Abstract
This study focuses on the chemotaxis, colonization and rice growth promoting ability of indole acetic acid (IAA) and 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase-producing rhizobacteria Rhizobium leguminosarum bv. trifolii SN10, previously isolated from root nodules of Trifolium alexandrium L. We show here that R. leguminosarum bv. trifolii SN10 promote the growth of four different rice varieties grown in India in terms of biomass, root branching and N content. In addition, using scanning electron microscopy and viable cell counts, we provide evidence that the bacteria successfully colonize the root surface of the rice variety which showed maximum growth promotion upon inoculation. Not only this, R. leguminosarum bv. trifolii SN10 exhibit a strong chemotaxis response towards the rice seed and root exudates despite the presence of a bacteriostatic phenolic compound, 7-hydroxycoumarin (umbelliferone). Further, R. leguminosarum bv. trifolii SN10 secretion of phytohormones such as IAA and ACC deaminase suggest the potential of the plant growth promoting rhizobacteria to be used as biofertilizer to enhance rice crop production in the subcontinent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen-fixing bacteria, especially Rhizobia and Bradyrhizobia, have been known for many years as specific beneficial symbionts for legumes. However, studies mainly in the last decade have revealed that roots of gramineous plant-like rice (Chaintreuil et al. 2000; Yanni et al. 1997), wheat (Hilali et al. 2001), sugarcane and maize (reviewed in Bhattacharjee et al. 2008) can also host as a third niche for the nitrogen-fixing bacteria. Such plant–bacteria association was found to have great potential in improving production of non-leguminous crops, manifested in terms of increased seedling vigor, yield, growth and nutrient uptake, photosynthetic activity, stomatal conductance and N content (reviewed in Bhattacharjee et al. 2008).
The benefits conferred by rhizobial association with non-legumes have been believed to be primarily the result of plant growth promoting rhizobacterial (PGPR) activities (Hilali et al. 2001; Yanni et al. 2001). PGPR exert their growth promoting effect by a wide variety of mechanisms. These include production of growth promoting compounds, such as, phytohormones (indole acetic acid (IAA), gibberellins and cytokinins) and siderophores or solubilization of mineral phosphates (Glick 1995; Nelson 2004). In addition, PGPR exert antagonism towards plant pathogens by releasing metabolites like antibiotics, cell wall lytic enzymes or by inducing defense systemic responses (Nelson 2004). PGPR interaction with host plant is complex and not yet fully understood. Plant root colonization by the bacteria is considered as a primary step towards the successful initiation of the plant–microbe interaction (Nelson 2004). Colonization in turn depends on bacterial motility and chemotaxis (specific migration towards chemicals) towards the plant root release or exudates (Bashan et al. 2004). Root exudates are a rich source of nutrients for rhizospheric bacteria that undergo chemotaxis and colonize the plant root surface (Bais et al. 2006). PGPR that make their way to the plant interior are termed endophytes. In either case, the PGPR exert their beneficiary effect on the plant in terms of growth and development.
One such PGPR isolated from rice (Oryza sativa L.), cultivated in the field of interior Egypt, was Rhizobium leguminosarum bv. trifolii (Yanni et al. 1997). This bacterium harbored the potential to improve growth and development of various rice and wheat varieties grown in Egypt. In India, rice forms the staple food crop for more than 60% of the population. With increasing demand, the need to increase production is leading to an excessive application of chemical fertilizers. In the light of growing environmental concerns associated with chemical fertilizer, rhizobial association with cereal is gaining importance as it promises to provide a cleaner path towards maintaining crop production targets. Considering the benefits that would arise from the interaction of rice and PGPR, in this study we have assessed the growth-promoting activities of a phytohormone-producing strain of R. leguminosarum bv. trifolii (isolated from Berseem clover) on rice. Furthermore, to understand this rice–Rhizobium interaction, we have investigated the involvement of rhizobial chemotaxis and plant root colonization.
Materials and methods
Plant seeds and bacterial strains
The strain SN10 of R. leguminosarum bv. trifolii used in this study was obtained from CCS Haryana Agricultural University, Hisar, India (Gaur et al. 2002). Escherichia coli DH5α strain was provided from Bethesda Research Laboratories (Gaithersburg, MD). The bacterial cultures were maintained on yeast extract mannitol (YEM) medium (Vincent 1970). Rice seeds were obtained from IARI, Delhi India. Seeds of Trifolium alexandrium L. were obtained from LSTM collection centre, Montpellier, France and IARI, New Delhi, India.
