Abstract
Andosols are characterised by high organic matter (OM) content throughout the soil profile, which is mainly due to the stabilisation of soil organic matter (SOM) by mineral interactions. The aim of the study was to examine whether there were differences in the chemical composition of mineral-associated SOM and free OM in the top A horizon and in the subsoil (horizons below the A11 horizon). Our experimental approach included the replicated sampling of a fulvic and an umbic Andosol under pine and laurel forest located on the island of Tenerife with a Mediterranean sub-humid climate. We determined the extent of the organo-mineral interactions by comparing the sizes of the light (free) and heavy (dense) soil fractions obtained by physical separation through flotation in a liquid with a density of 1.9 g cm–3. We determined the elemental and isotopic composition of both fractions and analysed their chemical composition by analytical pyrolysis. The elemental and isotopic composition showed similar values with depth despite the different vegetation and climatic conditions prevailing at the two sites. Carbon (C) stabilised by mineral interactions increased with depth and represented 80–90% of the total C in the lowest horizons. The heavy fractions mainly released N-containing compounds upon analytical pyrolysis, whereas lignin-derived and alkyl compounds were the principal pyrolysis products released from the light fractions of the top- and subsoil horizons. Principal component analysis showed that the chemical composition of OM stabilised by mineral interaction differs in the different horizons of the soil profile. In the A horizons, the chemical composition of this OM was similar to those of the light fractions, i.e. litter input. There was a gradual change in the bulk molecular composition from a higher contribution of plant-derived molecules in the light and heavy fractions of the A horizon to more microbial-derived molecules as well as black C-derived molecules at depth. We conclude that transport processes in addition to decomposition and possibly in situ ageing affect the chemical composition of mineral-associated OM in subsoils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carbon (C) sequestration in soils is one important strategy to counteract increasing atmospheric CO2 concentrations as it implies a transfer of atmospheric CO2 into the long-living soil organic matter (SOM) pools (Houghton and Goodale 2004). Three processes were introduced to explain C stabilisation in mineral soil (Sollins et al. 1996): (1) chemical recalcitrance of specific organic matter (OM) compounds, (2) protection of OM in soil aggregates and (3) physicochemical protection through the adsorption of OM onto soil minerals. This general concept may be extended by factors affecting microbial decay such as water saturation, extreme acidity and soil burial. Although all these factors inhibit SOM degradation, interaction with soil minerals may be the most important process able to stabilise C for centuries or longer (Kögel-Knabner et al. 2008). At present, we lack information about the C types stabilised by this process. Subsoils may be suitable models to study the composition of stabilised OM because in deep horizons, the interaction of organic C with soil minerals may result in the SOM being stabilised for centuries or millennia (Rumpel and Kögel-Knabner 2011). One difficulty with regard to the study of C in mineral subsoil horizons are concentrations <1%, as found in most soil types of temperate and tropical climates.
Andosols provide a unique opportunity to study OM stabilised in subsoil horizons because they accumulate high quantities of organic C (5–20% weight), estimated to be in the order of 25.4 kg m−2 in the upper 100 cm (Batjes 1996). Andosols cover 0.8% of the global land area, but store 5% of the world’s C (Dahlgren et al. 2004). The high C storage potential of Andosols may be related to their high content of allophane and amorphous Fe and Al oxides (Torn et al. 1997), which form organic matter complexes that are only slowly degradable by the soil microflora. It has been shown that SOM protection in Andosols should be other than physical because OM within soil aggregates was found to be of microbial origin (Buurman et al. 2007). Soil organic matter accumulation in Andosols seems to be related to extractable aluminium (Al3+; Matus et al. 2006). Its stabilisation in Andosols may be derived from the existence of short-range order minerals (allophane, imogolite and ferrihydrite) with considerable potential to form stable complexes with organic molecules (Shoji et al. 1993; Parfitt et al. 1997). In addition, Andosols show a peculiar structure made up of highly stable microaggregates where SOC, as organo-mineral and organometallic complexes (Warkentin and Maeda 1980), is protected from microbial mineralization by physicochemical processes. Recently, this protection mechanism was questioned and SOM protection related to the water saturation and anaerobic conditions in very fine pores (Buurman et al. 2007). For allophanic Andosols, which are considered as natural gels, a C sequestration mechanism related to the fractal structure of allophane aggregates was suggested (Chevallier et al. 2008, 2010).
