Abstract
Nitrogen isotope abundance (δ 15N) of paddy rice (Oryza sativa L.) grown for 110 days after transplanting (DAT) under field conditions with ammonium sulfate (AS with −0.4‰ as a synthetic fertilizer), pig manure compost (PMC with 15.3‰ as a livestock manure compost), and hairy vetch (HV with −0.5‰ as a green manure) was investigated to test the possible use of δ 15N technique in discriminating organically grown from conventionally grown rice. At 15 DAT, the δ 15N of whole rice decreased (P < 0.05) in the order of 10.5‰ for PMC > 5.5‰ for control (without N input) > 4.0‰ for HV > 1.8‰ for AS. This difference seemed to reflect primarily the δ 15N signal of N sources. Although differences in δ 15N of rice grown with isotopically distinct N inputs (i.e. PMC vs. AS and PMC vs. HV) became smaller over time, the difference (2.8 and 3.0‰ difference at harvest on 110 DAT, respectively) was still significant (P < 0.05). However, there was no distinguishable difference between AS and HV treatment after 42 DAT. Such effect of N inputs on δ 15N of whole rice was also observed for root, shoot, and grain at harvest. Therefore, our study suggests that it is possible to distinguish rice grown with manure composts from that grown with synthetic fertilizers. However, if green manure of preceding N2-fixing plants is used as the N source, δ 15N of rice may not be a good surrogate of N sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As organic farming practices without the use of synthetic fertilizers and pesticides produce safer food with minimum environmental impacts, market and social forces have created price premiums on organic produce (Mann 2003). To be labeled as organic products, the absence of synthetic pesticide and fertilizer use needs to be certified by qualified inspecting authorities (Codex Committee on Food Labelling 2001). The use of synthetic pesticides can be detected by pesticide residue analysis on soil and agricultural products. However, discriminating organic foods grown with non-synthetic fertilizers from those conventionally grown with synthetic fertilizers is not straightforward (Choi et al. 2002). Food companies are putting more efforts to strengthen reliability of their organic produce certification by adopting extra inspection systems that are not regulated by relevant laws (in the case of Korea, the Agricultural Products Quality Management Act).
One of the extra inspection systems potentially adoptable is using natural 15N abundance (15N/14N, expressed as δ 15N) of plant tissues to identify the use of synthetic fertilizers (Ro and Choi 2010). Choi et al. (2002) proposed the possible use of the δ 15N technique in the certification of organic products by showing a higher δ 15N of maize (Zea mays L.) grown with organic input (composted pig manure) than with synthetic fertilizer (urea). This technique is based on the fact that the δ 15N of synthetic fertilizers manufactured from atmospheric N2 using the Haber–Bosch process (<2.0‰) is usually lower than that of organic sources of N fertilizers (>5.0‰; e.g., manure/manure-based composts, seaweed-based materials, non-manure wastes of livestock, and fish-based materials) that are permitted in organic farming (Bateman et al. 2005; Bateman and Kelly 2007; Choi et al. 2003a; Choi et al. 2006; Rogers 2008; Schmidt et al. 2005).
Previous reports have shown that the application of organic fertilizers results in a higher δ 15N of crop than synthetic fertilizers application (Bateman et al. 2005; Choi et al. 2003a; Choi et al. 2006; Rogers 2008; Schmidt et al. 2005). The δ 15N of crops ranged from 0‰ to 5‰ for synthetic fertilizers and 3‰ to 15‰ for organic fertilizers. However, discriminating organic crops against conventional crops using a critical δ 15N range is not yet a straightforward task because there is sometimes an overlap in δ 15N values between organic and conventional crops. To improve the feasibility of the δ 15N technique for identifying organic vs. conventional crops, a crop δ 15N database encompassing a variety of crop species, N inputs, and soil types needs to be constructed (Bateman et al. 2007; Yun et al. 2006). In this context, δ 15N of paddy rice (Oryza sativa L.) grown with synthetic fertilizer and organic inputs should be added to the current crop δ 15N databases because rice is a staple food for more than half of the world’s population, particularly in many Asian countries, where the organic rice market is increasing (Coats 2003). However, to date, most studies have been conducted on vegetables such as Chinese cabbage, lettuce, tomato, and carrot (Bateman et al. 2005; Lim et al. 2007; Nakano et al. 2003; Rogers 2008; Schmidt et al. 2005; Šturm et al. 2011; Yun et al. 2006) and upland grain crops such as maize, canola, barley, and wheat (Choi et al. 2002, 2006).
Another gap in our current knowledge is the kind of organic N input used. Although various kinds of organic N sources are permitted in organic farming, most studies have been conducted with livestock manure including pig manure compost (Choi et al. 2002, 2003a; Yun et al. 2006), liquid pig manure (Lim et al. 2007), solid pig manure (Choi et al. 2006), chicken manure (Bateman et al. 2005), and cattle manure (Choi et al. 2006), which has distinctly higher δ 15N than synthetic fertilizers. Green manure plants that fix atmospheric N2 have served as a traditional N source for summer crops including paddy rice (Asagi and Ueno 2009; Ashraf et al. 2004). Due to the N2-fixing ability of green manure plants, we cannot eliminate the possibility of similar δ 15N signals to synthetic fertilizer, which may produce indistinguishable δ 15N signals between crops receiving green manure and synthetic fertilizer. Despite widespread organic crop rotation with green manure as a winter crop, there is little attention given to δ 15N of crops including rice grown with green manure.