Gnotobiotic rice growth promotion studies
De-hulled seeds of rice were surface-sterilized by soaking in 96% ethanol for 15 min followed by 0.2% HgCl2 for 5 min. The seeds were then rinsed several times and allowed to imbibe overnight in the dark in sterile water. To allow the germination of the seeds and to check contamination, the surface sterilized seeds were transferred to plates containing Cambel’s media (Chaintreuil et al. 2000). The plates were incubated in the dark at 28°C till the plantlets developed 1- to 2-cm long roots. Rice plantlets showing no contamination were soaked for 5 min in an axenic mid-log phase (OD600, ~0.6 to 0.8) rhizobial culture in YEM (Vincent 1970). As control, plants were inoculated with suspension of E. coli or heat-killed rhizobia in YEM. All the plantlets (inoculated and controls) were then transferred to 25- × 200-mm tubes (one seedling per tube) containing 20 ml of either N-free Jensen’s medium (Vincent 1970) or Jensen’s medium containing KNO3 (10 mM), supplemented with 0.8% agar. The plants were grown for 6 weeks in a growth chamber at 28°C, with a 12-h night and day cycle, adding nutrient solution (N-free Jensen’s medium and Jensen’s media with 10 mM KNO3 + 0.2% agar) every week, under aseptic conditions. Twenty plants were used in each experiment, and three independent experiments were performed. For the dry weight, both shoots and roots were oven-dried at 60°C until the weight became constant (~5 days). The N content of the shoot was estimated by the Kdjeldahl method (AOAC 1990).
Bacterial colonization counting and scanning electron microscopy
Rice roots from gnotobiotic seedlings (six plants) after 10 days of growth were carefully removed from each tube, excised, washed with sterile water, blotted dry, divided into two parts. One portion (three roots per plant) was used for viable cell count after taking the fresh weight. The number of bacteria present in the external rooting medium and on the root surface was determined by viable count method as described previously (Yanni et al. 2001). The CFU obtained from three roots in each of the three tubes were then averaged with the SD. The other portion was used for scanning electron microscopy (SEM). Roots for microscopic analysis were carefully rinsed with distilled deionised water and fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7) overnight. Tissues were then washed in 0.1 M phosphate buffer, four times for 15 min each. This was followed by distilled H2O wash 3 × 5 min. Tissues were dehydrated in graded ethanol series. Samples were then lyophilized for 3–4 h for critical drying. The moisture-free samples were coated with silver using the Sputter Coater E5100 (Polaron, England) by plasma coating. The thickness of the film was about 100–150 Å. The coated samples were then observed under SEM (Cambridge Setero Scan 360, UK).
Rice seed and root exudates collection
A hundred surface-sterilized seeds of O. sativa L. ssp. japonica cv. Kdulam, were soaked in 20 ml of sterile water overnight in the dark at 28°C. The solution thus obtained was termed as seed exudates for this study. For root exudates, the germinated seeds were grown on a paper support in 20 tubes containing liquid Jenson media (five seedlings per tube) for 10 days at 28°C with an 18-h light and 6-h dark period in gnotobiotic conditions. The strength of the media was reduced to 1/10 to minimize the interference of nutrient. The nutrient solution was filtered through a 0.2-μm Millipore filter. A batch of rice cultivar Kdulam was inoculated with R. leguminosarum bv. trifolii SN10. In order to remove the bacteria, root exudates obtained from this batch were centrifuged at 1,200 × g for 20 min at 4°C prior to filtration. The exudates were lyophilised and were kept at −20°C for later use.