Most studies on the chemical composition of SOM in Andosols have focussed on the analysis of fractions obtained by alkaline extraction (del Río et al. 1996; Nierop et al. 2005; Buurman et al. 2007; González-Pérez et al. 2007). In general, the results suggest that SOM in Andosols is enriched in carbohydrate- and chitin-derived compounds. When high aromaticity is found, it is usually related to charred plant remains from regular vegetation burning (Golchin et al. 1997). The lack of lignin moieties and the non-aryl nature of SOM in Andosols are probably favoured by an active biosynthesis of secondary compounds by soil biota (fungi and arthropods; Nierop et al. 2005; Buurman et al. 2007).
Using alkaline extraction procedures, it is not possible to distinguish between labile and stabilised OM. Physical fractionation of organic matter by size and/or density may be more appropriate for the separation of plant-derived ‘free’ OM and OM in interaction with the mineral phase (Turchenek and Oades 1979). Plant residues can be separated as particulate organic matter by flotation in density solutions or water, and organo-mineral complexes can be recovered in the heavy fraction (Spycher and Young 1977). Light and heavy fractions may present the extreme points of the decomposition continuum, and the characterisation of their chemical composition thus helps elucidate the changes occurring during microbial decay and stabilisation in different soil types (Grandy et al. 2007). For aerobic topsoils, a decomposition sequence has been postulated. According to this theory, the pathways of molecular C transformations in topsoil horizons lead to the accumulation of microbial-derived compounds in organo-mineral associations and similar chemical composition regardless of the soil type (Grandy and Neff 2008). At present, it is unknown whether this concept applies to OM in subsoil horizons, which are characterised by different processes concerning OM input and transformations compared with topsoils (Rumpel and Kögel-Knabner 2011).
In this study, two forest Andosols from the island of Tenerife (Canary Islands, Spain) were analysed. Samples were taken from all soil horizons and density fractionated into a light fraction containing free particulate OM and a heavy fraction containing mineral-bound OM. The chemical composition of the OM of these samples was characterised by analytical pyrolysis. The aim of our study was to compare the chemical composition of both OM fractions in top- and subsoil horizons in order to obtain information on the origin of OM stabilised by mineral interactions.
Material and methods
Sampling sites and soils
Andosols are well represented in the Canary Islands and are usually distributed between 700- and 1,500-m altitude within the influence area of alisio trade winds. This area has a thermo-Mediterranean mesophytic sub-humid bioclimate (Rivas-Martínez et al. 1993) with annual precipitation ranging between 500 and 900 mm, a mean annual temperature between 14°C and 16°C and a potential evapotranspiration of 750–800 mm year−1. These conditions favour the udic soil moisture regime and thermic soil temperature regime. The classification of the studied soils was done following the FAO system (IUSS Working Group WRB 2007). For this study, two representative soils with andic properties were selected. ‘Ravelo’ is a Fulvic Andosol (Ultic Fulvudand) located in a reforested area with Monterrey pine (Pinus radiata D. Don). ‘Las Lajas’ is an Umbric Andosol (Ultic Hapludand) under endemic humid subtropical laurel forest (Laurisilva; González-Pérez et al. 2007). Both sites have a fire history. General parameters of both soils are presented in Table 1.
Three horizons (A11, A12 and B) of the two soils were sampled. Samples were taken in triplicate from the different sides of the soil profile. Fraction separation and other analyses were performed on air-dried fine earth (<2 mm).
Density fractionation
Density fractionation was carried out with a potassium polytungstate solution with a density of 1.9 g cm–3 (Basile-Doelsch et al. 2007). Briefly, 2–5 g of soil was mixed with 20 mL of polytungstate. The soil suspension was centrifuged at 10×g for 10 min. The supernatant was removed by filtration and the procedure repeated two to five times until complete recovery of the light fraction. Afterwards, polytungstate was removed by washing with distilled water, and the two fractions were freeze-dried and ground for further analysis.
Elemental analysis
Organic C and N contents were determined by dry combustion using a CHN auto-analyser (CHN NA 1500, Carlo Erba) coupled to an isotopic ratio mass spectrometer (VG Sira 10), yielding the ratio of stable OC isotopes (δ13C). Stable N isotope ratios (δ15N) were determined with a CHN analyser coupled with an Isochrom III isotopic ratio mass spectrometer (Micromass-GVI Optima). The results for isotope abundance are reported in per mil (‰) relative to the Pee Dee Belemnite standard and relative to air N2 for δ13C and δ15N, respectively. Accuracy of the elemental analysis was ±0.1 mg g−1 for OC and ±0.05 mg g−1 for N content. Accuracy of isotope measurements was ±0.3‰.
