Abstract
A 15N dilution experiment was carried out to investigate effects of cultivation on the gross N transformation rate in coastal wetland zone. Microbial community composition was estimated by phospholipid fatty acid (PLFA) analysis and abundance of soil ammonia-oxidizing bacteria (AOB) was quantified by real-time polymerase chain reaction (PCR). Soil salinity decreased significantly, while total N increased after coastal wetland was cultivated. Microbial biomass (total PLFA), bacterial biomass, fungal biomass, and actinomycete biomass of the native coastal wetland soils were significantly (p < 0.05) lower than those of the cultivated soils whereas AOB population size also significantly increased after coastal wetland cultivation. Multiple regression analysis showed that total PLFA biomass and soil total N (TN) explained 97% of the variation of gross N mineralization rate in the studied soils (gross mineralization rate = 0.179 total PLFA biomass + 5.828TN − 2.505, n = 16, p < 0.01). Gross nitrification rate increased by increasing the soil AOB population size and gross mineralization rate (M) (gross nitrification rate = 3.39AOB + 0.18 M − 0.075, R 2 = 0.98, n = 16, p < 0.01). Management of salt discharge and mineral N fertilization during the cultivation of wetland soils might have changed composition of soil microflora and AOB population size, thus influencing mineralization and nitrification. Probably, the cultivation of coastal wetland soils increased the risk of N losses from soil through nitrate leaching and gas emission (e.g., N2O and NO).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal wetland, which is an important transitional ecosystem between terrestrial and ocean ecosystem, plays a crucial role in balancing and sequestrating C, N and P in the ecosystem and protecting biodiversity. However, human disturbance (mainly drainage and cultivation) along the coast has destroyed >80% of the region's coastal wetland area in Jiangsu province in China and thus increased concentration of reactive N in soil due to fertilization and changes in the soil N cycling. While net mineralization and net nitrification rates can provide an index of plant available N, they do not provide an understanding of the respective processes (Hart et al. 1994). Gross N transformation rates can provide important information on the mechanisms and dynamics of the internal N cycle, since they quantify the process rates. The 15 N dilution technique is the most widely used method to quantify gross N transformation rates in soil (Stark 2000) and it is based on the enrichment of the ammonium and nitrate pool with the stable isotope 15 N. However, few studies have been carried out to study the effects of cultivation on the gross N transformation rate in the coastal wetland zone.
Because soil N transformations are directly driven by microbial activity (Martens 1995), the production and consumption of inorganic N is a function of microbial biomass, microbial activity and exoenzyme production (Schimel and Bennett 2004). Determination of community level PLFA profiles can be useful in detecting changes of the composition of soil microbial communities to a variety of land uses or disturbances (Hedrick et al. 2000; Harris 2003), and some PLFA marker can be used as indicators of some soil microbial functional groups (Zak et al. 1994; Zelles 1997; Zogg et al. 1997). Ammonia oxidation is thought to be the rate-limiting reaction of nitrification in most ecosystems (Kowalchuk and Stephen 2001). In arable soils, most of the ammonia oxidization is carried out by autotrophic ammonia-oxidizing bacteria (AOB) (Barraclough and Puri 1995) and thus it is important to monitor the size and activity of AOB population under different soil N conditions. Real-time polymerase chain reaction (PCR), targeting 16S rRNA gene (Hermansson and Lindgren 2001) and amplification of ammonia monooxygenase gene amoA (Okano et al. 2004) have been successfully used to quantify AOB population size in soil. Changes in land use and intensive cropping can alter N cycling in soil, resulting in increased N losses (NO, N2O emission, NO −3 leaching) (Meng et al. 2005; Ding et al. 2010). It is hypothesized that land use/management changes of the coast wetland can change composition, activity and size of soil microbial communities, including AOB community, with effects on soil N transformations.
The objectives of this study were i) to compare the gross mineralization, nitrification and immobilization rates and the emissions of N2O and NO between cultivated coastal wetland soils and native coastal wetland soils and ii) to investigate the effects of coastal wetland cultivation on soil gross N transformation and N2O and NO production. Microbial community composition was estimated by phospholipid fatty acid (PLFA) analysis and the population size of AOB was quantified by real-time PCR.
