Abstract
The population of burrowing plateau zokors (Myospalax baileyi) was markedly increased in the Qinghai–Tibetan Plateau. The objective of this study was to investigate the effects of zokor foraging and mound-making disturbance on topsoil properties and organic C pools at an alpine site of the Qinghai–Tibetan Plateau. Surface (0–15 cm) soil samples were collected from mounds with different ages (3 months and 3, 6, and 15 years) and from undisturbed grassland. Above- and below-ground plant biomasses were depleted by zokors in newly created mounds (3 months). Plant cover and root biomass gradually recovered thereafter, but were still lower in the 15-year-old mounds than in the undisturbed soils. Organic C contents of coarse (>2 mm), soil (<2 mm), particulate (2–0.05 mm) fractions, and microbial biomass, organic C mineralization, β-glucosidase activity, urease activity, alkaline phosphatase activity, acid phosphatase activity, and soil aggregation were significantly lower in the 3, 6, and 15-year-old mound soils than in the undisturbed soils or newly created mound soils. Fifteen years after mound creation, the soil had only 12% of root biomass, 35% of coarse organic C, 83% of particulate organic C, 58% of microbial biomass C, 57% of 30-day respired C, and 45% of water-stable aggregate mean weight diameter, compared to values of the undisturbed soils. Our results suggested that foraging and mound-making by zokors have negative impacts on properties and organic matter content of the topsoil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
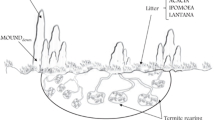
Over the past decades, alpine grasslands in the Qinghai–Tibetan Plateau have experienced severe soil degradation due to the activities of the increased rodent population, intensive farming activities, and climate change (Li 1997; Zhang 2001; Zhang et al. 2002; Zhou et al. 2005; Akiyama and Kawamura 2007; Li et al. 2008). Plateau zokors (Myospalax baileyi) are small (approximately 260–490 g) blind subterranean rodents inhabiting in the Qinghai–Tibetan plateau at an average density of 15 animals per hectare (Zhang et. al. 2003). Plateau zokors spend their lives solely in underground burrows system, and excavate tunnels from deep nests (2–2.5 m for females and 0.8–1.5 m for males) to the subsurface for foraging (Zhang et al. 2003), by using limbs and heads to dig and push the loosened soil to the surface (Su and Wang 1992); they also use strong incisors to hold roots and drag whole plants into the deep tunnel system (Zhang et al. 2003), and make mounds by digging, wriggling, pushing up, and mixing soil while foraging and eradicating the plants. After a mound is made and the plants are consumed, the zokors usually move to a new patch of grassland and make a new mound. As a result, the new zokor mounds are bare. Zokor mounds may cover as much as 15–20% of the soil surface (Zhang et al. 2003), significantly changing plant community structure, and decreasing farmland productivity (Zhang 2002). In addition, burrowing mammals can modify the environment, by generating habitats for other species, regulating community structure and dynamics, and altering ecosystem processes (Davidson and Lightfoot 2008). Generally, zokors’ biology and ecology and their effects on plant diversity have been studied (Zhang et al. 2003), whereas the effects of zokor mound on soil properties have been neglected. We hypothesize that both reduced plant-derived organic inputs by zokor foraging and mechanical disturbance and vertical translocation of soil in the mound-making process will significantly affect the properties of the topsoil where mounds are created. Soil organic matter maintains the stability of soil structure, serves as a reservoir of metabolic energy and nutrients, and determines the resilience of the system after it has been subjected to disturbance (Baldock and Nelson 2000). Soil microflora is essential for the functionality of soil, and its activity controls the cycling of energy and nutrients in soil (Hackl et al. 2005). Soil enzyme activities have been measured to monitor soil recovery processes (Carreira et al. 2008). Therefore, the measurement of changes in the soil organic matter pools, microbial biomass and activity, and soil aggregation, induced by zokor activity, can help in understanding the spatial heterogeneity of soil properties in the fields where zokors are inhabited.