Chemotaxis assay
The chemotactic responses of the bacterial species towards rice exudates were evaluated using: (1) swarm plate assay (Adler 1973) and (2) Pfeffer’s capillary tube assay (Adler et al. 1973). The exudates used for this assay were prepared by dissolving 10 mg of the lyophilized exudates in 0.5 ml of sterile distilled water. In these tests, T. alexandrium L. exudates were used as positive control while Phosphate Buffer Saline (PBS, pH 7.0) was used as negative control. Similarly, other chemotaxis assays were conducted by testing chemicals at a final concentration of 10 mM prepared in PBS (pH 7.0)
Detection and quantification of glucose, proline and flavonoid
Detection and quantification of d-glucose was performed using the glucose oxidase peroxidase method. This was done using the GOD–POD MerckotestR Glucose (Merck) as described in the user’s manual. For estimation of l-proline, acid ninhydrin was used as described before (Bates et al. 1973). For the detection of flavonoid compounds, lyophilized seed exudates and the root exudates were extracted with 80% methanol (~160 mg.ml−1) overnight at 4°C, with shaking. The flavonoids were purified by using a Sep-Pak C18 column (Waters) as described in the user’s manual. The high-performance liquid chromatography (HPLC) equipment used for the separation and characterization of the exudates consisted of a Hewlett-Packard 1090 M liquid chromatography system equipped with a diode array detector (200–400 nm). Chromatographic runs were performed on a Waters Symmetry C18 (250 × 4.6 mm) column. The solvent system and elution profiles used were as described previously (Mathesius et al. 1998). For mass spectrometry (JASCO-1585, Great Dunmow, UK Ltd.) electrospray spectra were obtained using the following: LC/MS system 2790 alliance equipped with a 996 photodiode array detector connected to a Q-TOF I (Micromass UK Ltd., Manchester) electrospray mass spectrometer operated in the positive ionization mode, electrospray; capillary voltage, 3 kV; cone voltage, 40 V; source block temperature, 150°C; desolvation temperature, 250°C. The mass range scanned was 50–1,500 Da, at a rate of 3 s per scan. The column and the conditions used to analyze the samples were the same as used for HPLC/DAD analysis. The synthetic flavonoid used in the identification 7-hydroxycoumarin was obtained from Indofine Chemical Company, Inc., USA. The standards were prepared as indicated by the provider and stored in dark bottles at −20°C.
Detection of 1-aminocyclopropane-1-carboxylic acid deaminase, indole acetic acid
The ACC deaminase activity test was performed as already described (Belimov et al. 2001). Estimation of IAA was done using Salkowski’s reagent as described previously (Patten and Glick 2002).
Umbelliferone tolerance test
Bacterial culture dilutions from 10−1 to 10−9 of either R. leguminosarum bv. trifolii SN10 or E. coli DH5α were prepared from a fresh culture at OD600nm = 1, in liquid YEM and plated on YEM containing plates. Sterile filter paper discs similar to those used for antibiograms were treated with 50 μl of umbelliferone concentrations ranging from 1 nM to 10 mM in solution in diluted NaOH (0.01 M), and were placed on the bacteria-plated YEM plates. Diluted NaOH without umbelliferone was used as control. Plates were incubated for 48 h at 30°C. Bacterial tolerance or sensitivity was measured by the radial inhibition of growth reported in millimeters at the lowest concentration tested.
Statistical analysis
Data were analyzed by ANOVA, and the means were compared following Fisher’s test of least significant difference to assess the effects of inoculation on plant growth promotion.
Results
IAA and ACC deaminase production by R. leguminosarum bv. trifolii SN10
In a culture filtrate, R. leguminosarum bv. trifolii SN10 previously isolated from roots of the leguminous plant T. alexandrium (Berseem clover) synthesize IAA to a final concentration of 2 ± 0.1 μg.ml−1. The IAA production by the bacteria increased in the presence of l-tryptophan in the media. In the presence of this amino acid at 100 μg.ml−1 in the media, the IAA production increased from 2 ± 0.1 μg.ml−1 to 25 ± 1 μg.ml−1. R. leguminosarum bv. trifolii SN10 were able to grow on medium containing ACC as the sole N source, confirming the ability to produce ACC deaminase. The ACC deaminase activity of R. leguminosarum bv. trifolii SN10 was measured to be 15 ± 2 (µmol NH +4 .mg-1 of protein.h-1).