14C activity
The 14C concentrations of the soil samples were measured at the Leibniz-Labor (University of Kiel) using accelerator mass spectrometry (AMS). For these measurements, CO2 was obtained from solid samples by combustion at 900°C. The CO2 was reduced to graphite which was subsequently analysed by accelerated mass spectrometry (Nadeau et al. 1998). The 14C activity was corrected for isotopic fractionation and the radiocarbon age reported according to Stuiver and Polach (1977). The AMS measurements are typically reproducible at 0.3 pMC (per cent modern carbon).
Analytical pyrolysis
Pyrolysis was performed using a double-shot pyrolyzer (Frontier Laboratories, model 2020) attached to a GC/MS system Agilent 6890N. Samples (5–8 mg) in a crucible capsule were placed in the micro-oven at an initial temperature of 100°C that was increased at a rate of 20°C min−1 to a final pyrolysis temperature of 500°C for 1 min. The temperature programme allowed for the removal of all volatile compounds through thermosorption before pyrolysis. The GC was equipped with a fused silica capillary column DB5 MS (J&W Scientific, 30-m × 250-μm × 0.25-μm film thickness); oven temperature was held at 50°C for 1 min and then increased up to 100°C at 30°C min−1, from 100°C to 300°C at 10°C min−1 and isothermal at 300°C for 10 min using a heating rate of 20°C min−1 in the scan modus. The carrier gas used was helium with a controlled flow of 1 mL min−1. The detector consisted of an Agilent 5973 mass-selective detector, and mass spectra were acquired with a 70-eV ionizing energy. The identification of individual compounds was carried out using single-ion monitoring for different homologous series, low-resolution mass spectrometry and comparison with published and stored data (NIST and Wiley libraries). The peak areas were calculated based on total abundance, considering the summation of the areas of all peaks as 100% of the total ion chromatogram (TIC). Peaks with over 1% total area were considered.
Statistical analysis
Principal component analysis (PCA) with 14 samples and 155 variables was performed after transforming the data using the Hellinger transform to circumvent problems associated with the Euclidean distances when the data matrices contain many zeros (Legendre and Gallagher 2001). Multivariate analysis was performed with ‘Vegan: Community Ecology Package’ (Oksanen et al. 2006) in R version 2.9.0 (R Development Core Team 2009).
Results and discussion
Elemental and isotopic content
Carbon content ranged from 120–200 g kg−1 in the A11 horizons to 16–30 g kg−1 in the Bw horizons (Table 2). Nitrogen (N) contents ranged from 1.7 to 14 g kg−1, leading to C/N ratios between 10 and 16 (Table 2). The values, especially in subsoil horizons, are much higher compared with other mineral soil types (Batjes 1996) and illustrate the capacity of Andosols to store high amounts of C. With increasing soil depth, the stable C and N isotope signatures became enriched in 13C and 15N, and in both soils, the 14C activity of SOM decreased from modern in the top A horizon to 75 and 78 pMC in the lowest soil horizon, corresponding to a radiocarbon age of around 2000 years BP (Table 2). Enrichment in stable C and N isotopes of SOM with increasing soil depth is observed in most studies and is often related to the fact that SOM in subsoil horizons is in general more transformed and enriched in strongly degraded plant material and/or microbial-derived compounds (e.g. Högberg 1997; Boström et al. 2007). This is corroborated by the low C/N ratios (Table 2). However, the changes of the stable C isotope signature with depth have also been related to the chemical composition of SOM, which depends on soil-inherent stabilisation mechanisms (Krull and Skjemstad 2003). The depth trends of 15N may be strongly controlled by ectomycorrhizal fungi activity, which was found to lead to a significant enrichment in 15N (Lindahl et al. 2007). However, whereas ectomycorrhizae may be present in pine forest, its occurrence in laurel forest is less evident as the aromatic oil produced by laurel may inhibit mycorrhizae growth (Hassiotis and Dina 2011). Considering the very similar changes with depth occurring for both soils, the 15N enrichment with depth may be best explained by turnover and accretion of 15N-enriched microbial compounds (Huygens et al. 2008).
