Abstract
Watermelon Fusarium wilt is one of the most severe soil-borne diseases caused by Fusarium oxysporum f. sp. niveum. In this study, the population of F. oxysporum was quickly monitored by real-time PCR and DNA array in watermelon Fusarium wilt infected soils treated with Paenibacillus polymyxa SQR21 enhanced bio-organic fertilizer (BIO) at the beginning of nursery growth and/or at the beginning of transplanting. The fungal community composition was investigated by molecular cloning and DGGE techniques. The real-time PCR results showed the F. oxysporum population in the rhizosphere soil decreased from 8.56 × 104 colony-forming units (cfu) g−1 rhizosphere soil to 9.41 × 103 cfu g−1 rhizosphere soil after BIO application and the DNA array detection signals of F. oxysporum population weakened. The difference between F. oxysporum abundance of BIO amended and not amended bulk soils was lower than 104 cfu g−1 soil. DGGE profile indicated that BIO application changed the fungal community structure in the rhizosphere soils; the molecular cloning data revealed that consecutive applications of BIO at nursery and transplanting stages not only decreased Ascomycota and increased Basidiomycota abundance in the rhizosphere soil but also caused the apperance of unique fungal group which were not found in the control. The beneficial fungi Chaetomium sp. Aspergillus penicillioides were found in the BIO amended treatment, while some harmful fungi such as F. oxysporum, Rhizoctonia solani, and Fusarium solani were only detected in the control. Data from this study indicated that BIO application can control watermelon Fusarium wilt by suppressing the population of F. oxysporum and changing the fungal community structure in the rhizosphere soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Watermelon is one of the most important fruits worldwide, but in many areas of China, its yield can be decreased (Ling et al. 2010; Wu et al. 2008) by a widespread diseases caused by the soil-borne pathogen Fusarium oxysporum f. sp. niveum (Booth 1971; Wu et al. 2008).
Chemical control is one commonly used strategy of disease control, but it is not environmentally friendly and has negative effects on some beneficial microbes (Minuto et al. 2006; Omar et al. 2006). It was reported that consecutive applications of chemical fertilizer lead to a decrease in some beneficial microbes and an increase in developed resistance of target pathogens (Goldman et al. 1994; Tjamos et al. 2004). For this reason, the importance of biological control has been increased over the years and bio-organic fertilizer (BIO) application has become one of the promising biological ways to control various soil-borne disease (Luo et al. 2010; Yang et al. 2011; Zhang et al. 2011). Up to now, many antagonistic microbes have been reported to biocontrol Fusarium wilt (Dijksterhuis et al. 1999; Zhang et al. 2011). Although composts can stimulate proliferation of antagonists in the rhizosphere and reduce the incidence of some soil-borne plant pathogens, it showed inconsistent levels of disease control and less control effectiveness than further manipulation of composts by inoculation or enrichment with specific antagonists (Borrero et al. 2004; Trillas et al. 2006; Zhao et al. 2010). Nowadays, it is widely reported that a combination of antagonistic microbes with mature compost may be more efficient in inhibiting disease than using single antagonistic microbial strains or compost alone (Cao et al. 2011; Huang et al. 2012; Qiu et al. 2012; Shen et al. 2013; Wang et al. 2013; Wei et al. 2011; Yin et al. 2011; Zhang et al. 2011).
Most studies of plant disease have focused on those fungi that affect the above-ground portion of plants (Burdon and Silk 1997). However, many of the most devastating diseases in agricultural systems are caused by below-ground pathogens. The lack of rapid, accurate, and reliable means by which plant pathogens can be detected and identified is one of the main limitations in integrated disease management (Njambere et al. 2011; Zhao et al. 2012). Traditionally, pathogen diagnostic mainly relies on morphological and/or cultural dependent methods, but all these methods are time consuming (Lievens and Thomma 2005; Zhang et al. 2008), because they usually take 1 week or longer to be completed (Zhao et al. 2012). The molecular methods such as real-time polymerase chain reaction (PCR) assay and DNA array have been developed for the rapid detection and quantification of pathogens from environment samples (Gilbert et al. 2008; Lievens and Thomma 2005), including soil samples (Lievens et al. 2003; Zhang et al. 2008). Analyzing fungal communities by denaturing gradient gel electrophoresis (DGGE) with separation of ITS gene or by cloning libraries have become the prevalent method to determine genetic diversity of the rhizosphere fungal communities (Anderson et al. 2003; White et al. 1990). It can reveal a more complete and distinctive picture of soil fungal composition and afford clear information of phylogeny and change in fungal community composition (Martin and Rygiewicz 2005).
The rhizosphere is known to be a research hotspot of plant–microbial interactions and a driving force of soil ecosystems (Fang et al. 2013). The role of fungi in rhizosphere soil is extremely complex and is fundamental to the soil ecosystems because fungi play a very important role in the decomposition of organic matter, enabling and facilitating the transport of nutrients from soil to plant (Bridge and Spooner 2001). Some fungi are known to cause a range of plant diseases, others are known to antagonize plant pathogens and stimulate plant growth, while some fungi can affect the composition of microbial communities by changing host plant physiology directly or indirectly (Marschner et al. 2001).