Materials and methods
Site description
The study was conducted at Gansu Agricultural University Alpine Grassland Experimental Station, located in a valley in Tianzhu Zang Autonomous County of Gansu Province. The study site (37°40′ N, 102°32′ E) has an altitude of 2,960 m, with a mean annual temperature of −0.1°C, mean annual precipitation of 416 mm, mean annual pan evaporation of 1,596 mm, and a plant growth period of 120–140 days during May–September. The soil at the site is a typic Cryrendoll according to USDA classification (Wu and Tiessen 2002).
Soil and root biomass sampling
Within a piece of enclosed grassland of about 120 × 150 m, we monitored the mound-making activity on a plot of 40 × 40 m since 1993. Mounds were labeled with the year they were created. The grassland had flat topography, grazed by sheep (Ovis aries) from November to March at about four sheep per hectare. In May 2008, recent (3 months old) and older mounds (3, 6, or 15-year-old) were randomly selected, with each type of mounds replicated three times. All selected mounds were intact since their origin and had a size of about 1 m2. On each replicate, four soil slices (100 × 50 mm in area and 150 mm in depth) were randomly collected using a spade and mixed to produce a composite sample. Litter was removed from the mound surface before soil sampling. Three composite samples of undisturbed soils were also collected as controls. Three subplots (about 6 × 6 m) were randomly selected within the monitored plot, and each subplot included mounds and undisturbed grasslands. On each subplot, four soil slices between mounds (with a distance of 1 m apart from mounds) were randomly taken to produce a composite sample in a similar way for mounds. At the time of soil sampling, the number and area of zokor mounds were measured in the plot.
After removal of roots, each soil sample was divided into three subsamples. One subsample was sieved (<5 mm) and stored at 4°C for a week before measuring microbial biomass C and enzyme activities, and organic C mineralization. Another subsample was air-dried at room temperature and pressed to pass a 2-mm sieve. The components retained on the 2-mm sieve were plant debris and some coarse minerals. They were oven-dried at 60°C, weighed, and then ground for measuring coarse (>2 mm) organic C concentration in soil. The portion passing the 2-mm sieve was used for measurements of soil mineral particle composition, pH value, soil organic C (<2 mm), and particulate organic C (2–0.05 mm). The remaining subsample was gently sieved to pass a 10-mm sieve, and aggregates retained on the 10-mm sieve were broken into sizes less than 10 mm by hands. This sample, less than 10 mm, was then air-dried for determination of soil aggregate stability (see below).
At the time of soil sampling, plant species and vegetation cover were determined for mounds and undisturbed grasslands. A soil column (200 × 200 mm at depth of 150 mm) was randomly excavated on each selected mound or subplot (for controls, about 6 × 6 m). After the soil was washed away, roots were collected by washing of soil and then weighed before being dried in oven at 60°C. Soil bulk density was determined for each selected mound or subplot (for controls) using a cutting ring (inner diameter 5.03 cm, volume 100 cm3; Institute of Soil Science, Academia Sinica 1978).
Organic C determination
The particulate soil fraction (2–0.05 mm) was extracted by shaking 20 g of air-dried (<2 mm) soil with 60 mL 0.5% (w/v) hexametaphosphate solution for 24 h. The dispersed soil was sieved (<0.05 mm) and sands and sand-sized organic materials (particulate fraction) retained on the sieve were oven-dried at 60°C and weighed.
The contents of soil organic C (<2 mm), particulate soil organic C (2–0.05 mm), and coarse organic C (>2 mm) were analyzed by the Walkley and Black dichromate oxidation method (Nelson and Sommers 1982). All samples were ground to pass a 0.25-mm sieve before organic C measurement.
Microbial biomass C determination
Fresh soil (<5 mm) equivalent to 20 g oven-dried weight was fumigated with ethanol-free chloroform at 25°C for 24 h (Joergensen and Brookes 1990). After removal of the fumigant, the soil was extracted with 0.5 M K2SO4 (1:4 soil-to-solution ratio) by shaking the soil slurry for 1 h on a horizontal shaker. The extracted solution was then filtered. Non-fumigated soil extracts were obtained in a similar way at the time fumigation commenced. The content of organic C of extracts was analyzed with a Multi C/N 3100 (Analytik jena, Germany). Microbial biomass C was estimated by the difference between organic C concentration in the fumigated and non-fumigated extracts and multiplying the difference by 2.22 (Joergensen and Brookes 1990).