Effect of R. leguminosarum bv. trifolii SN10 inoculation on rice growth
R. leguminosarum bv. trifolii SN10 were inoculated to four varieties of rice in the absence of basal N. The performance of the bacteria was scored after 30 days of growth in terms of plant biomass, root branching and increase in N content. Plants inoculated with heat-killed rhizobia in YEM were used as control. In N-free media, rhizobial inoculation led to an increase in total plant biomass (shoot and root) ranging from 19–61% when compared to heat-killed rhizobia inoculated plants used as the control (Fig. 1a). A similar pattern of increase was observed in the root branching of the four rice varieties upon rhizobial inoculation (Fig. 1b, d). There was minimal change in the N content of the inoculated plants in the absence of basal N in the media (Fig. 1c). Among the four varieties tested, performance of R. leguminosarum bv. trifolii SN10 was significantly high when inoculated to local variety Oryza sativa japonica cv. Kdulam where the increase of plant biomass was almost 60%. To investigate the effect of the presence of a N source on the performance of R. leguminosarum bv. trifolii SN10, we added 10 mM of KNO3 to the media. As O. sativa japonica cv. Kdulam showed maximum response to R. leguminosarum bv. trifolii SN10 inoculation, for the rest of the studies, we used this plant microbe combination. Without rhizobial inoculation, plant biomass was increased by 25% in plants grown in the presence of KNO3 compared to the control plants grown without a N source. However, the biomass increased two times upon the addition of R. leguminosarum bv. trifolii SN10 to KNO3-containing media when compared to the control (+KNO3; R. leguminosarum bv. trifolii SN10) (Table 1). Rhizobial inoculation also led to a change in root morphology in terms of root branching. When compared to the control, root branching increased to 64% in N-free medium, and to 82% in medium supplemented with N (Table 1). Of significant importance is the increase in N content in N-supplemented R. leguminosarum bv. trifolii SN10-inoculated plants. There was an increase of the shoot N content to 28% when compared to heat-killed rhizobial inoculation (Table 1).
Growth promotion of different rice varieties after 30 days of inoculation with R. leguminosarum bv. trifolii SN10 a total plant biomass; b root branching; c plant N content of the four rice varieties with and without rhizobial inoculation, DW dry weight; d root branching observed in O. sativa L. subsp. japonica cv. Kdulam after inoculation with R. leguminosarum bv. trifolii SN10
External colonization of rice roots by R. leguminosarum bv. trifolii SN10
PGPR are known to colonize plant roots while exerting their growth promoting effect (Nelson 2004). We therefore investigated the ability of R. leguminosarum bv. trifolii SN10 to colonize rice roots by dilution plating and SEM. Roots from inoculated rice were scored for adhering bacteria for 10 days. Uninoculated and E. coli-inoculated plants were used as control. Loosely attached bacteria were removed by washing rice roots thoroughly with water. Washing the roots reduced the number of colonies observed by dilution plating in E. coli-inoculated plants. In contrast, there was only a 10% reduction in the R. leguminosarum bv. trifolii SN10-inoculated plants. Overall, the colonization of R. leguminosarum bv. trifolii SN10 on rice roots were 95–100% more than the E. coli and uninoculated plants (Table 2). Colonization was further confirmed by SEM. The roots of 10-day-old rice-inoculated plants were densely populated with R. leguminosarum bv. trifolii SN10 (Fig. 2).
Chemotaxis towards rice root exudates
Colonization of rice roots by R. leguminosarum bv. trifolii SN10 prompted us to investigate the in vitro chemotactic motility of the bacteria towards rice root release (exudates) T. alexandrium L. is the natural leguminous host plant for R. leguminosarum bv. trifolii, so the root/seed exudates of the plant were used as positive control for this experiment. The average distance moved by R. leguminosarum bv. trifolii SN10 in rice (Kdulam) seed exudates containing plate was equivalent to the distance traveled in the presence of T. alexandrium L. seed exudates and root exudates. When exudates and free compounds were replaced by PBS in the assay, there was no swarm by R. leguminosarum bv. trifolii (Table 3). E. coli did not show any significant movement in the presence of the exudates. Further corroboration of these results was obtained by Pfeffer’s capillary tube (Table 3). Pfeffer’s capillary tube result showed that seed exudates attracted almost twofold higher bacteria than rice root exudates.