Density fractionation
Density fractionation was carried out to isolate SOM present as plant residues from SOM bound to soil minerals. The C content of the light fractions ranged in most cases between 20% and 30% (Table 3). These C contents were relatively low compared with those found by other authors for light fractions (e.g. Sollins et al. 1983, 2009) and could be due to the use of SPT with a density of 1.9 g cm−3, which might mean that the fraction included some inorganic material. Densities used in other studies to separate the light fraction from volcanic material ranged between 1.35 and 1.7 g cm−3 (Huygens et al. 2005, 2008; Sollins et al. 2006, 2009; Prior et al. 2007). In this study, a density of 1.9 g cm–3 was chosen because we wanted to be sure to remove all light materials and thus tolerated a higher amount of minerals present in this fraction. However, we consider the fraction >1.9 g cm–3 to contain mostly OM not associated with soil minerals (Basile-Doelsch et al. 2007). The carbon contents of the dense fractions were strongly reduced and decreased with depth in both soils (Table 3). Nitrogen followed a similar trend. The resulting C/N ratios ranged between 8 and 51 and were higher for the light fractions than for the heavy ones (Table 3), showing that higher amounts of relatively undecomposed plant material were contributing to these fractions. The much lower C/N ratio of SOM in the heavy fractions is in line with the protection of organic N by soil minerals against microbial decay (Nannipieri and Paul 2009). The unusually high C/N ratio of the light fractions from the B horizons may be explained by the N limitation of the system, where N-containing compounds are the first to be transformed by the soil microflora. In the top A horizon of both soils, much higher proportions of C and N were found in the light fractions compared with the heavy ones, which contained the mineral-bound SOM. In fact, the light fraction contributed 65–80% of SOM in the top A horizon (Table 3 and Fig. 1). High contribution of the light fraction to the total C in A horizons of volcanic soils was also found by other authors (Sollins et al. 1983). This proportion very much decreased with increasing soil depth, and the contribution of SOM in the heavy fractions increased to more than 90% in the deeper B horizon. A similar decrease of particulate organic matter contribution with increasing soil depth was recorded for Andosols (Spycher et al. 1983) as well as for other soil types (Kaiser et al. 2002; Jagadamma and Lal 2010). This may be explained by the fact that SOM in the subsoil is intimately associated with soil minerals and that fresh C input in the form of root material is scattered (Chabbi et al. 2009). Therefore, in subsoil horizons, organic matter transported by water or bioturbation may represent a higher proportion of the total input and may thus be more important as a precursor of stabilised SOM than particulate root material (Kaiser and Guggenberger 2000). In Andosols, physical transport of colloidal Fe/Al–humus complexes in deep soil horizons was found to be an important process increasing SOM (Osher et al. 2003). The association of SOM with the mineral phase has implications for the degree of stabilisation of SOM as measured by radiocarbon activity: a strong correlation between the 14C activity of bulk soil and the amount of C associated with the dense fractions was found for both soils (r 2 = 0.95, Fig. 2). Such a correlation is in line with the results from other authors, suggesting that the heavy fraction contains larger proportions of passive SOM (Prior et al. 2007). It has been stated that interaction with the mineral phase is the only SOM stabilisation mechanism which can lead to century-old or more stable C (Kögel-Knabner et al. 2008). In Andosols, which are regarded as natural gels (Chevallier et al. 2008), stabilisation by the formation of aggregates may be the dominant process even in subsoil horizons.