Organic C mineralization
The moisture of fresh soil (<5 mm), equivalent to 20 g oven-dried weight, was adjusted to 60% of the soil water-holding capacity; then, the soil was incubated in a sealed 500 mL jar for 30 days at 25°C in the dark. The evolved CO2 was absorbed by 0.6 M NaOH solution in vials, which were replaced at days 2, 5, 10, 20, and 30. The quantity of absorbed CO2 from decomposition was determined by back-titration with 0.25 M HCl in the presence of excess BaCl2 using phenolphthalein as an indicator. Incubation of each sample was duplicated. Three 500-mL jars with vials containing 0.6 M NaOH but no soil was incubated as blanks.
Enzyme assay
The activities of β-glucosidase, acid phosphatase, and alkaline phosphatase were assayed by colorimetric determination of p-nitrophenol (PNP) released when soil was incubated with p-nitrophonyl-β-d-glucosidase in pH 6.5 buffer, p-nitrophenyl phosphate in pH 6.5 buffer, and p-nitrophenyl phosphate in pH 11 buffer for 1 h (37°C), respectively (Tabatabai 1982; Hopkins et al. 2008). Fresh soil (<2 mm) equivalent to 0.5 g oven-dried weight was weighed into 30-ml glass vials containing 4 ml of buffer and 1 ml of substrate solution, and incubated. After incubation, 1 ml of 0.5 M CaCl2 and 4 ml of Tris buffer pH 12 were added in the β-glucosidase assay, and 1 ml of 0.5 M CaCl2 and 4 ml of 0.5 M NaOH were added in the acid and alkaline phosphatase assays. Immediately after filtration, the absorbance of enzyme extracts was measured at 400 nm. Urease activity was determined as described by Klose and Tabatabai (2000). Fresh soil (<2 mm), equivalent to 5 g oven-dried, was incubated with 9 ml 0.05 M tris buffer (pH 9.0) and 1 ml 0.2 M urea, as the substrate, at 37°C for 2 h, and then 35 ml of KCl–Ag2SO4 were added to stop the reaction. The NH +4 liberated was determined by steam distillation of 20 ml of the soil suspension with MgO for 4 min. All enzyme assays for each sample were duplicated and controls were included.
Soil aggregate stability and particle size distribution
A 0.25-mm sieve was used to separate 10–0.25 mm soil aggregates of the air-dried soil sample (<10 mm). Aggregates retained on the sieve were weighed and their percentage of the soil sample was calculated. About 30 g of 0.25−10 mm aggregates were placed on the top sieve of a stack of four sieves, with the sieve of 2 mm pore size followed by those with pore size of 1, 0.5, and 0.25 mm. Water level was adjusted so that aggregates on the top sieve were submerged just at the highest point of oscillation. After incubating aggregates in the water for 5 min, the apparatus oscillated for 15 min at a speed of 30 cycles per minute with an amplitude of 6 cm in the sieving action. Each fraction of stable aggregates was then oven-dried at 105°C for 24 h. After being soaked in water in a bowl and broken up using fingers, the coarse/sand particles were separated by sieving (2, 1, 0.5, and 0.25 mm), and were then oven-dried to correct the weight of true water-stable aggregates. The percentage of each water-stable aggregate fraction was calculated on whole soil oven-dry mass base. Mean weight diameter (MWD) was expressed as:
where Fi is the mass proportion of stable aggregate fraction i in whole soil sample and Di is the mean diameter of stable aggregate fraction i. The maximum mean diameter was 6 mm and the minimum 0.125 mm.
Soil particle size distribution was determined using the pipette method (Institute of Soil Science, Academia Sinica 1978).
Data analysis
One-way analysis of variance (ANOVA) was conducted to detect the differences in measured parameters between mounds of different ages using SPSS 10.0. Treatment means were separated using the least significant difference (LSD) at P ≤ 0.05.