Materials and methods
Site description
The study sites are located in Jiangsu province, China (33°24′–34°06′ N, 119°59′–120°33′ E), which covers an area of 2,795 km2 with a typical monsoon climate. The mean annual precipitation is 1,032 mm (30-year average), about 65% of which occurs from June to September. The mean annual temperature is 13.8°C (30-year average).
Sites and experimental plan
We chosen two cultivated soils: one was cropped continuously with Brassica rapa chinensis for about 9 years (B) and was annually fertilized with about 150 kg N ha−1 a−1; the other was cropped continuously with Hordeum uhulgare L for 14 years (H) and was annually fertilized with about 150 kg N ha−1 a−1. Two native coastal wetland soils: one with Spartina anglica (S) as the dominating vegetation, whereas the other had a dominating vegetation Phragmites australis (P). The cultivated and wetland soils were adjacent (within 1,000 m) and had similar altitude. Before wetland was cultivated, the dominating vegetations were S. anglica (S) and P. australis (P). Soil samples were collected in April 2010. In each site, a representative 500 m × 500 m plot was selected. Then, four grids (each about 10 m × 10 m, the distance among grids was not shorter than 200 m, so four soil samples were independently taken from each site) were randomly selected. From each grid, ten soil cores (dia. 5 cm) were taken (0–10 cm), pooled together, sieved (<3 mm), and stored at 4°C for less than 1 month before the experiment.
There were two treatments (each with three replicates): in one, the ammonium pool was labeled with (15NH4)2SO4 (20 at.% excess); in the other, the nitrate pool was labeled with K15NO3 (20 at.% excess). Fresh soil (30 g oven-dry basis) was put in a 250-ml Erlenmeyer flask. Two milliliters of (15NH4)2SO4 or K15NO3 solution was added to each flask at a rate of 50 μg NH +4 -N g−1 soil or 50 μg NO −3 -N g−1 soil. The soil was adjusted to 50% water filled pore space and incubated for 24 h at 25°C, which corresponds to the temperature at soil sampling. The flasks were sealed with silicone rubber stoppers. Soils were extracted at 0.5 and 24 h after fertilizer application for the determination of concentration and isotopic composition of NH +4 and NO −3 . Rates of N mineralization, nitrification and immobilization were calculated using the equations by Kirkham and Bartholomew (1954).
N2O and NO emission rates
Six incubation bottles with fresh soil (30 g oven-dry basis) were used to measure the N2O and NO emission rates at 6, 12, 24, 48, 96 and 144 h after the onset of incubation. Two milliliters of NH4NO3 solution was added to each flask at a rate of 50 μg NH +4 -N g−1 soil and 50 μg NO −3 -N g−1 soil. The bottles were allowed to aerate for approximately 30 min prior to being capped with a butyl rubber stopper. Three bottles treated with C2H2 (10 Pa) to inhibit autotrophic nitrification and the others (no C2H2) were used to measure the total N2O and NO emission. The incubation bottles were sealed and incubated for 4 h and the headspace gas was sampled using a syringe. Each gas sample was spitted with two subsamples, one for NO measurement and the other for N2O measurement. Prior to NO determination, the 20-ml gas samples were diluted to 1,000 ml using highly purified N2 (>99.9%). The sample NO concentration was measured using an EC 9841 T NO x analyzer (ECOTECH, chemiluminescence detector). The N2O concentration was determined by a gas chromatograph (Agilent 7890) equipped with an electron capture detector set at 300°C. Gas was passed through a 3-m long analytical column (i.d. 2 mm) packed with Porapak Q (80/100 mesh). The column temperature was maintained at 40°C and the carrier gas was argon–methane (5%) at a flow rate of 30 ml/min.