Analytical pyrolysis
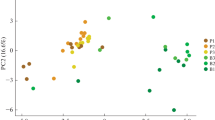
Analytical pyrolysis was carried out for the light fractions of A11 and A12 horizons, containing plant material. Unfortunately, the recovery of light fractions from B horizons was insufficient to realise this analysis. Additionally, the heavy fractions containing organo-mineral complexes have been examined. One representative pyrogram for each of the two fractions is presented in Fig. 3. The identified pyrolysis products derived from lignin, polysaccharides, aliphatic and N-containing compounds are presented in Table 4. The light fraction pyrogram contained more pyrolysis products than the pyrogram of the heavy fractions (Fig. 3). Heavy fractions of the subsoil horizons had the highest relative contribution of N-containing pyrolysis products, whilst the highest contribution of unspecific compounds was found in the light fraction of the topsoil horizon (Fig. 4). Lignin-derived pyrolysis products were found in higher proportions in the light fractions compared with the heavy fractions (Fig. 4). The nature of lignin-derived pyrolysis products recovered from the two soils differed (data not shown). The pyrograms of the Andosol under pine forest contained syringyl-type methoxy phenols, which are typical pyrolysis products of angiosperm lignin. In the pyrograms of the Andosol under laurel forest, additionally, vanillyl-type di-methoxy phenols, characteristic for gymnosperm lignin, were present. Polysaccharide-derived pyrolysis products contributed similarly to both density fractions from the top- and subsoil horizons. Black C-derived compounds, such as naphthalene and methyl-naphthalene, were most enriched in the heavy fraction of subsoil horizons. Analytical pyrolysis presents several advantages (i.e. multiple molecular information, small sample amount required), but also has drawbacks because special techniques of data exploitation are needed to treat the huge amount of data generated by this method. Moreover, several authors report artefacts due to the presence of soil minerals, which lead to new formation of pyrolysis products due to catalytic action and the retention of pyrolysis products (Zegouagh et al. 2004). The influence of soil minerals may be reduced in our case because Andosols are rich in organic C. The problem of exploitation of a huge data set was solved by applying statistical techniques specially adapted to this kind of data (Rumpel et al., 2009; see “Material and methods”). PCA showed that there were clear differences in the composition of the organic matter in the heavy and light fractions (Fig. 5). The heavy and light fractions were separated along the first axis (representing 33% of the total variability in the data) of the ordination plot. The PC loadings indicated that the difference was primarily due to a higher relative abundance of a number of N-containing pyrolysis products and compounds indicative of the presence of black C in the heavy fractions and relatively more molecules derived from lignin and alkyl compounds in the light fractions (Fig. 4). Although the origin of N-containing compounds is not entirely clear, a large part may be derived from chitin in soil fungi and micro/mesofauna (Stankiewicz et al. 1996) and also from amino acids and proteins (Bracewell and Robertson 1984; Chiavari and Galletti 1992; van Bergen et al. 1998).
Pyrograms from the light and heavy fractions of the A2 horizons (10–55 cm) of the soil sampled in ‘Las Lajas’. Peak labels refer to Table 4
There were also differences among horizons. In the light fractions, these were visible along the second ordination axis, which accounted for 24% of the total variability in the data. The separation was due principally to relatively more N-containing pyrolysis products in the A12 horizon and more alkyl, lignin-derived compounds or compounds of unspecific origin in the A11 horizons. Among the heavy fractions, the A11 horizon was separated from the A12 and B horizons. This indicates differences in the chemical composition of the OM present in close association with soil minerals (heavy fractions) in the top- and subsoil horizons despite a similar chemical composition of plant litter input (light fractions). The differences were visible along both ordination axes, but the reasons for the separation were less clear-cut. There were N-containing pyrolysis products in each horizon as well as compounds indicative of black C, alkyl and polysaccharide compounds (Fig 4). The good separation of A11 and A12/B horizons despite similar chemical compounds in these horizons might be explained by contrasting C inputs and the different nature and intensity of stabilisation processes in the subsoil horizons compared with the topsoil. Such a hypothesis is supported by the observation that the biotic conditions in terms of root growth, microbial activity, community composition and faunal activity are reduced in the subsoil compared with the A horizons (Fang and Moncrieff 2005; Taylor et al. 2002; Wilkinson et al. 2009). Root litter and/or root exudates most likely contribute more to the SOM stored in subsoil horizons as well as water-transported colloidal material or dissolved organic matter (Rumpel and Kögel-Knabner 2011). On the other hand, our results indicate that at the two sites, stable black C compounds produced by fire may have been subject to vertical transport either with water flow or bioturbation (Forbes et al. 2006) and accumulate in subsoil horizons, thus changing their pyrolysis signature. Moreover, the degree of stabilisation in terms of adsorption to the mineral phase or inclusion into the fractal matrix of Andosols must be considered. In topsoil, such a stabilisation might not be as strong as in subsoil horizons and might have affected less C compounds. Therefore, OM of the heavy fraction from subsoil may yield other products compared with those from topsoil upon pyrolysis.
The enrichment of N-containing compounds in the pyrograms of heavy fractions from A horizons was also found by Grandy and Neff (2008) who stated that lignin exerts little influence on more stable mineral-associated matter, which is mainly composed of microbially processed OM rather than plant-derived compounds. These authors suggested that the molecular dynamics of SOM in topsoil follows a decomposition sequence from plant litter to organo-mineral complexes, which contain microbial-derived material. Similar results were found in a pyrolysis study with density fractions of A horizons of two Andosols of different ages (80–10,000 years; Prior et al. 2007). These authors showed a shift from lignin-derived SOM to more polysaccharide-derived OM with increasing age. The results from our study suggest that for subsoil horizons, additional processes could influence the decomposition sequence. Moreover, because of the long mean residence times of SOM in subsoils, OM of the heavy fraction might have been subject to a more important ageing process than in topsoils.