Ideally, to test N isotopic response of rice to N inputs with various δ 15N, a long-term experiment employing different fertilization treatments needs to be conducted as various organic inputs are applied a couple of times in a single cropping season and return of plant residues including straw may influence δ 15N of rice in the next cropping season (Choi et al. 2006). However, for such a large-scale experiment, a preliminary study to obtain fundamental information on the variations in δ 15N of rice as affected by N inputs with various δ 15N may be helpful. Therefore, this study was conducted to test if the application of isotopically different N sources (i.e., synthetic fertilizer vs. composted manure) results in distinguishable δ 15N of paddy rice and to investigate if paddy rice grown with isotopically similar N sources (i.e., synthetic fertilizer vs. green manure) will show analogous δ 15N in a single cropping season.
Materials and methods
Nitrogen inputs
In this study, three kinds of N inputs were used, which were ammonium sulfate (AS) as synthetic fertilizer, pig manure compost (PMC) as livestock manure compost, and hairy vetch (HV) as green manure. Ammonium sulfate was purchased from a fertilizer manufacturing company (Namhae Chemical Ltd., Yeosu, Korea), and compost was obtained from the experimental livestock farming station at Chonnam National University. Before the initiation of the experiment, seeds of hairy vetch (Vicia villosa Roth.) were sown at the plots allocated for HV treatment (see the Experimental settings for details) at the recommended rate (10 g m−2). Five months later, hairy vetch (including roots) was harvested, weighed, and stored at 4°C until field application. The compost had a δ 15N value of 15.3‰ which was significantly (P < 0.05) higher than those of AS (−0.4‰) and HV (−0.5‰; Table 1).
Experimental settings
The experiment was conducted in the experimental rice paddy at Chonnam National University (126°53′ E, 35°10′ N), Gwangju, Republic of Korea. The climate is temperate with an annual mean temperature of 13.7°C over the past 30 years. Annual mean precipitation was approximately 1,520 mm, with over 60% of the annual precipitation occurring during the monsoon rainy season (June to August). The soil of the paddy field was classified as fine loamy, mesic family of Typic Hapludults in the USDA Soil Taxonomy. The δ 15N of soil was 6.4 ± 0.6‰ for total N, 4.1 ± 1.6‰ for NH +4 , and 5.3 ± 0.4‰ for NO -3 (Table 2).
Four treatments were laid out in a randomized complete block design with four replicates: control without N inputs (code: control), synthetic fertilizer with AS, livestock manure compost with PMC, and green manure with HV. In each block (4 × 15 m), plots were located 2 m apart, and each plot (1.5 × 1.5 m) was confined by inserting flexible plastic barriers (40 cm in height) into the plow pan layer of the soil (around 20 cm in depth) to minimize cross-contamination. Seven days before rice transplanting, N inputs, together with fused phosphate of which chemical composition is P2O5 19%, SiO2 27%, CaO 30%, and MgO 12% (4.5 g P2O5 m−2) and KCl (5.7 g K2O m−2), were mixed thoroughly with the soil surface layer (0∼20 cm). The total amount of N applied was 11 g m−2 (the recommend rate for rice cultivation) for AS, 22 g m−2 for PMC, and 9.3 g m−2 for HV treatments. For PMC, N was doubled to compensate the low N availability of livestock manure compost (Lim et al. 2010), and the lower HV application was due to low dry matter production of the green manure.
The total amounts of PMC and HV were applied as basal fertilization, but AS was applied in three dressings (5.5 g m−2 at transplanting, 3.3 g m−2 at tillering stage on 16 days after transplanting (DAT) and 2.2 g m−2 at panicle initiation stage on 43 DAT following the conventional fertilization practices.
Rice cultivation and sampling
Three 30-day-old rice seedlings (O. sativa L. subsp. Japonica) were transplanted into each plot with a hill spacing of 15 cm and a row spacing of 30 cm (a total of 50 hills per plot). The same rice seedlings were transplanted in the remaining spaces of the field to create canopy conditions. The plots were kept waterlogged (water depth, 3 cm) throughout the growing season except for a 7-day summer drainage period in the middle of July.
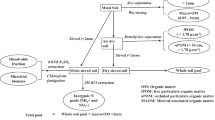
Rice samples (both above- and below-ground) were collected on 15 (the day before second fertilization), 42 (the day before third fertilization), 72, and 110 DAT (at maturity) from three randomly selected hills. Above-ground plants were collected, and the soils (a cylindrical shape with 5 cm in hill direction × 15 cm in row direction × 20 cm in depth with the hill as a center) were excavated intact with a shovel. Rhizosphere soils were separated from the excavated soils–roots mixture as much as possible by hands, and all the broken roots found during the separation were placed in plastic bags. The separated soils were mixed homogenously with hands, and around 100 g of the rhizosphere soils was used for chemical analysis, and the remaining was backfilled to each sampling pit. The roots including those in the plastic bags were covered with a net, and all visible root tissue was collected by washing soil particles out with tap water.
Plant samples collected on 15, 42, and 72 DAT were treated as single samples (i.e., not separated into each plant part), and those on 110 DAT were separated into grain (i.e., panicle), shoot (including green and dead leaves, leaf sheath and culm, and rootstocks) and root. Half of the grain was polished by hand removal of hull followed by milling. All the plant samples were oven-dried at 60°C to a constant weight. After dry weight measurement, plant samples were ground to coarse particles.
Chemical analyses and calculation
For the determination of total N concentration and δ 15N, each sample (soil, PMC, AS, and HV) was dried in different manners considering their NH +4 concentration that is susceptible to volatilization. Soil samples with a relatively low NH +4 concentration (9.1 mg N kg−1, see Table 2) were air-dried; PMC samples which have an NH +4 concentration as high as 327.2 mg N kg−1 (see Table 1) were freeze-dried (to minimize N loss during drying), and AS and HV samples oven-dried at 65°C to facilitate water removal. All the samples were ground to a fine powder with a ball mill (MM200, Retsch GmbH, Haan, Germany) and analyzed for concentration and the corresponding δ 15N using a continuous-flow stable isotope ratio mass spectrometer (CF-IRMS) linked to an elemental analyzer (IsoPrime-EA, Micromass, UK).