Results
Plant species and soil properties
The selected plot of 1,600 m2 included 123 zokor mounds with a total area of 134 m2. Zokor disturbance significantly affected the composition of plant species (Table 1). The dominant perennial species of the undisturbed grasslands completely disappeared in the 3-month-old mounds and changed to annual species in the 3-year-old mounds, compared with a mixture of annual and perennial species in the 6-year-old mounds and mainly perennial species in the 15-year-old mounds (Table 1). Although plant cover and root biomass increased since mounds had been formed, the 15-year-old mounds still had lower plant cover than the undisturbed grasslands and their root biomasses were only 12% of the undisturbed grasslands (Table 1).
Soil particle size distribution was not affected by zokor disturbance (Table 1). Mound-making activity loosened the topsoil such that the newly formed mounds (3 months old) were about 10–15 cm higher than the undisturbed soil. The changed micro-topography gradually flattened with time. Soil bulk density was significantly lower in the zokor mounds than that in the undisturbed grasslands (Table 1). Soil pH (1: 2.5 soil-to-water ratio) varied from 7.7 to 8.0 across mounds and the undisturbed grasslands and was not affected by zokor disturbance (data was not shown).
Organic C pools
Coarse soil organic C (>2 mm) concentration was significantly lower in the mound soils than in the undisturbed soils (Table 2). There was a recovery of coarse soil organic C concentration from the 3 years to the 15-year-old mounds, however, even 15 years after mound formation, coarse soil organic C concentration was only 35% of that of the undisturbed soils (Table 2). Concentrations of soil organic C (<2 mm) and particulate soil organic C (2–0.05 mm) were generally lower in the 3 to 15-year-old mound soils than in the undisturbed soils or 3-month-old mound soils (Table 2). The 3-month-old mounds had a higher soil organic C concentration than the undisturbed grasslands (Table 2). There was a recovery of soil organic C and particulate soil organic C concentrations in the 15-year-old mounds compared to the 6-year-old mounds, however, 15 years after mound creation, the soils had only 96% and 83% of soil and particulate soil organic C concentrations compared to the undisturbed soils (Table 2). Stocks (in the top 15-cm layer) of coarse soil organic C, soil organic C, and particulate soil organic C were significantly lower in the mound soils than in the undisturbed soils (Table 2).
Microbial biomass and activity
The level of microbial biomass C significantly decreased in soils from the 3-, 6-, and 15-year-old mounds compared with soils of the undisturbed grasslands or 3-month-old mounds (Table 2). Fifteen-year-old mounds showed a recovery in soil microbial biomass C compared with the 6-year-old mounds; however, microbial biomass C concentration of the 15-year-old mounds was only 58% of that of the undisturbed grasslands (Table 2). Soil respiration, the amount of CO2-C evolved in the 30-day incubation period was the highest in the undisturbed soils, followed by soil respiration evolved from the 3-month-old mounds, with the lowest values in soils of 3-, 6-, and 15-year-old mounds (Table 2). There was no recovery in soil respiration since mounds had been created; 15-year-old mounds only had 57% of the amount (882 g kg−1) of CO2-C evolved from the undisturbed soils. The 3 months and 3–15-year-old mound soils had lower urease activity, acid phosphatase activity and alkaline phosphatase activity than the undisturbed soils; the 3–15 years old mound soils had lower β-glucosidase activity than the undisturbed or 3 months old mound soils (Table 3). The 15-year-old mounds showed a recovery in soil urease activity and alkaline phosphatase activity compared to the 6-year-old mounds (Table 3).
Aggregation
Contents of dry-sieved aggregates and water-stable aggregates in soils of 3-, 6-, and 15-year-old mounds were reduced compared to the undisturbed and 3-month-old mound soils (Table 4). After wet-sieving, the percentages of water-stable aggregates greater than 1 mm were higher and those of water-stable aggregates lesser than 0.5 mm were generally lower, in the undisturbed and 3-month-old mound soils than those in the 3–15 years old mound soils, showing that the disturbance of mound-making transformed soil aggregates greater than 1 mm largely into aggregates lesser than 0.25 mm (Fig. 1). Mean weight diameter of water-stable aggregates was lower in all mound soils than in the undisturbed soils, and a recovery in the mean weight diameter occurred in the 15-year-old mound soils compared to the 6-year-old mound soils (Table 4).