In addition, sugars and amino acids earlier reported to be present in rice seed and root exudates in the first week of germination were individually tested (Bacilio-Jimenez et al. 2003). Among the free sugars and amino acids tested (listed in Table 3), R. leguminosarum trifolii SN10 showed a significant chemotactic response towards d-glucose (10 mM) and l-proline (10 mM) in both soft and capillary tube. R. leguminosarum bv. trifolii SN10 exhibited a positive response even at lower concentrations (2 and 5 mM) of d-glucose and l-proline. The bacterial strain exhibited a significantly lower chemotactic response to other amino acids and sugars in soft agar and capillary tube. However, E. coli exhibited a positive chemotaxis towards glucose (data not shown).
Detection of d-glucose and l-proline in rice root and seed exudates
A chemotaxis-promoting compound glucose was found to be an important constituent of O. sativa subsp. japonica cv. Kdulam seed exudates (1.16 ± 0.1 μg.mg−1 of seed exudates) and root exudates (0.25 ± 0.05 μg.mg−1 of root exudates). In addition, proline was also detected but at a lower concentration than glucose in both seed exudates (0.66 ± 0.05 μg.mg−1) and root exudates (0.25 ± 0.05 μg.mg−1). These concentrations corresponded from 13.2 to 23.2 mM of d-glucose and 5 mM of l-proline in root and seed exudates respectively, which are in the range of concentrations of free d-glucose and l-proline tested for positive chemotaxis of R. leguminosarum bv. trifolii SN10.
Rice root releases a phenolic compound that inhibit E. coli growth
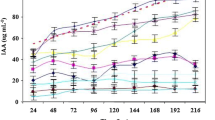
Phenolic compounds released by plants can act as biocontrol for microbial growth (Siqueira et al. 1991). We therefore investigated the rice root exudates for the presence of phenolic compounds that inhibited E. coli chemotaxis in seed and root exudates despite of the presence of a high concentration of glucose in the latter. HPLC-MS analysis indicated that a phenolic compound 7-hydroxycoumarin (retention time of 15.40 min and absorption maxima of 322.85 nm) is present at a detectable concentration in uninfected exudates (Fig. 3a). The mass spectrometry analysis of both molecular ions (M+H)+ and (M+AcN)+ of this compound gives m/z 163.06 and 204.08 which is in agreement with the structure of umbelliferone (data not shown). However, its concentration steeply increases from 15 ± 1.5 μg.g−1 (without rhizobia) to 58 ± 2 μg.g−1 in E. coli and R. leguminosarum bv. trifolii SN10-infected exudates (Fig. 3b). We therefore checked the sensitivity of R. leguminosarum bv. trifolii SN10 towards different concentrations of 7-hydroxycoumarin in vitro. At a high concentration of 10 mM, 7-hydroxycoumarin does not have any adverse effect on rhizobial survival. However, in the case of E. coli, hollow zones indicating bacterial inhibitions were observed at all concentrations tested.
Discussion
Over the years, the growing demand of rice has led to the indiscriminate use of chemical fertilizers around the world. With arising concerns about the effects of fertilizer use on the environment, the quest for non-consequential alternatives is being promoted. India is one of the largest rice producers in the world. In this context, we found that a clover symbiont synthesizing ACC deaminase and IAA, R. leguminosarum bv. trifolii SN10, could enhance growth of various varieties of rice grown in the subcontinent. In this association, R. leguminosarum bv. trifolii SN10 acts as a PGPR, undergoes chemotaxis and successfully colonizes the plant resisting the inhibitory effect of an antimicrobial compound released by the plant.
As a general feature, we observed that R. leguminosarum bv. trifolii SN10 isolated from the legume T. alexandrium, has stimulatory effects on different rice varieties evaluated in terms of shoot and root biomass and root branching. However, the effect of the bacteria is dependent on the variety of rice. Emphasis on the selection of the combination of bacteria and plant for obtaining optimum response has been made previously by other workers (Biswas et al. 2000a; Pedraza et al. 2010; Yanni et al. 2001). R. leguminosarum bv. trifolli are known to fix N in vitro as well as in association with legume (O'gara and Shanmugam 1978; Kishinevsky et al. 1992). However, it has been observed that R. leguminosarum bv. trifolii does not fix N in association with rice (Biswas et al. 2000; Yanni et al. 1997, 2001). Thus, the performance of the bacteria improved further when used in supplementation to N in the media. We can therefore speculate that IAA and ACC deaminase production by R. leguminosarum bv. trifolii SN10 may account for the observed increase in plant biomass in biotised plants (Glick 1995; Khalid et al. 2005; Shaharoona et al. 2006; Zahir et al. 2011). Root branching would provide an increased surface area for adsorption of nutrients (Yanni et al. 1997) in soil explaining the additional gain in rice plant biomass and N content upon R. leguminosarum bv. trifolii SN10 inoculation.