Soil analyses
Soil properties were determined as described by Zhang et al. (2009). Soil pH was measured in a 1:2.5 (v/v) soil to water ratio using a DMP-2 mV/pH detector (Quark Ltd., Nanjing, China). Soil organic C (SOC) was analyzed by wet digestion with H2SO4–K2Cr2O7 and total N was determined by semimicro Kjeldahl digestion using Se, CuSO4 and K2SO4 as catalysts. Ammonium and NO −3 were extracted with 2 M KCl at a soil/solution ratio of 1:5 on a mechanical shaker for 60 min at 300 rpm at 25°C. The extract was filtered through a filter paper and the concentrations of NH +4 and NO −3 were determined with a continuous flow analyzer (Skalar, Breda, Netherlands). Salinity was measured by simple electrical conductivity (EC) using a soil extract (1:5, soil:water). Isotopic composition of the NH +4 , NO −3 , and soil organic N was measured by mass spectrometry (Integra C N mass spectrometer). Ammonium and nitrate were separated for 15 N measurements by distillation with magnesium oxide and Devarda's alloy.
PLFA analyses
Microbial biomass was estimated as total extractable PLFA; PLFA profiles were analyzed to determine microbial community composition. Briefly, lipids were extracted from 0.5 g soil as already reported (Bligh and Dyer 1959; Yao et al. 2006). Fatty acids extracted from soil were automatically quantified and identified by the MIDI Sherlock software (MIDI, Newark, DE, USA). The abundance of individual fatty acid methyl esters was expressed as mole percentage. Nomenclature of fatty acids followed that used by Frostegård et al. (1993). The fatty acids i12:0, i13:0, i14:0, i15:0, a15:0, i16:0, i17:0, 17:0cy, 18:lm7c and 19:0cy were used as indicators of bacterial PLFAs. The unsaturated PLFAs 18:1ω9, 18:2 ω6,9 and 18:3ω6 were used as indicators of fungal biomass. The monoenoic and cyclopropane unsaturated PLFAs 10Me16:0, 10Me17:0, 10Me18:0, i12:0, i13:0, i14:0, i15:0, a15:0, i16:0, a16:0, i17:0 and a17:0 were chosen to represent gram-positive bacteria (G+). Finally, the branched, saturated PLFAs 3OH 11:0, 3OH i11:0, 12:0, 3OH 12:0, 3OH 13:0, 14:0, 3OH 15:0, 3OH i15:0, 16:0, 2OH 16:1, 3OH i16:0, 16:1 ω5, 16:1 ω7, 17:0, 3OH i17:0, 18:1 ω5, 18:1 w6, 18:1 ω7, cy17:0, cy19:0 and 11Me18:1ω7 were chosen to represent gram-negative bacteria (G−).
Real-time PCR
Soil total DNA extraction and purification has been described before (Chu et al. 2008). The primer pair amoA-1 F (forward) and amoA-2R (reverse) were used for PCR amplification targeting the gene amoA (Rotthauwe et al. 1997). The reaction mixture contained 25 μl of Platinum® SYBR® Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA, USA), 0.2 μM of each primer, 1 μl of ROX Reference Dye and 1 μl of 10-time diluted DNA template in a final volume of 50 μl. Real-time PCR was conducted using an ABI7000 (Applied Biosystem, Foster City, CA, USA) and the following thermal profile for amplification: 2 min at 50°C, 2 min at 95°C, 40 cycles of 15 s at 95°C and 30 s at 60°C. A standard curve based on known amoA copy numbers (10–106 copies μl−1) was generated using a purified PCR product obtained from the reference strain Nitrosospira multiformis ATCC25196. AOB population size was estimated by dividing the amoA copy number by 2.5, based on the fact that each AOB cell carries two or three nearly identical copies of the amoA gene (Norton et al. 2002).
Statistical analyses
ANOVA analysis was used to test the difference of soil properties and gross N reaction rate between native and cultivated coastal wetland soils. In order to determine the factors affecting soil mineral N production, we applied a multiple regression analysis using SPSS soft. We hypothesized that the various variables measured were correlated among themselves (multicollinearity). In order to reduce multicollinearity, a cross-correlation analysis was first applied. The colinearity issues were excluded, before multiple regression analysis was carried out. Analyses were conducted using SPSS 10.0 statistical package and differences were considered significant if p < 0.05.