In the previous study, we reported that the application of a novel BIO by fermenting mature composts with the antagonistic microbe Paenibacillus polymyxa can reduce the incidence of the Fusarium wilt disease by 60–100 % in pot experiments (Ling et al. 2010). Nevertheless, it is not known if the soil treated with P. polymyxa enhanced BIO can suppress F. oxysporum populations and change the compositions of soil fungal communities. We have hypothesized that application of P. polymyxa enhanced BIO can effectively control watermelon Fusarium wilt by reducing the F. oxysporum population and altering the composition of fungal communities in the rhizosphere soil. The results of this study can be important to monitoring soil born pathogens’ infection in a quick and accurate way, and an improved knowledge of the composition of fungal communities in the rhizosphere soil after BIO application can lead to a better understanding of fungal roles in these soil ecosystems.
Materials and methods
Organic fertilizer preparation
The organic fertilizer was composed of amino acid fertilizer and pig manure compost (1:1); the amino acid fertilizer was obtained from oil rapeseed cakes after enzyme hydrolysis by microbial fermentation at <50 °C for 7 days (Ling et al. 2010). The obtained organic fertilizer (BIO) was inoculated with the SQR21 (with high antagonistic efficiency against F. oxysporum causing cucumber and watermelon wilt disease) and incubated on fermentator for 6 days at 45 °C (Ling et al. 2010).
Soils
The nursery soil (300 g) for growing seedlings was sampled from a site without history of watermelon wilt disease, while the soil for the transplanted pot experiment was collected from the surface of 2 years mono-cultivated watermelon plots with serious watermelon Fusarium wilt disease located in Jiangyin country, Jiangsu province, China. The soil (sandy loam) has pH 6.8, organic C 12.0 g kg−1, total N 1.61 g kg−1, total P 0.37 g kg−1, and total K 6.15 g kg−1.
Seedling nursery
Watermelon seeds (Kangbing Jingxin) were surface-sterilized with 2 % NaClO for 3 min and rinsed in sterile water for several times and then put into 9-cm plates covered with sterile wet filter paper at 30 °C for their germination. The two treatments of the nursery soil were NCK (without BIO) and NBIO with BIO treated at 2 % (w:w). The NCK was treated with chemical fertilizer containing equivalent nutrients (192 mg N, 180 mg P2O5, and 54 mg K2O) of the 2 % BIO amendment (NBIO). The seedlings were grown under greenhouse conditions at temperature ranging from 26 to 35 °C.
Pot experiment and sampling
The seedlings with 3–4 true leaves were transplanted into pots which contained approximately 10 kg of the fresh soil from the watermelon wilt diseased field. In addition to the two nursery treatments, two more treatments (PCK and PBIO) were designed at the pot experiment stage with soils being amended without or with the BIO at a rate of 5 g kg−1. All four treatments are listed in Table 1. Three plant replicates were sampled randomly from four treatments. The rhizosphere soil from each plant was carefully collected by softly shaking by hands. The other soil samples obtained without shaking roots were considered as the bulk soils (Bakker and Schippers 1987).
DNA extraction and PCR amplification
DNA was extracted in triplicate from watermelon planted rhizosphere and bulk soil (0.5 mg) of each treatment by Ultra Clean™ Soil Kit (MOBIO Laboratories, Carlsbad, CA, USA). The extracts were subsequently pooled. The quality and the concentration of extracted DNA were determined by Nanovue (Gelife sciences, USA). The ITS region of the 18S rRNA gene was amplified with primers ITS4/ITS5 (Gardes and Bruns 1993; White et al. 1990) using a Thermocycler (Bio-Rad, icycler, USA). PCR was carried out in 50-μl reaction volume using 5 μl 10 × PCR Buffer (Mg2+ Free), 3-μl MgCl2 (25 mM, TaKaRa, Japan), 4-μl dNTP Mixture (each 2.5 mM), 1 unit Takara Taq DNA polymerase (Takara, Dalian, China), 2 μl each primer (10 pmol/μl), 4-μl template, and ddH2O to reach a volume of 50 μl. The used PCR cycling conditions were 95 °C for 5 min, 35 cycles at 95 °C for 50 s, 57 °C for 1 min, and 72 °C for 1 min, followed by final extension at 72 °C for 10 min. PCR products were purified according to the manufacturer’s protocol using the Axyprep™ DNA Gel Extraction Kit.
Real-time PCR amplification
Real-time PCR assays to quantify F. oxysporum f. sp. niveum DNA were conducted using the primer pair ITS1-F (Gardes and Bruns 1993) and AFP308R (10 mM) (Lievens et al. 2003). Real-time PCR amplification reactions were carried by SYBR® Premx Taq™ (2 × TaKaRa Bio technology Dalian Co., Ltd.). Reaction mixture contained 10 μl of SYBR® Premix ExTaq™ (2×), 0.5 μl of each primer, 0.5 μl of ROX Reference Dye II (50×), 2 μl extracted DNA, and double distilled water to reach a 25-μl reaction volume. Samples were preheated to 95 °C for 2 min and then followed by 40 amplification cycles at 94 °C for 15 s, 58 °C for 15 s, and extension at 72 °C for 10 s. After the final amplification cycle, specificity was examined by generating a dissociation curve after amplification.