In addition to phytohormone production by the bacteria, plant growth promotion also depends on the establishment of the inoculated strain in the rhizosphere. Thus colonization of the host plant by PGPR is a prerequisite for exerting a growth-promoting effect on the host plant (Larcher et al. 2003). A huge colonization on the surface of O. sativa japonica roots by R. leguminosarum bv. trifolii SN10 as against the E. coli-inoculated batch, indicates the specific rice root colonizing capacity of the bacteria. R. leguminosarum bv. trifolii SN10 adherence to the root was significant as loosely attached bacteria could be removed by washing. Our observation is thus in accordance with other studies which have shown that bacteria that promote plant growth also colonize the host plant (Larcher et al. 2003; Pedraza et al. 2010; Vande Broek et al. 1998).
It has been suggested that a prerequisite for the effective colonization of roots is positive chemotaxis towards root exudates (Vande Broek et al. 1998). A positive chemotaxis of R. leguminosarum bv. trifolii SN10 towards O. sativa japonica cv. Kdulam exudates therefore suggests that rice seed and root exudates release compounds that attract bacteria towards the plant leading to colonization. We also observed that R. leguminosarum bv. trifolii SN10 showed a significant chemotaxis towards glucose and proline which constituted considerable percentages of the seed and root exudates. The higher chemotaxis of R. leguminosarum bv. trifolii SN10 towards seed exudates than root exudates similar to the observation of Kato and Yasuhiro (2006) may be due to the higher concentration of glucose and proline in seed exudates. Most microorganisms use glucose, and in the case of proline as a source of C, thus E. coli showed positive chemotaxis towards glucose in the plate assay (Adler et al. 1973; Vílchez et al. 2000). Chemotaxis was also observed by some endophytic bacteria towards rice root exudates (Bacilio-Jimenez et al. 2003), Azospirillum towards strawberry (Pedraza et al. 2010) and Nostoc towards non-host Trifolium repens root extract (Nilsson et al. 2006). Our results suggest that in the case of this rice–Rhizobium interaction, biomolecules like glucose and proline function as major chemoattractants for the chemotaxis of R. leguminosarum bv. trifolii SN10.
The accumulation of high concentrations of 7-hydroxycoumarin upon inoculation with R. leguminosarum bv. trifolii SN10 in rice (O. sativa japonica bv. Kdulam) root exudates is a stress response towards microbial invasion. The coumarins are known to inhibit the growth and sporulation of fungal plant pathogens and to provide as well a defense tool against hostile microorganisms (Matern et al. 1999; Weinmann 1997). This high concentration of 7-hydroxycoumarin in these rice exudates produced upon microbial invasion might be the inhibitory compound that restricts E. coli chemotaxis (Duncan et al. 1998) as E. coli is sensitive to umbelliferone at any concentration.
The present results clearly indicate that R. leguminosarum bv. trifolii SN10, a symbiont of legumes can colonize rice root surfaces and confer beneficiary effects by enhancing root branching, increasing the biomass and N content of the plant, under laboratory conditions. Their colonization is subject to chemotaxis towards biomolecules released by rice root exudates. R. leguminosarum bv. trifolii SN10 confers the growth promoting activity to O. sativa japonica cv. Kdulam as a PGPR and resists the antimicrobial activity of the root release. Thus, R. leguminosarum bv. trifolii SN10 stands as a potential candidate for further field studies which would be helpful in extending their use as a PGPR for rice in India.