Mineral N (NH +4 and NO -3 ) concentrations of compost and soil samples were analyzed with the steam distillation method after extraction with 2 M KCl at 1:10 (w/v) ratio (Hauck 1982; Keeney and Nelson 1982). The N content in the distillates was potentiometrically determined by titration with 0.01 mol L−1 NaOH. After titration, the solution containing NH +4 was adjusted to pH 3 using 0.05 mol L−1 H2SO4 and evaporated to dryness at 65°C in an oven and analyzed for δ 15N using the CF-IRMS. Nitrogen isotope compositions were calculated as

where R sample and R standard are the ratio of 15N/14N for sample and standard (atmospheric N2), respectively. The reproducibility (n = 10) of the analytical procedure was checked with IAEA-N2 (ammonium sulfate, 20.3‰) for NH +4 and an internal reference material (6.8‰ for soil, 13.5‰ for compost, and 4.8‰ for plant samples) for total N as reference materials following the above-mentioned procedures, and it was better than 0.1‰.
For the sample collected at harvest (the final sampling), the integrated δ 15N values of the whole rice plant consisting of root, shoot, and grain were calculated using the following isotope mass balance equations (Karamanos and Rennie 1981):
where Nroot, Nshoot, Ngrain, and Nwhole are the N content of root, shoot, unpolished grain, and whole rice plant, respectively, and δ 15Nroot, δ 15Nshoot, δ 15Ngrain, and δ 15Nwhole are their corresponding δ 15N, respectively.
Statistical analysis
All the statistical analyses were performed with PASW Statistics 18 package (SPSS Inc., Chicago, IL, USA). For statistical analysis, data were tested for homogeneity of variance and normality of distribution. Dry matter data were normally distributed (P > 0.05) but N uptake at 72 and 110 DAT, and δ 15N of rice at 42 DAT were not. These data were log-transformed to achieve normal distribution and homogeneity of variance, and back-transformed means with 95% confidence limit are reported. Analysis of variance was performed to examine the effects of N inputs using the general linear model. When the treatment effects were significant, the means were separated by the Tukey's honestly significant difference test. The significance level was set at α = 0.05 for the mean separation.
Results
Dry matter and N uptake of rice
In all the treatments, the dry matter (DM) of rice increased linearly (r 2 > 0.98 and P < 0.01, regression equation of DM with DAT was not shown) with elapse of days after transplanting (Table 3). Comparing among the treatments, application of AS and HV resulted in a greater DM production than the control with varying statistical significance depending on sampling time (Table 3), meanwhile the effect of PMC application on increasing DM production over the control was not significant at α = 0.05. Overall, AS application produced the greatest DM, followed by HV and PMC applications (except at 15 DAT). At the final sampling time point (110 DAT), the DM of rice was in the order of AS (2,382.2 g m−2) > HV (1,744.0 g m−2) > PMC (1,183.9 g m−2) ≥ control (1,011.7 g m−2) at α = 0.05.
Temporal and inter-treatment variations in the amount of N assimilated by rice showed similar patterns to DM (Table 3). At 110 DAT, the amount of N uptake was greatest in AS (15.2 g m−2), followed by HV (9.3 g m−2), then PMC (7.5 g m−2), and finally the control (6.1 g m−2; P < 0.05).
Temporal change and intra-plant variations in δ 15N of rice
At 15 DAT, the δ 15N of rice was significantly (P < 0.05) different among treatments, 10.5‰ for PMC > 5.5‰ for control > 4.0‰ for HV > 1.8‰ for AS (Fig. 1). However, at 42 DAT, differences in δ 15N of rice among treatments became smaller and that between AS and HV treatments was insignificant (P > 0.05), as a result of a decrease in δ 15N of rice in the PMC treatment (8.5‰) and a concomitant increase in δ 15N in the AS treatment (4.6‰). Thereafter, the δ 15N of rice in PMC and AS treatments showed very small fluctuation and the final δ 15N values at 110 DAT were 8.2‰ and 5.4‰, respectively. Meanwhile, the δ 15N of rice grown without N input (control) and with HV did not change dramatically at 42 DAT but showed gradual increasing patterns with time from 5.5‰ (15 DAT) to 6.7‰ (110 DAT) for control and from 4.0‰ to 5.2‰ for HV treatment during the same period.
Temporal changes in δ 15N of whole rice plants grown with different N sources. Values are the means of four replicates with standard errors (vertical bars). Treatment effects were all significant at α = 0.05. Data on the same days after treatment (DAT) with different lowercase letters are significantly different at α = 0.05
At 110 DAT (harvest), the δ 15N (8.2‰) of whole rice with PMC was significantly (P < 0.05) higher than that (6.7‰) of the control. Meanwhile, application of AS and HV resulted in similar δ 15N of whole rice (5.4‰ for AS and 5.2‰ for HV) that is lower than the control. The effect of N inputs on δ 15N of each rice part was similar to that on whole rice, and the value for each part was highest in PMC and lowest in AS and HV treatments (Fig. 2). Comparing the δ 15N of rice parts, grain δ 15N ranged from 5.3‰ to 7.1‰ for unpolished and from 4.6‰ to 6.1‰ for polished, and those of shoot and root were between 7.8‰ and 8.6‰ and between 7.7‰ and 9.7‰, respectively. Within the same N treatments, δ 15N of grain for control and PMC in which N availability was relatively low as shown by the low N uptake (Table 3) was significantly (P < 0.05) lower than those of shoot and root but not for AS and HV treatments (Table 3).