Discussion
Foraging and mound-creating by zokors changed plant species and significantly reduced the below-ground plant biomass, soil organic matter pools, microbiological activity, and soil aggregation. After the original vegetation had been devastated in the mounds, new species gradually appeared and their numbers increased. Soil organic C pools, microbial biomass and activity (urease and alkaline phosphatase activities), and soil aggregation (water-stable aggregate mean weight diameter) recovered with the age of mounds but the recoveries in soil organic C pools lagged behind those of vegetation and root biomass, and the recoveries of microbial biomass, microbial activity, and soil aggregation lagged behind those of soil organic C pools.
The flattening of mounds after formation with time was due to the soil compactness after mound formation, possibly in response to grazing animal trampling and precipitation. This was consistent with the higher values of soil bulk density of the undisturbed grasslands and 3- to 15-year-old mounds than that of 3-month mounds. The complete depletion of root biomass in the top 15 cm of soil by plateau zokor foraging or transferring to deeper burrows of food storage in the soil of 3-month-old mounds probably occurred because plateau zokors have broad diets with feeding roots and shoots of annual and perennial grasses, forbs, and shrubs (Wang et al. 2000). The recovery in root biomass depended on the presence of plant species.
The organic matter level in a soil is the result of a balance between plant-derived C inputs and soil organic matter outputs mainly through mineralization. The reductions of contents of coarse organic C (>2 mm), soil organic C (<2 mm), and particulate organic C (2–0.05 mm) in 3-, 6-, and 15-year-old mound soils compared to the undisturbed or the 3-month-old mound soils may be due to the significant decrease in plant C input under degraded vegetation and to the stimulated decomposition favored by soil loosening and decreased soil aggregation. Non-compacted soil is known to have a higher organic C mineralization rate than compacted soil due to a favorable soil aeration (De Neve and Hofman 2000; Tan and Chang 2007). Disaggregation induced by mechanical disturbances such as tillage generally increases mineralization due to exposure of protected organic matter to microbial degradation (Kristensen et al. 2003). Coarse soil organic C concentration increased in the 15-year-old mounds compared to the 3-year-old mounds, and soil organic C and particulate organic C levels increased in the 15-year-old mounds compared to values of 6 years old mounds, likely due to the input of organic C into the soils from improved vegetation, with the input exceeding the output by organic C mineralization. Higher soil organic C concentration in the 3-month-old mounds than in the undisturbed grasslands was likely as a result of significantly decreased soil bulk density (0.59 Mg m−3) of the 3-month-old mounds compared to the undisturbed grasslands (bulk density 0.83 Mg m−3), i.e., a sampling depth of 15 cm in the 3-month-old mounds was equivalent to an 11-cm sampling depth in the undisturbed grasslands.
Soil microbial biomass and activity were related to changes in vegetation cover (Baldrian et al. 2008) because they are stimulated by inputs from plants litter and rhizodeposition (Kuzyakov and Domanski 2000; Blagodatskaya and Kuzyakov 2008); both plant inputs contain labile organic C pools, coarse and particulate organic C fractions (Christensen 1992). In this study, decreased microbial biomass C and soil respiration in the 3- to 15-year-old mounds than in the undisturbed soils and the recovery in microbial biomass in the 15-year-old mounds compared to the 6-year-old mounds could be related to the degraded vegetation by mound-making and to the recovery in vegetation after mound formation. Soil enzymes are secreted by roots or released by soil organisms fed with organic substrates derived from roots and residues in the soil (Vinotha et al. 2000). Decreased urease-, acid-phosphatase-, alkaline-phosphatase-, and glucosidase activities of mound soils compared to the undisturbed soils, and recoveries of urease- and alkaline-phosphatase activities in the 15-year-old mounds compared to the 6-year-old mounds could be explained by the response of microbial biomass and microbial activity to changes in vegetation (Nannipieri et al. 2003; Acosta-Martínez et al. 2008).