References
Adler J (1973) A method for measuring chemotaxis ad use of the method to determine optimum conditions forchemotaxis by Escherichia coli. J Gen Microbiol 74:77–91
Adler J, Gerald L, Hazelbauer DMM (1973) Chemotaxis towards sugars in Escherichia coli. J Bacteriol 115:824–847
AOAC (1990) Official method of analysis of the association of official analytical chemist, 15 Ed, Arlington, VA, pp. 552–553
Bacilio-Jimenez M, Aguilar-Flores S, Ventura-Zapata E, Perez-Campos E, Bouquelet S, Zenteno E (2003) Chemical characterization of root exudates from rice (Oryza sativa) and their effects on the chemotactic response of endophytic bacteria. Plant Soil 249:271–277
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Bashan Y, Holguin G, de-Bashan LE (2004) Azospirillum-plant relationships: physiological, molecular, and environmental advances (1997–2003). Can J Microbiol 50:521–577
Bates LS, Waldron RP, Teare TD (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Belimov AA, Safronova VI, Sergeyeva TA, Egorova TN, Matveyeva VA, Tsyganov VE, Borisov AY, Tikhonovich IA, Kluge C, Preisfeld A, Dietz KJ, Stepanok VV (2001) Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 47:642–652
Bhattacharjee RB, Singh A, Mukhopadhyay SN (2008) Use of nitrogen-fixing bacteria as biofertilizer for non-legumes: prospects and challenges. Appl Microbiol Biotechnol 80:199–209
Biswas JC, Ladha JK, Dazzo FB, Yanni YG, Rolfe BG (2000a) Rhizobial inoculation influences seedling vigor and yield of rice. Agron J 92:880–886
Biswas JC, Ladha JK, Dazzo FB (2000b) Rhizobia inoculation improves nutrient uptake and growth of lowland rice. Soil Sci Soc Am J 64:1644–1650
Chaintreuil C, Giraud E, Prin Y, Lorquin J, Ba A, Monique G, De Lajudie P, Dreyfus B (2000) Photosynthetic bradyrhizobia are natural endophytes of the African wild rice Oryza breviligulata. Appl Environ Microbiol 66:5437–5447
Duncan SH, Flint HJ, Stewart CS (1998) Inhibitory activity of gut bacteria against Escherichia coli O157 mediated by dietary plant metabolites. FEMS Microbiol Lett 164:283–288
Gaur YD, Rewari RB, Bhatnagar RS, Narayan KP, Sen AN (2002) Sinorhizobium meliloti and Rhizobium leguminosarum bv. trifolii in Indian soils and their inoculation response. Indian J Microbiol 42:23–28
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 4:109–117
Hilali A, Prévost D, Broughton WJ, Antoun H (2001) Effets de l'inoculation avec des souches de Rhizobium leguminosarum biovar trifolii sur la croissance du blé dans deux sols du Maroc. Can J Microbiol 47:590–593
Kato K, Yasuhiro A (2006) Potential of seed and root exudates of the common bean Phaseolus vulgaris L. for immediate induction of rhizobial chemotaxis and nod genes. J Soil Sci Plant Nutr 52:432–437
Khalid A, Arshad M, Zahir Z (2005) Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96:473–480
Kishinevsky D, Leshem Y, Friedman Y, Krivatz G (1992) Yield and nitrogen fixation of berseem clover as a potential winter forage crop under semiarid conditions. Arid Soil Res Rehab 6:261–270
Larcher M, Muller B, Mantelin S, Rapior S, Cleyet-Marel JC (2003) Early modifications of Brassica napus root system architecture induced by a plant growth-promoting Phyllobacterium strain. New Phytol 160:119–125
Matern U, Luer P, Kreusch D (1999) Biosynthesis of coumarins. In: Barton D, Nakanishi K, Meth-Cohn O, Sankawa U (eds) Comprehensive natural products chemistry, vol 1, polyketides and other secondary metabolites including fatty acids and their derivatives. Elsevier, Oxford, pp 623–637
Mathesius U, Bayliss C, Weinman JJ, Schlman HRM, Spaink HP, Rolfe BJ, McCully ME, Djordjevic MA (1998) Flavonoids synthesized in cortical cells during nodule initiation are early developmental markers in white clover. Mol Plant Microbe Interact 1:1223–1232