δ 15N of root, shoot, unpolished grain (rough rice), and polished grain of rice sampled at harvest (110 DAT). Values are the means of four replicates with standard errors (vertical bars). Treatment effects were all significant at α = 0.05. Data on the same days after treatment (DAT) with different lowercase letters are significantly different at α = 0.05
Concentrations and δ 15N of soil mineral N
Across the treatments, the concentration of NH +4 varied between 3.4 and 6.4 mg kg−1 during the sampling time (Fig. 3a). Significant treatment effect was observed during the growth period until 72 DAT, and NH +4 concentration was consistently (P < 0.05) higher in AS than in other treatments. Meanwhile, NO -3 concentration was consistently lower than 2 mg kg−1 during the course of the experiment (Fig. 3b).
The δ 15N of soil NH +4 fluctuated from 5.4‰ to 9.6‰ (Fig. 4). Overall, soil amended with PMC had a relatively higher (P < 0.05) δ 15N than those amended with other treatments throughout the experiment (Fig 4). However, differences in δ 15N among other N inputs were not significant (P > 0.05). The δ 15N of NO -3 was not determined due to a sustained low concentration of below 2 mg kg−1.
Discussion
Dry matter production and N uptake of rice
The percent increment of DM and N uptake relative to the control were 17.0% and 24.2% for PMC, 135.5% and 150.6% for AS, and 72.4% and 54.1% for HV, respectively (calculated with the data in Table 3). The significant greater DM yield and N uptake of rice grown with AS than those with PMC and HV seemed to reflect their different N availability. The amounts of plant N derived from inputs calculated by using difference method (Cassman et al. 1998), i.e., the difference in N uptake between control and each N input treatment, were 1.5, 9.1, and 3.3 g N m−2 for PMC, AS, and HV treatments, respectively. These amounts correspond to 6.7%, 83.1%, and 35.3% of the applied N inputs, respectively. Apparently, lower uptake efficiency of livestock manure compost-N by rice than that of chemical fertilizer-N was consistent with the result of Nishida et al. (2005), reporting 17% of N uptake efficiency for swine manure compost and 34% for ammonium sulfate, and this is generally ascribed to low mineralization of compost-N due to stabilization of organic compost-N via humification during composting (Kim et al. 2008). The N uptake efficiency of PMC observed in this study (6.7%) is relatively low but within the ranges of percent N mineralization (1.2% to 65.0%) of a variety of livestock manure compost (Lim et al. 2010; Miller et al. 2008; Nendel et al. 2004). Meanwhile, the relatively high N uptake efficiency of AS in this study than the values (less than 50%) reported for chemical fertilizer (Bronson et al. 2000) could be attributed both to split application and to the confined experimental conditions that can minimize loss of applied N. The difference method used in this study could also lead to over-estimation of N uptake efficiency as N fertilization can stimulate uptake of soil N over the control due to so-called “priming effect” (Jenkinson et al. 1985).
Compared with AS, however, low uptake efficiency of HV by rice was different from other studies (Asagi and Ueno 2009; Diekmann et al. 1993) that reported no significant difference between chemical fertilizer and green manure. In this study, HV application was conducted at 7 days before transplanting (i.e., 6 days earlier than AS application) as recommended by Korean government to avoid gas damage to young seedling that is in contrast with other studies in which green manure and chemical fertilizer were applied at the same time. The early incorporation of HV into soil could lower N uptake efficiency because green manure is easily decomposable and thus some portion of N derived from HV might have been lost before rice transplanting (Thorup-Kristensen 1994). Magid et al. (2001) reported that most mineralization of green manure estimated by CO2 evolution occurred rapidly during the first 4 days of incorporation into soil.
Temporal changes in rice δ 15N
Plant δ 15N is determined by δ 15N of the N source (soil and N inputs) and isotopic fractionation associated with N transformation and subsequent loss that results in 15N enrichment of the substrate (i.e., mineral N) for plant uptake (Evans 2001). Due both to isotope mixing of added N with indigenous soil N and to the isotopic fractionation, plant δ 15N is likely to deviate from the 15N signature of applied N (Choi et al. 2002; Högberg 1997). In our study, the significantly (P < 0.05) different isotope signatures among treatments at 15 DAT (Fig. 1) show that the N source effect is greater than the N isotopic fractionation effect in the early growth period, leading to greater δ 15N difference among treatments (10.5‰ for PMC > 5.5‰ for control > 4.0‰ for HV > 1.8‰ for AS) than in the later growth period (Fig. 1).
When the plant δ 15N at 15 DAT was compared with the δ 15N of the N source, rice grown with PMC had a lower δ 15N (10.5‰) than the applied N input (15.3‰) while that receiving AS or HV had a higher δ 15N (1.8‰ and 4.0‰, respectively) than the applied N inputs (−0.4‰ and −0.5‰, respectively) due primarily to isotope mixing with soil N, which has an intermediate δ 15N (6.4‰). In spite of the similar isotope signature of AS and HV, δ 15N of rice grown with AS showed a lower δ 15N than that with HV, possibly due to a greater contribution of 15N-depleted N input to total plant N in AS treatment than that in HV treatment, as indicated by greater N uptake in AS (1.0 g m−2) than in HV (0.5 g m−2) treatments at 15 DAT (Table 1).