Results
Soil properties
The soil salinity and the ratio of soil organic C to total N decreased significantly (p < 0.05), while the content of total N increased after coastal wetland was cultivated (Table 1). However, the soil pH and soil organic C contents did not change significantly after coastal wetland was cultivated.
Soil microbial community and soil ammonia-oxidizing bacteria (AOB)
Total PLFA biomass (the sum of the 35 fatty acids that were identified in the samples), bacterial biomass, fungal biomass, and actinomycete biomass of the native coastal wetland soils were significantly lower than those of the cultivation soils (p < 0.05) (Table 2). However, the percentages of bacterial, fungal, and actinomycetes biomasses to total PLFA biomasses did not change significantly after cultivation of coastal wetland. The total PLFA biomass exponentially increased by decreasing soil salinity (y = 44.1e −2.2x, R 2 = 0.91, n = 16, p < 0.01). The total PLFA biomass was positively correlated with soil total N content (p < 0.01).
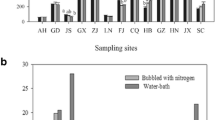
AOB population sizes in the cultivated soils (B, and H) were 14, and 27 times, respectively, higher than those of the native coastal wetland soils (Fig. 1). The AOB population size also exponentially increased by decreasing soil salinity (y = 2.03e −2.2x, R 2 = 0.82, n = 16, p < 0.01).
Gross N reactions
The gross N mineralization rates were 1.71 ± 0.36, 1.48 ± 0.37, 8.25 ± 0.1.14, and 10.56 ± 1.82 mg kg−1 d−1 for soil S, P, B, and H, respectively (Table 3). The cultivation of coastal wetland significantly (p < 0.05) increased the gross N mineralization rate. The multiple regression analysis showed that total PLFA biomass and soil total N (TN) explained 97% of the variation of gross N mineralization rate in the studied soils (gross N mineralization rate = 0.179 total PLFA biomass + 5.828TN − 2.505, n = 16, p < 0.01). The standardized coefficients indicated that the total PLFA biomass (0.508) played a more important role in controlling N mineralization than TN (0.493). The gross ammonium immobilization rate also increased significantly after cultivation of native coast wetland soil (p < 0.01) and was positively correlated with the gross N mineralization (p < 0.01).
Similar to N mineralization, the cultivation of coastal wetland also significantly (p < 0.05) increased the gross nitrification rates, which were 0.51 ± 0.11, 0.51 ± 0.17, 4.13 ± 0.67, and 7.37 ± 1.86 mg kg−1 d−1 for S, P, B, and H, respectively. The multiple regression analysis showed that the gross nitrification rate increased by increasing the soil AOB population size and gross mineralization rate (M) (gross nitrification rate = 3.39AOB + 0.18 M − 0.075, R 2 = 0.98, n = 16, p < 0.01). The standardized coefficients indicated that the soil AOB population size (0.763) played a more important role in affecting nitrification than gross N mineralization rate (0.247). However, gross immobilization of nitrate rate did not change after coastal wetland was cultivated.
N2O and NO emission rate
The comparison of results between control and the inhibition with 10 Pa C2H2 indicated that the emission of N2O and NO in the present investigation was mainly produced via autotrophic nitrification (Table 4). The cultivation of coast wetland significantly (p < 0.05) increased the emission of N2O and NO from soil. The N2O emission rate logarithmically increased by increasing the gross nitrification rate (p < 0.01); similarly, NO emission rate linearly increased by increasing the gross nitrification rate (p < 0.01).
Discussion
The gross N mineralization rate significantly increased after coast wetland was cultivated, probably due to the increase in soil microbial biomass (i.e., total PLFA biomass) and soil total N. Nitrogen mineralization is mainly due to soil microbes (Martens 1995) and this may explain the positive and significant correlation between soil microbial biomass and N mineralization. The significant and negative relationship between soil microbial biomass (i.e., total PLFA biomass) and soil salinity suggests that salt management of soil is important in affecting N cycling in the studied soils. The reduction of soil salinity can provide a suitable environment for soil microbes (Setia et al. 2010). The soil organic N pool being the direct source of mineralized N can be the predictive of N mineralization (Booth et al. 2005). The increase in mineral N fertilization in arable soils can increase plant growth and thus input of crop residues and roots to soil with the increased soil organic N content.