DNA array probes and array development
Specific oligomer probes used in this study to detect the genus Fusarium (Fgn1 and Fgn2) and the species F. oxysporum (Fo1, Fo2, Fox1, Fox2), Verticillium dahliae (Vda1), Verticillium albo-atrum (Val2), Verticillium spp. (Vgn1, Vgn2), Fusarium solani, and Rhizoctonia solani were previously designed and validated by Lievens et al. (2003) and Zhang et al. (2007) (Table 2). All oligomers were dissolved at a concentration of 50 μM in spotting buffer (4 μM sodium carbonate buffer, pH 8.4, 3× SSC [1 × SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.01 % Nlauroyl sarcosine, and 0.004 % bromophenol blue), and each detector oligonucleotide probe was spotted onto Hybond N+ nylon membranes (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA) with four replicates per each probe using a 96-pin tool (V&P Scientific Inc., San Diego, CA, USA). To assess the specificity of detection by the array (Fig. 1), ITS4 (A1), ITS5 (A2), ITS2 (A3), and internal controls from ITS2-1 (A4) to ITS2-5 (A8), which differ from ITS2 at one base of the probes, were spotted to test the cross-reaction against the array (Table 2); negative control were double distilled water (C5) and spotting buffer (C6). The spotted membranes were air dried for 10 min and fixed by UV exposure at 240 mJ/cm2. After incubation in a 0.5 % SDS at 60 °C for 1 h, membranes were rinsed with 100 mM Tris/HCl (pH 8.0) for 5 min and kept moist at 4 °C until used. The ITS amplicons from soil samples were labeled and hybridized using the Gene Images AlkPhos Direct Labeling and Detection System with CDP-Star (Amersham Biosciences) following the manufacturer’s protocol. Chemiluminescence was detected using Kodak Biomax Light film. Developed films were scanned with a Hewlett-Packard 5300C ScanJet and read by ImageJ 1.33u (National Institutes of Health, MD)(Zhang et al. 2007; Njambere et al. 2011).
DNA array design and hybridization results of fungal communities of rhizosphere soil. Positive controls ITS4, ITS5, and ITS2 were spotted in A1, A2, and A3. Internal controls ITS2-1 to ITS2-5 were spotted in A4, A5, A6, A7, and A8. Negative controls (buffer and ddH2O) were also spotted in C5 and C6. Specific probes for Fusarium sp. (B1 and B2), F. oxysporum (B3, B4, B5, and B6), Verticillium spp. (B7 and B8), Verticillium dahliae (C1), Verticillium albo-atrum (C2), Fusarium solani (C3), and Rhizoctonia solani (C4) were spotted four times on the array respectively. NCK + PCK, both nursery and pot soil were untreated; NCK + PBIO, the pot soil was treated with bio-organic fertilizer but not the nursery soil; NBIO + PCK, the nursery soil was treated with bio-organic fertilizer but not the pot soil; NBIO + PBIO, both nursery and pot soil were treated with bio-organic fertilizer
Cloning ITS fragment of NCK + PCK and NBIO + PBIO rhizosphere soils
Genomic DNA of rhizosphere soil from NCK + PCK and NBIO + PBIO was amplified with the primer pair ITS1F/ITS4 by the Taq PCR Enzyme Mix (Takara, Dalian, China); PCR products from three separate amplifications per treatments were pooled and used in one cloning reaction, resulting in a total of two cloning reactions. PCR products were purified with Qiaquick gel extraction kit (Qiagen) and cloned into pMD19-T Vector (TaKaRa Biotechnology Dalian Co., Ltd.); the colonies of transformed E. coli were isolated from LB media, after incubation for 16 h at 37 °C; the clones were transferred into LB broth and then plasmids were isolated and treated with enzymes (Alu I, Hinf I, and Dra I from New England Bio Lab, Beverly, MA); DNA fragments were then resolved on 2 % agarose. The clones with different band types were sequenced and aligned by Clustal.
DGGE analysis
DGGE analysis was performed using the D-Code DGGE system (Bio-Rad); DGGE profiles were obtained with ITS1-F (Gardes and Bruns 1993) and ITS2 (Anderson et al. 2003; White et al. 1990) primers. A GC clamp (5′-CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG G-3′) (Muyzer et al. 1993) was added to the 5′ end of the ITS1-F primer to stabilize the melting behavior of DNA fragments. Twenty microliters of PCR products (400 ng) were loaded onto 8 % (w/v) polyacrylamide gels (37.5:1 of 40 % acrylamide/bis-solution, Bio-Rad Laboratories) with denaturing gradients ranging from 25 to 55 % for the fungal DNA. DGGE was performed in 1 × Tris-acetate-EDTA buffer (40 mM Tris-acetate, 1 mM EDTA, pH 8.0) at 60 °C at a constant voltage of 80 V for 16 h; then, DGGE fingerprint were silver stained.