At 42 DAT, δ 15N of rice grown with compost decreased by 2.0‰ and that with AS increased by 2.8‰, and thereafter their values remained almost constant (Fig. 1). These temporal changes in δ 15N of crop grown with compost and synthetic fertilizer have been reported by Choi et al. (2002) who ascribed the pattern primarily to increasing contribution of soil-derived N of which δ 15N is lower than that of compost but higher than that of synthetic fertilizer to total plant N with time (Tables 1 and 2). Meanwhile, 15N enrichment of plant-available mineral N due to N isotopic fractionation associated with N transformation and loss might also play a critical role in temporal increases of rice δ 15N particularly for AS treatment in which mineral N availability was higher than other treatment (Fig. 3) as 15N enrichment of the remaining soil N due to N loss is proportional to N availability (Choi et al. 2003b; Högberg 1997). Besides, changed N turnover rates by different N inputs (inorganic vs. organic) might have influenced δ15N of mineral N and subsequent plant δ 15N, e.g., Billings et al. (2002) reported that increased microbial activity due to sufficient C substrate under elevated CO2 resulted in 15N-enrichment of soil mineral N and plant tissue. In the present study, therefore, the higher δ 15N of NH +4 (Fig. 4) and rice (Fig. 1) treated with PMC could be attributed partially to increased N turnover by the application of organic-C enriched compost. However, such effect was not observed for HV treatment, suggesting that δ 15N rather than chemical composition (e.g., organic-C content) of N inputs might influence δ 15N of available N and subsequent that of rice more greatly.
The δ 15N of plant is often correlated with that of available N (mineral N) for root uptake (Billings et al. 2002; Choi et al. 2003a). However, in our study, the temporal pattern of δ 15N of NH +4 which is the predominant mineral N form available for plant uptake in paddy soils did not match with that of rice, suggesting that the δ 15N signal of mineral N is not always a conservative surrogate of the N source because the mineral N pool is very dynamic, and thus its δ 15N is susceptible to change by many processes including volatilization and nitrification (for NH +4 ) and denitrification (for NO -3 ; Choi and Ro 2003; Choi et al. 2003b). Although microbial immobilization–remineralization of N (Billings et al. 2002; Monaco et al. 2010) and physico-chemical fixation of NH +4 (Nieder et al. 2011) are also important fates of NH +4 in soils, N isotopic fractionation associated with these processes are generally negligible (Högberg 1997). For example, N isotopic fractionation factors (α) associated with N mineralization, immobilization, and ion exchange are generally lower than 1.002, but those with NH3 volatilization, nitrification, and denitrification are as high as 1.035 (Högberg 1997). In an incubation study without plants, Choi and Ro (2003) investigated the changes in δ 15N of NH +4 and NO -3 in water-saturated and unsaturated soils after adding (NH4)2SO4 (δ15N = −2.6‰) and found that δ 15N of NH +4 sharply increased to around 50.0‰ with a rapid decrease of NH +4 concentration within 7 days under water-unsaturated nitrifying conditions. Meanwhile, under water-saturated conditions, NH +4 concentration decreased at a slower rate, and δ 15N of the remaining NH +4 increased to around 20.0‰ at most during the 36-day incubation due to retardation of nitrification under anaerobic conditions as in paddy soils. Another reason for the discrepancy of the temporal pattern of δ 15N between plant and NH +4 is that plant δ 15N is an integrated δ 15N value for the whole plant growth period but δ 15N of mineral N is an instantaneous value at the time of soil sampling. For example, the δ 15N of NH +4 in AS-treated soil represents the value of the remaining NH +4 after rice uptake of applied AS because soil sampling (i.e., on 15 and 42 DAT) was made the day before next fertilization in our study.
Rice δ 15N with N inputs at harvest
The δ 15N of various N inputs have been reported by Bateman and Kelly (2007); the mean value was −0.2‰ for synthetic fertilizer, 8.1‰ for manure/compost, 2.5‰ for seaweed-based materials, 5.9‰ for non-manure wastes of livestock, and 7.1‰ for fish-based materials in their study. Among the various N inputs, the types of N inputs tested for crop δ 15N are classified into liquid manure (LM), solid manure (SM), manure compost (MC), synthetic fertilizer (SF), and non-synthetic fertilizer/non-manure (NSF/NM) type such as hornmeal and corn steep liquor (Bateman et al. 2005; Choi et al. 2002, 2003a, 2006; Georgi et al. 2005; Lim et al. 2007; Nakano et al. 2003; Yun et al. 2006).
Summarizing the previous studies on δ 15N variations of crops as affected by various types of N inputs, the δ 15N of crops ranged from 5.6‰ to 14.9‰ for LM, from 6.0‰ to 12.0‰ for MC, from 5.7‰ to 10.0‰ for NSF/NM, from 2.2‰ to 6.0‰ for SM, from 0.7‰ to 7.7‰ for no N input (i.e. control in the experiments), and from −4.0‰ to 5.5‰ for SF (Fig. 5). The δ 15N of rice in our study are within (for control, PMC, and AS) or adjacent to (for HV) the range reported for the corresponding N input regime as depicted in Fig. 5. The wide variations in δ 15N of crops grown with non-synthetic N inputs are ascribed primarily to different N availability and δ 15N of N inputs. Therefore, when other experimental conditions are the same, application of LM (high δ 15N and high N availability) is likely to result in highest crop δ 15N followed by MC (high δ 15N and low N availability), NSF/NM (intermediate δ 15N and high N availability), and SM (low δ 15N and high N availability; Bateman et al. 2005; Choi et al. 2006; Georgi et al. 2005; Lim et al. 2007). Meanwhile, a wide range of δ 15N of crops grown with the same type of N input is attributed to many factors, such as fertilization rate, N availability of inputs, weather conditions, soil characteristics, crop species, and other management practices (Bateman and Kelly 2007; Choi et al. 2006; Rogers 2008; Yun et al. 2006).