In addition to mechanical disruption by mound-making, decreases in root biomass, organic C fractions and microbial activity can be responsible for the reduction of soil aggregation and stability in the zokor mounds compared to the undisturbed grasslands. It is well established that organic fractions, root biomass, and related microbial activity mediate soil aggregation (Tisdall and Oades 1982; Caravaca et al. 2005).
Conclusions
Mound-making by plateau zokors decreased vegetation and root biomass, and reduced soil organic matter pools, microbial mass and activity, and soil aggregation in the top layer where zokor foraging and mound-making took place in the field. It may take more than 15 years for a complete recovery of degraded soil organic matter pools, microbial activity, and aggregation to the original state.
References
Acosta-Martínez V, Acosta-Mercado D, Sotomayor-Ramírez D, Cruz-Rodríguez L (2008) Microbial communities and enzymatic activities under different management in semiarid soils. Applied Soil Ecol 38:249–260. doi:1016/j.apsoil.2007.10.012
Akiyama T, Kawamura K (2007) Grassland degradation in China: methods of monitoring, management and restoration. Grassl Sci 53:1–17. doi:10.1111/j.1744-697x.2007.00073.x
Baldock JA, Nelson PN (2000) Soil organic matter. In: Malcolm ES (ed) Handbook of soil science. Boca Raton pp B-25–D-72
Baldrian P, Trögl J, Frouz J, Šnajdr J, Valášková V, Merhautvá V, Cajthaml T, Herinková J (2008) Enzyme activities and microbial biomass in topsoil layer during spontaneous succession in spoil heaps after brown coal mining. Soil Biol Biochem 40:2107–2115. doi:10.1016/j.soilbio.2008.02.019
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131. doi:10.1007/s00374-008-0334-y
Caravaca F, Alguacil MM, Torres P, Roldán A (2005) Plant type mediates rhizosperic microbial activities and soil aggregation in a semiarid Mediterranean salt marsh. Geoderma 124:375–382. doi:10.1016/j.geoderma.2004.05.010
Carreira JA, Viñegla B, Garćia-Ruiz R, Ochoa V, Hinojosa MB (2008) Recovery of biochemical functionality in polluted flood-plain soils: the role of microhabitat differentiation through revegetation and rehabilitation of the river dynamics. Soil Biol Biochem 40:2088–2097. doi:10.1016/j.soilbio.2008.01.021
Christensen BT (1992) Physical fractionation of soil and organic matter in primary particle size and density separates. Adv Soil Sci 20:1–90
Davidson AD, Lightfoot DC (2008) Burrowing rodents increase landscape heterogeneity in desert grassland. J Arid Environ 72:1133–1145. doi:10.1016/j.jaridenv.2007.12.015
De Neve S, Hofman G (2000) Influence of soil compaction on carbon and nitrogen mineralization of soil organic matter and crop residues. Biol Fertil Soils 30:544–549
Hackl E, Pfeffer M, Donat C, Bachmann G, Zechmeister-boltenstern S (2005) Composition of microbial communities in the mineral soil under different types of natural forest. Soil Biol Biochem 37:661–671. doi:10.1016/j.soilbio.2004.08.023
Hopkins DW, Sparrow AD, Shillam LL, English LC, Dennis PG, Novis P, Elberling B, Gregorich EG, Greenfield LG (2008) Enzymatic activities and microbial communities in an Antarctic dry valley soil: Responses to C and N supplementation. Soil Biol Biochem 40:2130–2136. doi:10.1016/j.soilbio.2008.03.022
Institute of Soil Science, Academia Sinica (1978) Physical and chemical analytical methods of soil. Shanghai Science Technology, Shanghai, China
Joergensen RG, Brookes PC (1990) Ninhydrin-reactive nitrogen measurements of microbial biomass in 0.5 M K2SO4 soil extracts. Soil Biol Biochem 22:1023–1027
Klose S, Tabatabai MA (2000) Urease activity of microbial biomass in soils as affected by cropping systems. Biol Fertil Soils 31:191–199