Conclusion
The characterisation of the molecular composition of SOM in light and dense fractions isolated from the top- and subsoil horizons of two Andosols showed that mineral-bound SOM in the heavy fractions of A horizons and all light fractions, containing mostly fresh plant material, have a similar composition. As indicated by elemental analysis, SOM of heavy fractions in subsoils is enriched in N and has a contrasting molecular composition. Pyrograms of the heavy fraction of subsoil horizons show a decreased relative contribution of alkyl compounds and lignin-derived compounds and increased contribution of black C-derived compounds compared with those of topsoils. Nitrogen-containing compounds are the most important ones in heavy fractions of subsoil horizons. Principal component analysis suggests that the OM composition of light fractions and heavy fractions does not show abrupt changes, but shows a gradual change from a higher contribution of plant litter in both density fractions of the topsoil horizon to more microbial-derived OM and extremely stable OM compounds like black C in the stabilised mineral-associated OM of subsoils. It is not clear whether these changes are related to a different origin (in situ formation or input of transported material) or a result of an in situ ageing process. Recently, it has been shown that different OM pools react differently upon climate change (von Lützow and Kögel-Knabner 2009). How these changes affect the fate of OM in mineral association within the A horizon and deeper in the soil profile is still unknown.
References
Basile-Doelsch I, Amundson R, Stone WEE, Borschneck D, Bottero JY, Moustier S, Masin F, Colin F (2007) Mineral control of carbon pools in a volcanic soil horizon. Geoderma 137:477–489
Batjes NH (1996) Total carbon and nitrogen in the soils of the world. Eur J Soil Sci 47:151–163
Boström B, Comstedt C, Ekblad A (2007) Isotope fractionation and 13C enrichment in soil profiles during the decomposition of soil organic matter. Oecologia 153:89–98
Bracewell JM, Robertson GW (1984) Quantitative comparison of the nitrogen-containing pyrolysis products and amino acid composition of soil humic acids. J Anal Appl Pyrol 6:19–29
Buurman P, Peterse F, Almendros G (2007) Soil organic matter chemistry in allophanic soils: a pyrolysis-GC/MS study of a Costa Rican Andosol catena. Eur J Soil Sci 58:1330–1347
Chabbi A, Kögel-Knabner I, Rumpel C (2009) Stabilised carbon in subsoil horizons is located in spatially distinct parts of the soil profile. Soil Biol Biochem 41:256–271
Chevallier T, Woignier T, Toucet J, Blanchart E, Dieudonne P (2008) Fractal structure in natural gels: effect on carbon sequestration in volcanic soils. J Sol–Gel Sci Techn 48:231–238
Chevallier T, Woigner T, Toucet J, Blanchart E (2010) Organic carbon stabilization in the fractal pore structure of Andosols. Geoderma 159:182–188
Chiavari G, Galletti GC (1992) Pyrolysis gaschromatography mass spectrometry of amino acids. J Anal Appl Pyrol 24:123–137
Dahlgren RA, Saigusa M, Ugolini FC (2004) The nature, properties and management of volcanic soils. Adv Agron 82:113–182
del Río JC, Martín F, Gonzalez-Vila FJ (1996) Thermally assisted hydrolysis and alkylation as a novel pyrolytic approach for the structural characterization of natural biopolymers and geomacromolecules. Trac-Trend Anal Chem 15:70–79
Fang C, Moncrieff JB (2005) The variation of soil microbial respiration with depth in relation to soil carbon composition. Plant Soil 268:243–253
Forbes MS, Raison RJ, Skjemstad JO (2006) Formation, transformation and transport of black carbon (charcoal) in terrestrial and aquatic ecosystems. Sci Total Environ 370:190–206
Golchin A, Clarke P, Baldock JA, Higashi T, Skjemstad JO, Oades JM (1997) The effects of vegetation and burning on the chemical composition of soil organic matter in a volcanic ash soil as shown by 13C NMR spectroscopy. I. Whole soil and humic acid fraction. Geoderma 76:155–174