Statistical analyses
Similarly, the microbial communities were expressed using the unweighted pair group method with mathematical averages (UPGMA). Quantity one 4.6.3 (Bio-Rad, USA) was used to construct UPGMA dendrograms and Cluster analyses. The DGGE bands were visualized with UV transillumination and photographed; average intensities of individual bands were analyzed using Quantity One image analysis software, version 4.6.3 (Bio-Rad Laboratories). Shannon index (H′) was calculated from DGGE profile (Girvan et al. 2003) using the formula H′ = −∑P i ln pi, where pi is the percent contribution of the microbial species (intensity of the band i⁄total intensity of all bands in the lane) and ln p i is the natural log of p i . Equitability index (J) was also calculated using the formula J = H′⁄ (ln n i ), where J is the Shannon Weaver equitability index and ln n i is the natural log of the total number of species in a fingerprint. Statistical significance was determined by the analysis of replicate samples using analysis of variance (Anova) test. For all experiments a P value of <0.05 was required to establish significant differences between unamended and amended samples.
Results
Detection of F. oxysporum in soils of four treatments by real-time PCR
The real-time quantitative results indicated there was a significant difference (P > 0.05) in CFU levels of F. oxysporum in the rhizosphere soil of BIO treated (NBIO + PCK and NBIO + PBIO) or untreated (NCK + PCK and NCK + PBIO) nursery cups. The abundance of F. oxysporum in nursery cups treated without BIO was 8.56 × 104 cfu g−1 rhizosphere soil in the NCK + PCK treatment and 8.08 × 104 cfu g−1 rhizosphere soil in the NCK + PBIO treatment, whereas, the abundance of F. oxysporum in nursery cups treated with BIO was 16.19 × 103 and 9.41 × 103 cfu g−1 rhizosphere soil in the treatments NBIO + PCK and NBIO + PBIO respectively. Then the NBIO + PBIO treatment showed the lowest F. oxysporum abundance 9.41 × 103 cfu g−1 soil in the rhizosphere soil. In the bulk soils of the four treatments, the number of F. oxysporum ranged from 5.72 × 104 cfu g−1 soil to 3.93 × 104 cfu g−1 soil (Table 3), without significant differences.
Validation of the DNA array
The membrane-based DNA array was validated with DNA extracted from rhizosphere soils of the four treatments. Universal oligonucleotide ITS4, ITS5, and ITS2 (Fig. 1, A1, A2, A3) were used as control being supposed to detect the presence of any fungal species with consistent positive signals in the four treatments; four internal controls ITS2_1 to ITS2_5 (Fig. 1, A4 to A8) that differed from primer ITS2 at one or two bases were not supposed to detect any fungal species with negative signals in the four treatments except A8; which had a very weak cross-hybridization signal. However, there were differences in signal intensities of pathogens between treatments without (Fig. 1, NCK + PCK and NCK + PBIO) or with (Fig. 1, NBIO + PCK and NBIO + PBIO) BIO application to the nursery cups. The signal intensities of B1, B2 (Fusarium sp.) and B3, B4, B5, B6 (F. oxysporum) in BIO treatments to the nursery cups (Fig. 1, NBIO + PCK and NBIO + PBIO) were weaker than those of treatments without BIO application to the nursery cups (Fig. 1, NCK + PCK and NCK + PBIO). Intensities of B1, B2 (Fusarium sp.) and B3, B4, B5, B6 (F. oxysporum) in the NBIO + PBIO treatment displayed the lowest signals among the four treatments (Fig. 1). The signal intensities of C3 (Fusarium solani) and C4 (Rhizoctonia solani) of four treatments were positive and showed the same trend of B1, B2 (Fusarium sp.) and B3, B4, B5, B6 (F. oxysporum) (Fig. 1). No positive signals were detected in the B7, B8 (Verticillium spp.), C1 (Verticillium dahliae) and C2 (Verticillium albo-atrum) position, whereas C5 and C6 displayed negative signals in the four treatments (Fig. 1).
ITS clone libraries of NCK + PCK and NBIO + PBIO rhizosphere soils
A total of 129 fungal sequences were obtained from the NCK + PCK (66 sequences) and NBIO + PBIO (63 sequences) clone libraries, which were classified as 21 types of OTUs after treatment with three enzymes. The Ascomycota was the dominant taxon in the NCK + PCK (35 of 66) and NBIO + PBIO (38 of 63) clone libraries, and the Zygomycota (23 of 66) was the second dominant taxon in the NCK + PCK while the Basidiomycota (13 of 63) became the second dominant taxon in the NBIO + PBIO rhizosphere soil (Table 4). Nine out of 66 sequences had 99 % similarity with sequences of F. oxysporum in the NCK + PCK treatment, while there was only one clone showing 99 % similarity with sequences of F. oxysporum in the NBIO + PBIO treatment.
Investigation at the genus level showed that uncultured Mortierella accounted for 29 % of the total fungal communities in the NCK + PCK rhizosphere soil (Table 4), suggesting that this was an important soil fungal group in the watermelon Fusarium wilt rhizosphere soil. On the contrary, the presence of Rhodotorula cresolica increased whereas the presence of F. oxysporum and other pathogens decreased in the NBIO + PBIO rhizosphere soil. The results of cloning libraries also indicated that consecutive applications of BIO at nursery and transplanting stages resulted in the presence of fungal group species which were not found in the NCK + PCK ITS clone library; these were uncultured Hypocreales, Fusarium merismoides, Bionectria ochroleuca, Neonectria sp., uncultured Plectosphaerella, Chaetomium sp., and Aspergillus penicillioides; both Chaetomium sp. and Aspergillus penicillioides were considered beneficial fungi. The presence of harmful fungi F. oxysporum, Rhizoctonia solani, Fusarium solani, and Fusarium sp. decreased or was suppressed in the NBIO + PBIO treatment. There were nine fungal species that were found in both clone libraries, at varing proportion (Table 4, in bold).