Kristensen HL, Debosz K, McCarty GW (2003) Short-term effects of tillage on mineralization of nitrogen and carbon in soil. Soil Biol Biochem 35:979–986. doi:10.1016/S0038-0717(03)00159-7
Kuzyakov Y, Domanski G (2000) Carbon input by plants into soil [review]. J Plant Nutr Soil Sci 163:421–431
Li B (1997) The rangeland degradation in north China and its preventive strategy. Scientia Agricultura Sinica 30:1–9
Li XL, Yuan QH, Wan LQ, He F (2008) Perspectives on livestock production systems in China. Rangeland J 30:211–220. doi:10.1071/RJ08011
Nannipieri P, Ascher J, Ceccherini MT, Landi L, Pietramellara G, Renella G (2003) Microbial diversity and soil functions. Eur J Soil Sci 54:655–670. doi:10.1046/j.1365-2389.2003.00556.x
Nelson DW, Sommers LE (1982) Total carbon, organic carbon and organic matter. In: Page AL, Miller RH (eds) Methods of soil analysis, part 2, chemical and microbiological properties, 2nd edn. American Society of Agronomy, Madison, WI, pp 539–577
Su J, Wang Z (1992) Studies on the population energetic of plateau zokor I. Average daily metabolic rate and burrowing metabolic rate. Acta Theriologica Sinica 12:200–206
Tabatabai MA (1982) Soil enzymes. In: Page AL, Miller RH (eds) Methods of soil analysis, part 2. Chemical and microbiological properties, 2nd edn. American Society of Agronomy, Madison, WI, pp 903–943
Tan X, Chang SX (2007) Soil compaction and forest litter amendment affect carbon and nitrogen mineralization in boreal forest soil. Soil Tillage Res 93:77–86. doi:10.1016/j.still.2006.03.017
Tisdall JM, Oades JM (1982) Organic matter and water-stable aggregates in soils. J Soil Sci 33:141–163
Vinotha SP, Parthasarathi K, Ranganathan LS (2000) Enhanced phosphatase activity in earthworm casts is more of microbial origin. Curr Sci India 79:1158–1159
Wang Q, Zhou W, Wei W, Zhang Y, Fan N (2000) The burrowing behavior of Myospalax baileyi and its relation to soil hardness. Acta Theriologica Sinica 20:277–283
Wu RG, Tiessen H (2002) Effect of land use on soil degradation in alpine grassland soil, China. Soil Sci Soc Am J 66:1648–1655
Zhang Z (2001) Significance, problems and suggestion of alpine pasture animal husbandry in Tibet, China. Partacultural Science 18:1–5
Zhang Y (2002) Effects of plateau zokors on vegetation characteristics and productivity of alpine meadow. Acta Theriologica Sinica 22:201–210
Zhang Z, Guo Z, Wu S (2002) Problems facing to prataculture in western alpine regions and its sustainable development. Acta Partaculture Sinica 11:29–33
Zhang Y, Zhang Z, Liu J (2003) Burrowing rodents as ecosystem engineers: the ecology and management of plateau zokors Myospalax fontanierii in alpine meadow ecosystems on the Tibetan Plateau. Mammal Rev 33:284–294
Zhou H, Zhao X, Tang Y, Gu S, Zhou L (2005) Alpine grassland degradation and its control in the source region of the Yangtze and Yellow Rivers, China. Grassl Sci 51:191–203. doi:10.1111/j.1744-697x.2005.00028.x
Acknowledgments
This work was financed by Globe Environmental Fund (052456CHA-GS-Y-4), “973” program (2007CB106804) and innovation group project of China Ministry of Education. We would like to thank Mr. Changlin Xu for his constructive assistance with mound marking and experiment management since 1993.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X.G., Zhang, M.L., Li, Z.T. et al. Dynamics of soil properties and organic carbon pool in topsoil of zokor-made mounds at an alpine site of the Qinghai–Tibetan Plateau. Biol Fertil Soils 45, 865–872 (2009). https://doi.org/10.1007/s00374-009-0398-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-009-0398-3