Distribution of δ 15N in crops as affected by the type of N inputs applied (LM liquid manure, MC manure compost, SM solid manure, NI no input, SF synthetic fertilizer, NSF/NM non-synthetic fertilizer/non-manure). Data adopted from Choi et al. (2002; 2003a), Bateman et al. (2005), Choi et al. (2006), Yun et al. (2006), Nakano et al. (2003), Lim et al. (2007), Georgi et al. (2005). Error bars indicate the range of δ 15N and boxes correspond to the most frequently observed range of values. Solid circle represents the δ 15N of whole rice based on the N input category observed in this study
In our study, the difference in δ 15N of whole rice between treatments with PMC (8.2‰) and AS (5.4‰) at harvest was 2.8‰, suggesting that δ 15N of rice can be exploited in discriminating rice grown with manure compost and synthetic fertilizer. Fortunately, we observed higher δ 15N of soil NH +4 in PMC treatment than in AS treatment (Fig. 4). The difference between manure-based N inputs and synthetic fertilizer (2.8‰) detected in our study is within the range (around 2‰ to 6‰ difference) reported for other crops, for example, less than that for Chinese cabbage (Brassica campestris L.) and chrysanthemum (Chrysanthemum morifolium Ramatuelle) grown with liquid pig manure and urea (>6.0‰ difference) (Lim et al. 2007), similar for carrot (Daucus carota L.) grown with chicken manure and NH4NO3 (3‰ to 4‰ difference; Bateman et al. 2005), and greater for maize grown with pig manure compost and urea (<2‰ difference; Choi et al. 2002). This variation is due primarily to differences in N availability and δ 15N of N inputs as previously mentioned. In general, the higher the δ 15N and N availability of the organic fertilizer, the higher the δ 15N in crop tissues (Choi et al. 2006).
Meanwhile, the δ 15N of whole rice grown with AS and HV was not different although the amount of rice N derived from AS (9.1 g N m−2) was higher than that from HV (3.3 g N m−2), and both are not isotopically different. This result suggests that the δ 15N technique is not applicable in discriminating conventional farming with synthetic fertilizer from organic farming with green manure. The δ 15N of N derived from inputs (δ 15NNDFI) was calculated by using the isotope mass balance equations (Karamanos and Rennie 1981).
where TNT and TNcontrol are the N content of rice treated with N inputs and the control, respectively, and δ 15NT and δ 15Ncontrol are the corresponding δ 15N of rice. The δ 15N of N derived from AS (δ 15NNDFAS) and HV (δ 15NNDFHV) were 4.6‰ and 2.6‰, respectively. The higher δ 15NNDFAS and δ 15NNDFHV than the corresponding δ 15N of N inputs (−0.4‰ for AS and −0.5‰ for HV, see Table 1) could be attributed to 15N enrichment of the remaining N via N isotopic fractionation during N loss, and such patterns have been widely reported for several crop species (e.g., Choi et al. 2002, 2003a, 2006). However, for AS treatment, the “priming effect” seemed to contribute to the higher δ 15NNDFAS as root DM at the harvest was significantly (P < 0.001, data not shown) higher in AS treatment (592.8 g m−2) than others (204.4, 236.7, and 428.5 g m−2 for control, PMC, and HV, respectively). According to the “priming effect” theory (Jenkinson et al. 1985), chemical fertilizer enhances root proliferation and thus uptake of soil N particularly when the root growth is not restricted as in the present study (Choi et al. 2001). In this study, δ 15N of total soil and rice in control was 6.4‰ and 8.2‰ (Table 2; Fig. 1) and thus increased uptake of soil-derived N which is more enriched with 15N compared with AS-derived N could lead to estimation of δ 15NNDFAS at a higher value than the real δ 15N of N derived from AS.
All the rice parts (root, shoot, and grain) showed similar δ 15N patterns to the total rice plant, with the highest δ 15N in PMC treatments followed by the control and then HV or AS (Fig. 2). Among the rice parts, grain showed the smallest isotopic difference (1.8‰ for unpolished and 1.1‰ for polished) between PMC and AS treatments compared with other parts (3.7‰ for root and 3.5‰ for shoot). This seemed to be related with rice organ development and associated N uptake and use patterns. Rice plants assimilate N from soils and store N in shoot during the active growing period, and at the grain filling stage (i.e., reproductive growth stage), parts of N are supplied from N stored in the shoot (internal N reservoir) through remobilization and the other parts from the soil through root uptake (external soil N; Kano 1995; Thorup-Kristensen 1994). Since N availability of PMC-treated soil was much lower than that of AS-treated soil as indicated by N uptake data (Table 3), reliance of rice on an internal N source (shoot N) for grain filling might have been greater when it was grown with PMC than that with AS. Remobilization and redistribution of stored N in the plant causes 15N depletion in the sink (grain for rice) due to N isotopic fractionation associated with enzymatic remobilization of stored N (Choi et al. 2002; Shearer and Kohl 1986). In our study, the lower δ 15N of grain than shoot in relatively N-limited soils as in the control and PMC treatments evidenced N isotopic fractionation during the N allocation from shoot to grain, and Choi et al. (2002) also reported similar pattern with maize. Therefore, the smallest isotopic difference of grain between PMC and AS treatments could be ascribed to the greater contribution of intra-plant redistribution of N to grain N of PMC treatment that led to a lower δ 15N of grain.