González-Pérez JA, Arbelo CD, González-Vila FJ, Rodríguez-Rodríguez A, Almendros G, Armas CM, Polvillo O (2007) Molecular features of organic matter in diagnostic horizons from andosols as seen by analytical pyrolysis. J Anal Appl Pyrolysis 80:369–382
Grandy AS, Neff JC (2008) Molecular C dynamics downstream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci Total Environ 404:297–307
Grandy AS, Neff JC, Weintraub MN (2007) Carbon structure and enzyme activities in alpine and forest ecosystems. Soil Biol Biochem 39:2701–2711
Hassiotis CN, Dina EI (2011) The effects of laurel (Laurus nobilis L.) on development of two mycorrhizal fungi. Int Biodeter Biodegr 65:628–634
Högberg P (1997) Tansley review No. 95. 15N natural abundance in soil–plant systems. New Phytol 137:179–203
Houghton RA, Goodale CL (2004) Effects of land-use change on the carbon balance of terrestrial ecosystems. Geoph Monog Series 153:85–98
Huygens D, Boeckx P, Van Cleemput O, Oyarzun C, Godoy R (2005) Aggregate and soil organic carbon dynamics in South Chilean Andisols. Biogeosciences 2:159–174
Huygens D, Denef K, Vandeweyer R, Godoy R, Van Cleemput O, Boeckx P (2008) Do nitrogen isotope patterns reflect microbial colonization of soil organic matter fractions? Biol Fertil Soils 44:955–964
IUSS Working Group WRB (2007) World Reference Base for Soil Resources 2006, first update 2007. World Soil Resources Reports No. 103. FAO, Rome
Jagadamma S, Lal R (2010) Distribution of organic carbon in physical fractions of soils as affected by agricultural management. Biol Fertil Soils 46:543–554
Kaiser K, Guggenberger G (2000) The role of DOM sorption to mineral surfaces in the preservation of organic matter in soils. Org Geochem 31:711–725
Kaiser K, Eusterhues K, Rumpel C, Guggenberger G, Kögel-Knabner I (2002) Stabilisation of organic matter by soil minerals—investigations of density and particle-size fractions of two acid forest soils. J Plant Nutr Soil Sc 165:451–459
Kögel-Knabner I, Guggenberger G, Kleber M, Kandeler E, Kalbitz K, Scheu S, Eusterhues K, Leinweber P (2008) Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry. J Plant Nutr Soil Sc 171:61–83
Krull ES, Skjemstad JO (2003) δ13C and δ15N profiles in 14C-datied Oxisol and Vertisols as a function of soil chemistry and mineralogy. Geoderma 112:1–29
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Hogberg P, Stenlid J, Finlay RD (2007) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol 173:611–620
Matus F, Amigo X, Kristiansen SM (2006) Aluminium stabilization controls organic carbon levels in Chilean volcanic soils. Geoderma 132:158–168
Nadeau M-J, Grootes PM, Schleicher M, Hasselberg P, Rieck A, Bitterling M (1998) Sample throughput and data quality at the Leibniz-Labor AMS Facility. Radiocarbon 40:239–245
Nannipieri P, Paul EA (2009) The chemical and functional characterization of soil N and its biotic components. Soil Biol Biochem 41:2357–2369
Nierop GJ, van Bergen PF, Buurman P, van Lagen B (2005) NaOH and Na4P2O7 extractable organic matter in two allophanic volcanic ash soils of the Azores Islands—a pyrolysis GC/MS study. Geoderma 127:36–51
Oksanen J, Kindt R, Legendre P, Hoara R (2006) VegaV: Community Ecology Package, Version 1.8-3
Osher LJ, Matson PA, Amundson R (2003) Effect of land use change on soil carbon in Hawaii. Biogeochemistry 65:213–232
Parfitt RL, Theng BKG, Whitton JS, Shepherd TG (1997) Effects of clay minerals and land use on organic matter pools. Geoderma 75:1–12
Prior CA, Baisden WT, Bruhn F, Neff JC (2007) Using a soil chronosequence to identify soil fractions for understanding and modeling soil carbon dynamics in New Zealand. Radiocarbon 49:1093–1102
R Development Core Team (2009) R: a language and environment for statistical computing. http://www.Rproject.org
Rivas-Martínez S, Wildpret W, Díaz TE, Pérez de Paz PL, del Arco M, Rodríguez O (1993) Itinera Geobot. 75