DGGE fingerprints of four treatments rhizosphere soil ITS fragment
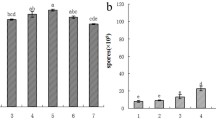
A distinct fungal DGGE pattern was observed in the BIO rhizosphere soil of watermelon plants, and there were clear differences in the intensity and number of bands in the DGGE fingerprints of the four treatments (Fig. 2a). The DGGE bands were clustered with Quantity One computer software (version 4.6.3, Bio-Rad, p < 0.05); three distinct clusters were observed (Fig. 2b). The fungal communities of treatments without BIO application in either nursery or transplanted pot (NCK + PCK) and with only BIO application in the pot (NCK + PBIO) were in the same cluster, while the fungal communities of only BIO treatment in the nursery (NBIO + PCK) and the BIO treatment in both the nursery and pot (NBIO + PBIO) formed two different clusters (Fig. 2b). These results confirmed that changes occurred in the composition of fungal communities of different treatments and that the BIO application to both nursery and pot soil had greater effects on composition of soil fungal communities than other treatments.
The DGGE profiles (a) and the UPGMA cladograms based on Dice similarity of the composition of fungal communities in watermelon rhizosphere soil (b). #1, 2, and 3 were three replicates of NCK + PCK rhizosphere soil; #4, 5, and 6 were three replicates of NCK + PBIO rhizosphere soil; #7, 8, and 9 were three replicates of NBIO + PCK rhizosphere soil; and #10, 11, and 12 were three replicates of NBIO + PBIO rhizosphere soil. NCK + PCK, both nursery and pot soil were untreated; NCK + PBIO, the pot soil was treated with bio-organic fertilizer but not the nursery soil; NBIO + PCK, the nursery soil was treated with bio-organic fertilizer but not the pot soil; NBIO + PBIO, both nursery and pot soil were treated with bio-organic fertilizer
In fungal community, the Shannon–Wienner index, the equitability index, and number of bands indicating fungal communities were calculated (Table 5). The DGGE profile showed high Shannon–Wienner diversity indices (Table 5) due to high numbers of bands with different intensities. The number of detected bands ranged from 13 to 22 bands but only four bands were present in all samples. The difference between band intensity was assumed to be due to differences in the abundance of the target species. In the BIO treatments (NBIO + PCK and NBIO + PBIO), Shannon Weaver (H′) diversity values were lower than those of the BIO nursery unamended soil samples (NCK + PCK and NCK + PBIO); Shannon Weaver diversity indices of NCK + PCK and NCK + PBIO treatments were 2.86 and 2.82 respectively, while those of the NBIO + PCK and NBIO + PBIO rhizosphere soils were 1.94 and 1.57. The NBIO + PBIO treatment showed the lowest Shannon Weaver (H′) diversity value (1.57) among four treatments. Equitability indexes (J′) of the four treatments showed the same trend with Shannon Weaver (H′) indexes of the four treatments (Table 5), again indicating that BIO application could change fungal diversity and evenness.
Discussion
Plant defense responses to soil-borne pathogens and their roots interactions with soil microbial communities depend on plant species (Lang et al. 2012; Ling et al. 2012; Luo et al. 2010; Wu et al. 2008) because root exudation can be highly specific (Alabouvette et al. 2006; Prieto et al. 2011). BIO (a combination of organic fertilizers with antagonistic microbes) has been reported to have positive effects on controlling soil-borne diseases in many crops such as rice (Yin et al. 2011), cucumber (Chen et al. 2012; Cao et al. 2011; Huang et al. 2012; Qiu et al. 2012), tomato (Wei et al. 2011), banana (Shen et al. 2013; Wang et al. 2013; Zhang et al. 2011), cotton (Lang et al. 2012), potato (Ding et al. 2013), tobacco (Liu et al. 2013), and watermelon (Ling et al. 2012). However, the relationship linking pathogen, plant disease, and composition of soil fungi communities after BIO application to soil are poorly understood.
We have reported that a P. polymyxa strain can enhance the suppressiveness of BIO against Fusarium wilt disease by 60 to 100 % and when BIO was applied to nursery and transplanted soils it significantly promoted the growth by 7.27 g plant−1 (Ling et al. 2010). Without BIO application (NCK + PCK), the culturable abundance of F. oxysporum population in the watermelon rhizosphere soil was as high as 8.56 × 104 cfu g−1 soil with a high disease incidence (100 %). The most effective treatment in reducing F. oxysporum abundance in the rhizosphere soil was the BIO application in both nursery and transplanted soil (NBIO + PBIO), confirming previous results (Ling et al. 2010) and indicating that the abundance of the F. oxysporum population in the rhizosphere soil is a key factor in the incidence of watermelon disease. The abundance of F. oxysporum in the bulk soil of watermelon was stable among four treatments accounting for about 103 cfu g−1 soil probably because the rhizosphere of watermelon may represent an excellent environment for the fast-growing Fusarium species (Timmusk et al. 2005). It has been suggested that the antagonists P. polymyxa SQR21 of the BIO can utilize root exudates of watermelon to support its activities (Ling et al. 2010), and thus, it is a more competitive and effective root colonizer than most native microbial species (Haggag and Timmusk 2008; Trillas et al. 2006). Additionally, antibiosis produced by P. polymyxa may prevent F. oxysporum f. sp. nevium colonization of the roots (Dijksterhuis et al. 1999; Timmusk et al. 2005) and thus reducing the incidence of Fusarium wilt (Ling et al. 2010; Raza et al. 2008).
