL, Bent E, Borneman J, Majumder A, Allison SD, Treseder KK (2010) Functional diversity in resource use by fungi. Ecology 91:2324–2332
Mokhtar M, El-Mougy N (2014) Bio-compost application for controlling soil-borne plant pathogens–a review. Population 4:61–68
Moll J, Goldmann K, Kramer S, Hempel S, Kandeler E, Marhan S, Ruess L, Krüger D, Buscot F (2015) Resource type and availability regulate fungal communities along arable soil profiles. Mol Ecol 70:390–399
Moore-Landecker E (2008) Zygomycota and glomeromycota. In: Encyclopedia of Life Sciences (ELS). John Wiley & Sons, Ltd., Chichester
Mundra S, Bahram M, Tedersoo L, Kauserud H, Halvorsen R, Eidesen PB (2015) Temporal variation of Bistorta vivipara-associated ectomycorrhizal fungal communities in the High Arctic. Mol Ecol 24:6289–6302
Nakase T, Jan-ngam H, Tsuzuki S, Lee F-L, Jindamorakot S, Potacharoen W, Tanticharoen M, Takashima M (2004) Two new ballistoconidium-forming yeast species, Bullera melastomae and Bullera formosana, found in Taiwan. Syst Appl Microbiol 27:558–564
Nguyen NH, Song Z, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248
Noll M, Matthies D, Frenzel P, Derakshani M, Liesack W (2005) Succession of bacterial community structure and diversity in a paddy soil oxygen gradient. Environ Microbiol 7:382–395
Ou SH (1985) Rice diseases. Commonwealth Mycological Institute, Kew, pp 247–299
Paungfoo-Lonhienne C, Yeoh YK, Kasinadhuni NRP, Lonhienne TG, Robinson N, Hugenholtz P, Ragan MA, Schmidt S (2015) Nitrogen fertilizer dose alters fungal communities in sugarcane soil and rhizosphere. Sci Rep 5:8678
Pickles BJ, Genney DR, Potts JM, Lennon JJ, Anderson IC, Alexer IJ (2010) Spatial and temporal ecology of Scots pine ectomycorrhizas. New Phytol 2010:755–768
Pietikäinen J, Pettersson M, Bååth E (2005) Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiol Ecol 52:49–58
Ravnskov S, Larsen J, Olsson PA, Jakobsen I (1999) Effects of various organic compounds on growth and phosphorus uptake of an arbuscular mycorrhizal fungus. New Phytol 141:517–524
Revsbech N, Pedersen O, Reichardt W, Briones A (1999) Microsensor analysis of oxygen and pH in the rice rhizosphere under field and laboratory conditions. Biol Fertil Soils 29:379–385
Rousk J, Bååth E (2011) Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiol Ecol 78:17–30
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60
Song G, Chen R, Xiang W, Yang F, Zheng S, Zhang J, Zhang J, Lin X (2015) Contrasting effects of long-term fertilization on the community of saprotrophic fungi and arbuscular mycorrhizal fungi in a sandy loam soil. Plant Soil Environ 61:127–136
Sterkenburg E, Bahr A, Brandström Durling M, Clemmensen KE, Lindahl BD (2015) Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol 207:1145–1158
Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, Milchunas DG, Pennings S (2005) Functional-and abundance-based mechanisms explain diversity loss due to N fertilization. Proc Natl Acad Sci U S A 102:4387–4392
Sun R, Dsouza M, Gilbert JA, Guo X, Wang D, Guo Z, Ni Y, Chu H (2016) Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ Microbiol 18:5137–5150
Team RC (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna 2012 Open access available at: http://cran.r-project.org
Thormann MN (2006) Diversity and function of fungi in peatlands: a carbon cycling perspective. Can J Soil Sci 86:281–293
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Treseder KK, Allen EB, Egerton Warburton LM, Hart MM, Klironomos JN, Maherali H, Tedersoo L (2018) Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: a trait-based predictive framework. J Ecol 106:480–489
Walters D, Bingham I (2007) Influence of nutrition on disease development caused by fungal pathogens: implications for plant disease control. Ann Appl Biol 151:307–324
Wang J, Xue C, Song Y, Wang L, Huang Q, Shen Q (2016) Wheat and rice growth stages and fertilization regimes alter soil bacterial community structure, but not diversity. Front Microbiol 7:1207
Wang J, Ni L, Song Y, Rhodes G, Li J, Huang Q, Shen Q (2017) Dynamic response of ammonia-oxidizers to four fertilization regimes across a wheat-rice rotation system. Front Microbiol 8:630
Wei C, Yu Q, Bai E, Lü X, Li Q, Xia J, Kardol P, Liang W, Wang Z, Han X (2013) Nitrogen deposition weakens plant–microbe interactions in grassland ecosystems. Glob Chang Biol 19:3688–3697
Xue C, Penton CR, Zhu C, Chen H, Duan Y, Peng C, Guo S, Ling N, Shen Q (2018) Alterations in soil fungal community composition and network assemblage structure by different long-term fertilization regimes are correlated to the soil ionome. Biol Fertil Soils 54:95–106
Yoshida S (1981) Fundamentals of rice crop science, vol 279. International Rice Research Institute, Los Banos
Zinati GM (2005) Compost in the 20th century: a tool to control plant diseases in nursery and vegetable crops. HortTechnology 15:61–66
Funding
This work was financially supported by the National Basic Research Program of China (2015CB150500 and 2013CB127403), Jiangsu Science and Technology Department (BK20150059), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, and the 111 project (B12009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Rhodes, G., Huang, Q. et al. Plant growth stages and fertilization regimes drive soil fungal community compositions in a wheat-rice rotation system. Biol Fertil Soils 54, 731–742 (2018). https://doi.org/10.1007/s00374-018-1295-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-018-1295-4