Rumpel C, Chabbi A, Nunan N, Dignac MF (2009) Impact of landuse change on the molecular composition of soil organic matter. J Anal Appl Pyrol 85:431–434
Rumpel C, Kögel-Knabner I (2011) Deep soil organic matter—a key but poorly understood component of terrestrial C cycle. Plant Soil 338:143–158
Shoji S, Nanzyo N, Shirato Y, Ito T (1993) Chemical kinetics of wheathering in young Andisols from Northeastern Japan using soil age normalized to 10°C. Soil Sci 155:53–60
Sollins P, Spycher G, Topik C (1983) Processes of soil organic matter accretion at a mudflow chronosequence, Mt Shasta California. Ecology 64:1273–1282
Sollins P, Homann P, Caldwell BA (1996) Stabilization and destabilization of soil organic matter: mechanisms and controls. Geoderma 74:65–105
Sollins P, Swanston C, Kleber M, Filley T, Kramer M, Crow S, Caldwell BA, Lajtha K, Bowden R (2006) Organic C and N stabilization in a forest soil: evidence from sequential density fractionation. Soil Biol Biochem 38:3313–3324
Sollins P, Kramer MG, Swanston C, Lajtha K, Filley T, Aufdenkampe AK, Wagai R, Bowden RD (2009) Sequential density fractionation across soils of contrasting mineralogy: evidence for both microbial- and mineral-controlled soil organic matter stabilization. Biogeochemistry 96:209–231
Spycher G, Young JL (1977) Density fractionation of water dispersable soil organic mineral particles. Commun Soil Sci Plan 8:37–48
Spycher G, Sollins P, Rose S (1983) Carbon and nitrogen in the light fraction of a forest soil—vertical distribution and seasonal patterns. Soil Sci 135:79–87
Stankiewicz BA, van Bergen PF, Duncan IJ, Carter JF, Briggs DEG, Evershed RP (1996) Recognition of chitin and proteins in invertebrate cuticles using analytical pyrolysis/gas chromatography/mass spectrometry. Rapid Commun Mass Sp 10:1747–1757
Stuiver M, Polach HA (1977) Discussion: reporting of 14C data. Radiocarbon 19:355–363
Taylor JP, Wilson B, Mills MS, Burns RG (2002) Comparison of microbial numbers and enzymatic activities in surface soils and subsoils using various techniques. Soil Biol Biochem 34:387–401
Torn MS, Trumbore SE, Chadwick OA, Vitousek PM, Hendricks DM (1997) Mineral control over soil carbon storage and turnover. Nature 389:170–173
Turchenek LW, Oades JM (1979) Fractionation of organo-mineral complexes by sedimentation and density techniques. Geoderma 21:311–343
van Bergen PF, Flannery MB, Poulton PR, Evershed RP (1998) Organic geochemical studies of soils from the Rothamsted Classical experiments: III. Nitrogen-containing organic matter in soil from Geescroft wilderness. ACS Symposium Series, vol. 707. In: Stankiewicz BA, van Bergen PF (eds) Nitrogen-containing macromolecules in the bio- and geosphere. Oxford University Press, New York, pp 321–338
Von Lützow M, Kögel-Knabner I (2009) Temperature sensitivity of soil organic matter decomposition—what do we know? Biol Fertil Soils 46:1–15
Warkentin BP, Maeda T (1980) In: Theng BKG (ed) Soils with variable charge. New Zealand Society of Soil Science, Lower Hutt, New Zealand, pp 281–301
Wilkinson MT, Richards PJ, Humphreys GS (2009) Breaking ground: pedological, geological and ecological implications of soil bioturbation. Earth Sci Rev 97:257–272
Zegouagh Y, Derenne S, Dignac MF, Baruiso E, Mariotti A, Largeau C (2004) Demineralisation of a crop soil by mild hydrofluoric acid treatment. Influence on organic matter composition and pyrolysis. J Anal Appl Pyrolysis 71:119–135
Acknowledgements
Financial support was provided by EGIDE under the framework of the French–Spanish exchange programme Piccasso. The authors thank Master’s student AnnIsabelle Scian for the laboratory work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rumpel, C., Rodríguez-Rodríguez, A., González-Pérez, J.A. et al. Contrasting composition of free and mineral-bound organic matter in top- and subsoil horizons of Andosols. Biol Fertil Soils 48, 401–411 (2012). https://doi.org/10.1007/s00374-011-0635-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-011-0635-4