Disease management can be improved with rapid and accurate pathogen detection (Njambere et al. 2011; Zhao et al. 2012). The quantification by real-time PCR showed that F. oxysporum survives poorly when BIO was applied into nursery and pot soil. The probes for detecting non-target pathogens such as Verticillium dahliae, Verticillium albo-atrum, and Verticillium spp. (Lievens et al. 2003; Zhang et al. 2007) were also included in the DNA assay aiming to assess the specificity and sensitivity of this assay. Even though the DNA assay system used in this study displayed a very weak cross-hybridization of ITS2-5 (Table 4, A8), it detected and monitored the target pathogen F. oxysporum DNA. The weak cross-hybridization might be due to highly similar sequences where the mismatched base was located near the end or in a string of the identical bases. This might be prevented by designing dimeric oligonucleotide probes (Njambere et al. 2011; Zhang et al. 2008). The oligonucleotide-based DNA array detection system used in this study is reliable and effective for F. oxysporum identification even when multiple pathogens are present in the same soil sample.
Disease suppression can function as an indicator for a stable and healthy soil ecosystem (Van Bruggen and Semenov 1999), and microbial structural and functional diversity in soil may be important for soil health (Visser and Parkinson 1992). The ability of P. polymyxa SQR21 to inhibit plant pathogens and to promote plant growth has been documented (Ryu et al. 2006; Timmusk et al. 2005), but its effects on composition of soil fungi communities are poorly known. The DGGE and clone library results showed that application of BIO changed the composition of fungal community of the rhizosphere zone. The detectable band number in the NBIO + PBIO treatment was only 13, indicating that the fungal species richness (n) was lower than the other treatments as well as the Shannon Weaver (H′) diversity values and equitability (J).
However, the BIO application in both nursery and pot increased the proportion of beneficial fungi such as Chaetomium sp. and Aspergillus penicillioides and decreased the proportion of some harmful fungi compared with unamended soil (Table 3). Chaetomium sp. (Suyanto et al. 2003) is a thermophilic fungus, which can decompose palm-oil mill fibers, while Aspergillus penicillioides is a halophile salt tolerant fungal species which inhibit most fungal species (Pitt and Hocking 2009; Tamura et al. 1999). Among the detected pathogenic fungi, F. oxysporum, Rhizoctonia solani, and Fusarium solani were found to co-exist in the rhizosphere soil and both abundances decreased with BIO application. As a result, the presence of pathogenic fungi and potential pathogenic fungi to watermelon was negatively affected by the BIO treatment (Ling et al. 2010). Further, the possible protection mechanism involved in this study may attributed to the increased activities of antioxidases and pathogenesis-related proteins in plants by BIO (Dijksterhuis et al. 1999; Ling et al. 2010), leading to enhanced plant systemic acquired resistance to the pathogen. In addition, the formulation of P. polymyxa SQR21 with organic matters changed the microbial composition of the watermelon rhizosphere soil (Dijksterhuis et al. 1999; Raza et al. 2008), and this may have affected soil nutrient availability to watermelons by changing soil enzyme activities (Yang et al. 2007).
In conclusion, our work showed that the application of P. polymyxa SQR21 inoculated BIO not only changed the composition of fungal communities but also significantly reduced Fusarium wilt disease symptoms by reducing the F. oxysporum population in the rhizosphere soil. It is important to have detected changes in the composition of fungal communities of the rhizosphere soil after BIO application because stability and composition of soil microbial communities are important indicators for soil health and sustainability. The underlying mechanisms of P. polymyxa SQR21 enhanced BIO application to control Fusarium wilt need to be further studied.