In arable soils, most of the ammonia oxidization may be carried out by autotrophic ammonia-oxidizing bacteria (AOB) (Barraclough and Puri 1995). The present investigation demonstrated that AOB population size significantly increased after coastal wetland was cultivated (Fig. 1), probably because cultivation reduced the soil salinity, providing also a suitable environment for ammonia-oxidizing bacteria. The other important factor affecting soil AOB population size was mineral N fertilization in arable soil. The increased AOB population size by N fertilization has been observed in both laboratory (Okano et al. 2004) and field experiments (Mendum et al. 1999; Chu et al. 2008). In addition, the increase in N mineralization provided more substrate for ammonia-oxidizing bacteria. Since ammonia oxidation is the rate-limiting step of nitrification and most of its activity is carried out by AOB in arable soils (Killham 1986; Barraclough and Puri 1995), it can be assumed that the nitrification was regulated by AOB in the aerobic soils. Gross nitrification rate increased by increasing the soil AOB population size and gross N mineralization rate (M) (gross nitrification rate = 3.39AOB + 0.18 M − 0.075, R 2 = 0.98, n = 16, p < 0.01). The gross nitrification rate, however, did not affect nitrate immobilization, which was very low. Therefore, the cultivation of coastal wetland can increase the risk of N losses through nitrate leaching.
NO and N2O emissions, important N losses, are produced in soils by nitrification and denitrification. Probably in our studies both N2O and NO were mainly produced by nitrification, since the N2O and NO emission rate increased by increasing the gross nitrification rate. In addition, out results indicated that fertilization increased NO and N2O emission compared to unfertilized soils (Smith et al. 1998; Smart et al. 1999; Meng et al. 2005; Ding et al. 2010).
Conclusion
The cultivation of coastal wetland significantly increased gross N mineralization, nitrification, and N2O and NO production rates. Management of salt discharge and mineral N fertilization during the cultivation of wetland soils might have affected soil microbial biomass and AOB population size with effects on N mineralization and nitrification after cultivation of coastal wetland soils. Therefore, the cultivation of coastal wetland soils can increase the risk of N losses from soil through nitrate leaching and gas emission (e.g., N2O and NO). Future studies are needed to measure the field water dynamics, denitrification rate, nitrification rate and N immobilization in situ and to better understand the underlying mechanisms of the effects of cultivation of coastal wetland on N cycle.
References
Barraclough D, Puri G (1995) The use of 15 N pool dilution and enrichment to separate the heterotrophic and autotrophic pathways of nitrification. Soil Biol Biochem 27:17–22
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Booth MS, Stark JM, Rastetter EB (2005) Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol Monogr 75:139–157
Chu HY, Fujii T, Morimoto S, Lin XG, Yagi K (2008) Population size and specific nitrification potential of soil ammonia-oxidizing bacteria under long-term fertilizer management. Soil Biol Biochem 40:1960–1963
Ding W, Yagi K, Cai Z, Han F (2010) Impact of long-term application of fertilizers on N2O and NO production potential in an intensively cultivated sandy loam soil. Water Air Soil Pollut. doi:10.1007/s11270-010-0328-x
Frostegård A, Bååth E, Tunlid A (1993) Shifts in the structure of soil microbial communities in limed forests as revealed by phospholipid fatty acid analysis. Soil Biol Biochem 25:723–730
Harris JA (2003) Measurements of the soil microbial community for estimating the success of restoration. Eur J Soil Sci 54:801–808
Hart SC, Stark JM, Davidson EA, Firestone MK (1994) Nitrogen mineralization, immobilization, and nitrification. In: Hart SC, Stark JM, Davidson EA, Firestone MK (eds) Methods of soil analysis part 2: microbiological and biochemical properties. Soil Science Society of American, Madison, WI, pp 985–1018