Not only δ 15N of N sources (fertilizer-N and soil N) but also N assimilation pattern can influence intra-plant patterns of δ 15N (Choi et al. 2002). For example, a significant intra-plant variation of δ 15N can be found when NO -3 rather than NH +4 is the predominant N source because primary NO -3 assimilation can occur in both roots and leaves and NO -3 not assimilated in the root and thus translocated to leaves would be enriched in 15N because it experienced N isotopic fractionation during the assimilation mediated by nitrate reductase in the root (Evans et al. 1996; Yoneyama and Kaneko 1989). Meanwhile, little intra-plant variations of δ 15N are observed when NH +4 is the predominant N source as in this study (paddy conditions), because NH +4 is likely to be assimilated immediately after entering roots due to its toxic accumulation within the plant (Bloom 1988). Because the predominant mineral N species in our study is NH +4 due to the waterlogged soil conditions (Fig. 3), therefore, the contribution of the N assimilation pattern to the intra-plant variations of δ 15N could be assumed to be negligible.
In conclusion, although this study was conducted for a single cropping season, the results suggest a possibility of using δ 15N analysis in discriminating rice grown with manure composts from that with synthetic fertilizers; however, when organic N input (HV in our study) of which δ 15N signal is similar to synthetic fertilizers (AS) is used, rice δ 15N may not serve as a surrogate of N sources. We believe that our study provides fundamental data on δ 15N variations of rice plants as affected by different N inputs, and these data can be considered in designing a long-term experiment. Although small isotopic difference of grain, the commercial product of rice cultivation, between PMC and AS treatments was observed in this single cropping experiment, this may need to be further tested in a long-term experiment as continuous applications of organic inputs may enrich soil N with 15N and thus lead to annual increases of plant δ 15N. It may also be necessary to consider investigating δ 15N of other rice parts (i.e., whole rice, root, and shoot) samples for more reliable certification.
References
Asagi N, Ueno H (2009) Nitrogen dynamics in paddy soil applied with various 15N-labelled green manures. Plant Soil 322:251–262
Ashraf M, Mahmood T, Azam F, Qureshi RM (2004) Comparative effects of applying leguminous and non-leguminous green manures and inorganic N on biomass yield and nitrogen uptake in flooded rice (Oryza sativa L.). Biol Fertil Soils 40:147–152
Bateman AS, Kelly SD (2007) Fertilizer nitrogen isotope signatures. Isot Environ Healt Stud 43:237–247
Bateman AS, Kelly SD, Jickells TD (2005) Nitrogen isotope relationships between crops and fertilizer: implications for using nitrogen isotope analysis as an indicator of agricultural regime. J Agric Food Chem 53:5760–5765
Bateman AS, Kelly SD, Woolfe M (2007) Nitrogen isotope composition of organically and conventionally grown crops. J Agric Food Chem 55:2664–2670
Billings SA, Schaeffer SM, Zitzer S, Charlet T, Smith SD, Evans RD (2002) Alterations of nitrogen dynamics under elevated carbon dioxide in an intact Mojave Desert ecosystem: evidence from nitrogen-15 natural abundance. Oecologia 131:463–467
Bloom AJ (1988) Ammonium and nitrate as nitrogen sources for plant growth. ISI Atlas Sci 1:55–59
Bronson KF, Hussain F, Pasuquin E, Ladha JK (2000) Use of 15N-labeled soil in measuring nitrogen fertilizer recovery efficiency in transplanted rice. Soil Sci Soc Am J 64:235–239
Cassman KGS, Peng S, Olk DC, Ladha JK, Reichardt W, Doberman A, Singh U (1998) Opportunities for increased nitrogen use efficiency from improved resource management in irrigated rice systems. Field Crops Res 56:7–38
Choi WJ, Ro HM (2003) Difference in isotopic fractionation of nitrogen in water-saturated and unsaturated soils. Soil Biol Biochem 35:483–486
Choi WJ, Jin SA, Lee SM, Ro HM, Yoo SH (2001) Corn uptake and microbial immobilization of 15N-labeled urea-N in soil as affected by composted pig manure. Plant Soil 235:1–9
Choi WJ, Lee SM, Ro HM, Kim KC, Yoo SH (2002) Natural 15N abundances of maize and soil amended with urea and composted pig manure. Plant Soil 245:223–232
Choi WJ, Ro HM, Hobbie EA (2003a) Patterns of natural 15N in soils and plants from chemically and organically fertilized uplands. Soil Biol Biochem 35:1493–1500
Choi WJ, Ro HM, Lee SM (2003b) Natural 15N abundances of inorganic nitrogen in soil treated with fertilizer and compost under changing soil moisture regime. Soil Biol Biochem 35:1289–1298
Choi WJ, Arshad MA, Chang SX, Kim TH (2006) Grain 15N of crops applied with organic and chemical fertilizers in a four-year rotation. Plant Soil 284:165–174
Coats B (2003) Global rice production. In: Smith CW, Dilday RH (eds) Rice Origin, History, Technology and Production. Wiley, Hoboken, NJ, pp 247–470
Codex Committee on Food Labelling (2001) Guidelines for the Production, Processing. Marketing and Labelling of Organically Produced Foods, Rome, Italy
Diekmann KH, Datta SK, Ottow JCG (1993) Nitrogen uptake and recovery from urea and green manure in lowland rice measured by 15N and non-isotope techniques. Plant Soil 148:91–99