References
Alabouvette C, Olivain C, Steinberg C (2006) Biological control of plant diseases: the European situation. Eur J Plant Pathol 114:329–341
Anderson IC, Campbell CD, Prosser JI (2003) Potential bias of fungal 18S rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil. Environ Microbiol 5:36–47
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth stimulation. Soil Biol Biochem 19:451–457
Booth C (1971) The genus Fusarium. Eastern Press, London, pp 147–149
Borrero C, Trillas MI, Ordovás J, Tello J, Avilés M (2004) Predictive factors for the suppression of Fusarium wilt of tomato in plant growth media. Am Phytopathol Soc 94:1094–1101
Bridge P, Spooner B (2001) Soil fungi: diversity and detection. Plant Soil 232:147–154
Burdon JJ, Silk J (1997) Sources and patterns of diversity in plant pathogenic fungi. Phytopathology 87:664–669
Cao Y, Ling N, Yang XM, Chen LH, Shen QR (2011) Bacillus subtilis SQR9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol Fertil Soils 47:495–506
Chen LH, Huang XQ, Zhang FG, Zhao DK, Yang XM, Shen QR (2012) Applications of Trichoderma harzianum SQR-T037 bioorganic fertilizer significantly affect the microbial communities of continuously cropped soil of cucumber. J Sci Food Agric 92:2465–2470
Dijksterhuis J, Sanders M, Gorris LGM, Smid EJ (1999) Antibiosis plays a role in the context of direct interaction during antagonism of Paenibacillus polymyxa towards Fusarium oxysporum. J Appl Microbiol 86:13–21
Ding CY, Shen QR, Zhang RF, Chen W (2013) Evaluation of rhizosphere bacteria and derived bio-organic fertilizers as potential biocontrol agents against bacterial wilt (Ralstonia solanacearum) of potato. Plant Soil 366:453–466
Fang SZ, Liu D, Tian Y, Deng SP, Shang XL (2013) Tree species composition influences enzyme activities and microbial biomass in the rhizosphere: a rhizobox approach. PloS ONE 8:e61461
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes, application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118
Gilbert CA, Zhang N, Hutmacher RB, Davis RM, Smart CD (2008) Development of a DNA-based macroarray for the detection and identification of Fusarium oxysporum f. sp. vasinfectum in cotton tissue. J Cotton Sci 12:165–170
Girvan MS, Bullimore J, Pretty JN, Osborn AM, Ball AS (2003) Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soil. Appl Environ Microbiol 69:1800–1809
Goldman GH, Hayes C, Harman GE (1994) Molecular and cellular biology of biocontrol Trichoderma spp. Trends Biotechnol 12:478–482
Haggag WM, Timmusk S (2008) Colonization of peanut roots by biofilm-forming Paenibacillus polymyxa initiates bio-control against crown rot disease. J Appl Microbiol 104:961–969
Huang XQ, Zhang N, Ying XY, Yang XM, Shen QR (2012) Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol Res 167:135–143
Lang JJ, Hu J, Ran W, Xu YC, Shen Q (2012) Control of cotton Verticillium wilt and fungal diversity of rhizosphere soils by bio-organic fertilizer. Biol Fertil Soils 48:191–203
Lievens B, Thomma BPHJ (2005) Recent developments in pathogen detection arrays: implications for fungal plant pathogens and use in practice. Phytopathology 95:1374–1380
Lievens B, Brouwer M, Vanachter ACRC, Lévesque CA, Cammue BPA, Thomma BPHJ (2003) Design and development of a DNA array for rapid detection and identification of multiple tomato vascular wilt pathogens. FEMS Microbiol 223:113–122
Ling N, Xue C, Huang QW, Yang XM, Xu YC, Shen QR (2010) Development of a mode of application of bioorganic fertilizer for improving the biocontrol efficacy to Fusarium wilt. Biocontrol 55:673–683
Ling N, Zhang WW, Tan SY, Huang QW, Shen QR (2012) Effect of the nursery application of bioorganic fertilizer on spatial distribution of Fusarium oxysporum f. sp niveum and its antagonistic bacterium in the rhizosphere of watermelon. Appl Soil Ecol 59:13–19
Liu YX, Shi JX, Feng YG, Yang XM, Li X, Shen QR (2013) Tobacco bacterial wilt can be biologically controlled by the application of antagonistic strains in combination with organic fertilizer. Biol Fertil Soils 49:447–464
Luo J, Ran W, Hu J, Yang XM, Xu YC, Shen QR (2010) Application of bio-organic fertilizer significantly affected fungal diversity of soils. Soil Sci Soc Am J 74:2039–2048
Marschner P, Crowley DE, Lieberei R (2001) Arbuscular mycorrhizal infection changes the bacterial 16S rDNA community composition in the rhizosphere of maize. Mycorrhiza 11:297–302
Martin KJ, Rygiewicz PT (2005) Fungal-specific PCR primers developed for analysis of the ITS region of environmental DNA extracts. BMC Microbiol 5:28
Minuto A, Davide S, Garibaldi A, Gullino ML (2006) Control of soil-borne pathogens of tomato using a commercial formulation of Streptomyces griseoviridis and solarization. Crop Prot 25:468–475
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Njambere EN, Clarke BB, Zhang N (2011) Dimeric oligonucleotide probes enhance diagnostic macroarray performance. J Microbiol Meth 86:52–61
Omar I, O’Neill T, Rossall S (2006) Biological control of Fusarium crown and root rot of tomato with antagonistic bacteria and integrated control when combined with the fungicide carbenda-zim. Plant Pathol 55:92–99