Hedrick DB, Peacock A, Stephan JR, Macnaughton SJ, Brüggemann J, White DC (2000) Measuring microbial community diversity using polar lipid fatty acid and denaturing gradient gel electrophoresis data. J Microbiol Meth 41:235–248
Hermansson A, Lindgren PE (2001) Quantification of ammonia oxidizing bacteria in arable soil by real-time PCR. Appl Environ Microbiol 67:972–976
Killham K (1986) Heterotrophic nitrification. In: Prosser JI (ed) Nitrification. IRL Press, Oxford, UK, pp 117–126
Kirkham D, Bartholomew WV (1954) Equations for following nutrient transformations in soil, utilizing tracer data. Soil Sci Soc Am Pro 18:33–34
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529
Martens R (1995) Current methods for measuring microbial biomass C in soil: potentials and limitations. Biol Ferti Soils 19:87–99
Mendum TA, Sockett RE, Hirsch PR (1999) Use of molecular and isotopic techniques to monitor the response of autotrophic ammonia-oxidizing populations of the subdivision of the class Proteobacteria in arable soils to nitrogen fertilizer. Appl Environ Microbiol 65:4155–4162
Meng L, Ding WX, Cai ZC (2005) Long-term application of organic manure and nitrogen fertilizer on N2O emissions, soil quality and crop production in a sandy loam soil. Soil Biol Biochem 37:2037–2045
Norton JM, Alzerreca JJ, Suwa Y, Klotz MG (2002) Diversity of ammonia monooxygenase operon in autotrophic ammonia-oxidizing bacteria. Arch Microbiol 177:139–149
Okano Y, Hristova KR, Leutenegger CM, Jackson LE, Denison RF, Gebreyesus B, Lebauer D, Scow KM (2004) Application of real-time PCR to study effects of ammonia-oxidizing bacteria in soil. Appl Environ Microbiol 70:1008–1016
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Setia R, Marschner P, Baldock J, Chittleborough D (2010) Is CO2 evolution in saline soils affected by an osmotic effect and calcium carbonate? Biol Ferti Soils 46:781–792
Smart DR, Stark JM, Diego V (1999) Resource limitations to nitric oxide emissions from a sagebrush steppe ecosystem. Biogeochemistry 47:63–86
Smith KA, McTaggart IP, Dobbie KE, Conen F (1998) Emissions of N2O from Scottish agricultural soils as a function of fertilizer N. Nutr Cycl Agroecosys 52:123–130
Stark JM (2000) Nutrient transformations. In: Sala OE, Jackson RB, Mooney HA, Howarth RW (eds) Methods in ecosystem science. Springer, New York, pp 215–234
Yao HY, Bowman D, Shi W (2006) Soil microbial community structure and diversity in a turfgrass chronosequence: land-use change versus turfgrass management. Appl Soil Ecol 34:209–218
Zak JC, Willig MR, Moorhead DL, Wildman HG (1994) Functional diversity of microbial communities: a quantitative approach. Soil Biol Biochem 26:1101–1108
Zelles L (1997) Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 35:275–294
Zhang JB, Cai ZC, Cheng Y, Zhu TB (2009) Denitrification and total nitrogen gas production from forest soils of Eastern China. Soil Biol Biochem 41:2551–2557
Zogg GP, Zak DR, Ringelberg DB, MacDonald NW, Pregitzer KS, White DC (1997) Compositional and functional shifts in microbial communities due to soil warming. Soil Sci Soc Am J 61:475–481
Acknowledgement
This work was funded by the Ministry of Land and Resources, Special Funding Project for Research in Public Sector (research project “Carbon Abatement Effect and Regulation Research in Land Use Planning, 200511033) and the National Key Technology RCD Program (research project “Incremental and Economic Land Consoremental and Economic Land Consolidation Technological Development in Main Food Producing Area of central China, 2008BAB38B06)”. We would like to thank the reviewers and editors for their time and comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jin, X., Huang, J. & Zhou, Y. Impact of coastal wetland cultivation on microbial biomass, ammonia-oxidizing bacteria, gross N transformation and N2O and NO potential production. Biol Fertil Soils 48, 363–369 (2012). https://doi.org/10.1007/s00374-011-0631-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-011-0631-8