Evans RD (2001) Physiological mechanisms influencing plant nitrogen composition. Trends Plant Sci 6:121–126
Evans RD, Bloom AJ, Sukrapanna SS, Ehleringer JR (1996) Nitrogen isotope composition of tomato (Lycopersicon esculentum Mill. Cv. T-5) grown under ammonium or nitrate nutrition. Plant Cell Environ 19:1317–1323
Georgi M, Voerkelius S, Rossmann A, Graβmann J, Schnitzler WH (2005) Multielement isotope ratios of vegetables from integrated and organic production. Plant Soil 275:93–100
Hauck RD (1982) Nitrogen-isotope ratio analysis. In: Page AL (ed) Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties. SSSA and ASA, Madison, WI
Högberg P (1997) Tansley Review no. 95. 15N natural abundance in soil-plant systems. New Phytol 137:179–203
Jenkinson DD, Fox RH, Rayner JH (1985) Interactions between fertilizer nitrogen and soil nitrogen—the so called ‘priming’ effect. J Soil Sci 36:425–444
Kano H (1995) Major essential elements. In: Matsuo T, Kimazawa K, Ishii R, Ishihara K, Hirata H (eds) Science of the Rice Plant. Food and Agriculture Policy Research Center, Tokyo, Japan, pp 310–401
Karamanos RE, Rennie DA (1981) The isotope composition of residual fertilizer nitrogen in soil columns. Soil Sci Soc Am J 45:316–321
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL (ed) Methods of soil analysis. Part 2: chemical and microbiological properties. SSSA and ASA, Madison, WI
Kim YJ, Choi WJ, Lim SS, Kwak JH, Chang SX, Kim HY, Yoon KS, Ro HM (2008) Changes in nitrogen isotopic compositions during composting of cattle feedlot manure: effects of bedding material type. Bioresour Technol 99:5452–5458
Lim SS, Choi WJ, Kwak JH, Jung JW, Chang SX, Kim HY, Yoon KS, Choi SM (2007) Nitrogen and carbon isotope responses of Chinese cabbage and chrysanthemum to the application of liquid pig manure. Plant Soil 295:67–77
Lim SS, Kwak JH, Lee SI, Lee DS, Park HJ, Hao X, Choi WJ (2010) Compost type effects on nitrogen leaching from Inceptisol, Ultisol, and Andisol in a column experiment. J Soils Sediment 10:1517–1526
Magid J, Henriksen O, Thorup-Kristensen K, Mueller T (2001) Disproportionately high N-mineralization rates from green manures at low temperatures – implications for modeling and management in cool temperate agro-ecosystems. Plant Soil 228:73–82
Mann S (2003) Why organic food in Germany is a merit good. Food Policy 28:459–469
Miller JJ, Beasley BW, Chanasyk DS, Larney FJ, Olson BM (2008) Short-term nitrogen leaching potential of fresh and composted beef cattle manure applied to disturbed soil cores. Compost Sci Util 16:12–19
Monaco S, Sacco D, Borda T, Grignani C (2010) Field measurement of net nitrogen mineralization of manured soil cropped to maize. Biol Fertil Soils 46:179–184
Nakano A, Uehara Y, Yamauchi A (2003) Effect of organic and inorganic fertigation on yields, δ15N values, and δ13C values of tomato (Lycopersicon esculentum Mill. cv. Saturn). Plant Soil 255:343–349
Nendel C, Reuter S, Kubiak R, Neider R (2004) Nitrogen mineralization from mature bio-waste compost in vineyard soils. 1. Long-term laboratory incubation experiments. J Plant Nutr Soil Sci 94:159–167
Nieder R, Benbi DK, Scherer HW (2011) Fixation and defixation of ammonium in soils: a review. Biol Fertil Soils 47:1–14
Nishida M, Moriizumi M, Tsuchiya K (2005) Changes in the N recovery process from 15N-labeled swine manure compost and rice bran in direct-seeded rice by simultaneous application of cattle manure compost. Soil Sci Plant Nutr 51:577–581
Ro HM, Choi WJ (2010) Method for discriminating organic agricultural products from conventional agricultural products by using nitrogen isotope index. US Patent Publication No. US 7670842 B2.
Rogers KM (2008) Nitrogen isotopes as a screening tool to determine the growing regimen of some organic and nonorganic supermarket produce from New Zealand. J Agric Food Chem 56:4078–4083
Schmidt HL, Roβmann A, Voerkelius S, Schnitzler WH, Georgi M, Graβmann J, Zimmermann G, Winkler R (2005) Isotope characteristics of vegetables and wheat from conventional and organic production. Isot Environ Healt Stud 41:223–228
Shearer G, Kohl DH (1986) N2-fixation in field settings: estimations based on natural abundance. Aus J Plant Physiol 13:699–756
Šturm M, Kacjan-Maršić N, Jojen S (2011) Can δ15N in lettuce tissues reveal the use of synthetic nitrogen fertilizer in organic production? J Sci Food Agric 91:262–267
Thorup-Kristensen K (1994) The effect of nitrogen catch crop species on the nitrogen nutrition of succeeding crops. Fert Res 37:227–234
Yoneyama T, Kaneko A (1989) Variations in the natural abundance of 15N in nitrogenous fractions of komatsuna plants supplied with nitrate. Plant Cell Physiol 30:957–962
Yun SI, Ro HM, Choi WJ, Chang SX (2006) Interactive effects of N fertilizer source and timing of fertilization leave specific N isotopic signatures in Chinese cabbage and soil. Soil Biol Biochem 38:1682–1689
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD; KRF-2008-313-F00005).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yun, SI., Lim, SS., Lee, GS. et al. Natural 15N abundance of paddy rice (Oryza sativa L.) grown with synthetic fertilizer, livestock manure compost, and hairy vetch. Biol Fertil Soils 47, 607–617 (2011). https://doi.org/10.1007/s00374-011-0571-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-011-0571-3