Pitt JI, Hocking AD (2009) Fungi and food spoilage. In: Pitt JI, Hocking AD (eds) Aspergillus and related teleomorphs, 3rd edn. Springer, New York, pp 275–295
Prieto LH, Bertiller MB, Carrera AL, Olivera NL (2011) Soil enzyme and microbial activities in a grazing ecosystem of Patagonian Monte, Argentina. Geoderma 162:281–287
Qiu MH, Zhang RF, Xue C, Zhang SS, Li SQ, Zhang N, Shen QR (2012) Application of a novel bioorganic fertilizer can control Fusarium wilt by regulating microbial community of cucumber rhizosphere soils. Biol Fertil Soils 48:807–816
Raza W, Yang W, Shen QR (2008) Paenibacillus polymyxa: antibiotics, hydrolytic enzymes and hazard assessment. J Plant Pathol 90:419–430
Ryu CM, Kim J, Choi O, Kim SH, Park CS (2006) Improvement of biological control capacity of Paenibacillus polymyxa E681 by seed pelleting on sesame. Biol Control 39:282–289
Shen ZZ, Zhong ST, Wang YG, Mei XL, Wang BB, Li R, Ruan YZ, Shen QR (2013) Induced soil microbial suppression of banana Fusarium wilt disease using compost and bio fertilizers to improve yield and quality. Eur J Soil Biol 57:1–8
Suyanto OT, Yazaki S, Mimura Ui AS (2003) Isolation of a novel thermophilic fungus Chaetomium sp. nov. MS-017 and description of its palm-oil mill fiber-decomposing properties. Appl Microbiol Biotechnol 60:581–587
Tamura M, Kawasaki H, Sugiyama J (1999) Identity of the xerophilic species Aspergillus penicillioides: integrated analysis of the genotypic and phenotypic. J Gen Appl Microbiol 45:29–37
Timmusk S, Grantcharova N, Wagner HGE (2005) Paenibacillus polymyxa invades plant roots and forms biofilm. Appl Environ Microbiol 71:7292–7300
Tjamos EC, Tsitsiyannis DI, Tjamos SE, Antoniou PP, Katinakis P (2004) Selection and screening of endorhizosphere bacteria from solarized soils as biocontrol agents against Verticillium dahliae of solanaceous hosts. Eur J Plant Pathol 110:35–44
Trillas MI, Casanova E, Corxarrera L, Ordovas J, Borrero C, Aviles M (2006) Composts from agricultural waste and the Trichoderma asperellum strain T-34 suppress Rhizoctonia solani in cucumber seedlings. Biol Control 39:32–38
Van Bruggen AHC, Semenov AM (1999) A new approach to the search for indicators of root disease suppression. Aust J Plant Pathol 28:4–10
Visser S, Parkinson D (1992) Soil biological criteria as indicators of soil quality: soil micro-organisms. Am. J. Alternative Agric 7:33–37
Wang BB, Yuan J, Zhang J, Shen ZZ, Zhang MX, Li R, Ruan YZ, Shen QR (2013) Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana. Biol Fertil Soils 49:435–446
Wei Z, Yang XM, Yin SX, Shen QR, Ran W, Xu YC (2011) Efficacy of Bacillus-fortified organic fertilizer in controlling bacterial wilt of tomato in the field. Appl Soil Ecol 48:152–159
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Wu HS, Yang XM, Fan JQ, Miao WG, Ling N, Shen QR (2008) Suppression of Fusarium wilt of watermelon by a bio-organic fertilizer containing combinations of antagonistic microorganisms. Bio Control 54:287–300
Yang R, Tang J, Chen X, Hu S (2007) Effects of coexisting plant species on soil microbes and soil enzymes in metal lead contaminated soils. Appl Soil Ecol 37:240–246
Yang XM, Chen LH, Yong XY, Shen QR (2011) Formulations can affect colonization and biocontrol efficiency of Trichoderma harzianum SQR-T037 against Fusarium wilt of cucumbers. Biol Fertil Soils 47:239–248
Yin SX, Dong YH, Xu YC, Huang QW, Shen QR (2011) Upland rice seedling wilt and microbial biomass and enzyme activities of compost-treated soils. Biol Fertil Soils 47:303–313
Zhang N, Geiser DM, Smart CD (2007) Macroarray detection of solanaceous plant pathogens in the Fusarium solani species complex. Plant Dis 91:1612–1620
Zhang S, Raza W, Yang XM, Hu J, Huang QW, Xu YC, Liu X, Ran W, Shen QR (2008) Control of Fusarium wilt disease of cucumber plants with the application of a bioorganic fertilizer. Biol Fertil Soils 4:1073–1080
Zhang N, Wu K, He X, Li SQ, Zhang ZH, Shen B, Yang XM, Zhang RF, Huang QW, Shen QR (2011) A new bioorganic fertilizer can effectively control banana wilt by strong colonization of Bacillus subtilis N11. Plant Soil 344:87–97
Zhao Q, Dong C, Yang X, Mei X, Ran W, Shen Q, Xu Y (2010) Biocontrol of Fusarium wilt disease for Cucumismelo melon using bio-organic fertilizer. Appl Soil Ecol 47:67–75
Zhao S, Clarke BB, Shen Q, Zhang L, Zhang N (2012) Development and application of a TaqMan real-time PCR assay for rapid detection of Magnaporthe poae. Mycologia 104:1250–1259
Acknowledgments
The authors wish to extend their appreciation to Dr. Luo Jia for his assistance in the laboratory analyses. This work was financially co-sponsered by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), by the National Natural Science Foundation of China for Youth (31301809), by the 111 project (B12009), and by the Chinese Ministry of Agriculture (201103004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, S., Liu, D., Ling, N. et al. Bio-organic fertilizer application significantly reduces the Fusarium oxysporum population and alters the composition of fungi communities of watermelon Fusarium wilt rhizosphere soil. Biol Fertil Soils 50, 765–774 (2014). https://doi.org/10.1007/s00374-014-0898-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-014-0898-7