Nelson LM (2004) Plant growth-promoting rhizobacteria (PGPR): prospects for new inoculants. Crop Manag. doi:101094/Cm-2004-0301-05-RV
Nilsson M, Rasmussen U, Bergman B (2006) Cyanobacterial chemotaxis to extracts of host and nonhost plants. FEMS Microbiol Ecol 55:382–390
O'gara F, Shanmugam KT (1978) Mutant strains of clover rhizobium (Rhizobium trifolii) that form nodules on soybean (Glycine max). Proc Natl Acad Sci USA 75:2343–2347
Patten CL, Glick B (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microb 68:3795–3801
Pedraza RO, Motok J, Salazar SM, Ragout A, Mentel MI, Tortora ML, Guerrero Molina MF, Winik BC, DíazRicci JC (2010) Growth-promotion of strawberry plants inoculated with Azospirillum brasilense. World J Microbiol Biotechnol 26:265–272
Shaharoona B, Arshad M, Zahir ZA (2006) Effect of plant growth promoting rhizobacteria containing ACC-deaminase on maize (Zea mays L.) growth under axenic conditions and on nodulation in mung bean (Vigna radiata L.). Lett Appl Microbiol 42:155–159
Siqueira JO, Nair MG, Hammerschmidt R, Safir GR, Putnam AR (1991) Significance of phenolic compounds in plant-soil-microbial systems. Crit Rev Plant Sci 10:63–121
Vande Broek A, Lambrecht M, Vanderleyden J (1998) Bacterial chemotactic motility is important for the initiation of wheat root colonization by Azospirillum brasilense. Microbiology 144:2599–2606
Vílchez S, Molina L, Ramos C, Ramos JL (2000) Proline catabolism by Pseudomonas putida: cloning, characterization, and expression of the put genes in the presence of root exudates. J Bacteriol 182:91–99
Vincent JM (1970) A manual for practical study of root nodule bacteria. IBP Handbook no. 15. Blackwell, Oxford
Weinmann I (1997) History of the development and applications of coumarin and coumarin-related compounds. In: O’Kennedy R, Thornes RD (eds) Coumarins: biology, applications and mode of action. Wiley, New York, pp 1–22
Yanni YG, Rizk RY, Corich V, Squartini A, Ninke K, Philip-Hollingsworth S, Orgambide G, de Bruijn FJ, Stoltzfus J, Buckley D, Schmidt TM, Mateos PF, Ladha JK, Dazzo FB (1997) Natural endophytic association between R. legumionosarum bv. trifolli and rice root and assessment of its potential to promote rice growth. Plant Soil 194:99–114
Yanni YG, Rizk RY, Abd El-Fattah FK, Squartini A, Corich V, Giacomini A, deBruijn FJ, Rademaker J, Maya-Flores J, Ostrom P, Vega-Hernandez M, Hollingsworth RI, Martinez-Molina E, Ninke K, Philip-Hollingsworth S, Mateos PF, Velasquez E, Triplett E, Umali-Garcia M, Anarna JA, Rolfe BG, Ladha JK, Hill J, Mujoo R, Ng PK, Dazzo FB (2001) The beneficial plant growth-promoting association of Rhizobium leguminosarum bv. trifolii with rice roots. Aust J Plant Physiol 28:845–870
Zahir ZA, Zafar-ul-Hye M, Sajjad S, Naveed M (2011) Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC deaminase for coinoculation with Rhizobium leguminosarum to improve growth, nodulation and yield of lentil. Biol Fertil Soils 47:457–466
Acknowledgements
We are thankful to Dr. V. Sikka of CCS Haryana Agricultural University, Hisar, India for providing the Rhizobium leguminosarum bv. trifolii SN10 strain for this work. We are also thankful to Sujay Bhattacharjee for his editorial help. One of the authors, RBB, is thankful to the Council of Scientific and Industrial Research, India for the Ph.D. fellowship and “The Embassy of France” in India for the “Sandwich Program” Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhattacharjee, R.B., Jourand, P., Chaintreuil, C. et al. Indole acetic acid and ACC deaminase-producing Rhizobium leguminosarum bv. trifolii SN10 promote rice growth, and in the process undergo colonization and chemotaxis. Biol Fertil Soils 48, 173–182 (2012). https://doi.org/10.1007/s00374-011-0614-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-011-0614-9