Abstract
Water extractable organic matter (WEOM) derived from fresh- or early-stage decomposing soil amendment materials may play an important role in the process of organic matter accumulation. In this study, eight WEOM samples extracted with a 40:1 (v/w) water to sample ratio from alfalfa (Medicago sativa L.), corn (Zea mays L.), crimson clover (Trifolium incarnatum L.), hairy vetch (Vicia villosa L.), lupin (Lupinus albus L.), soybean (Glycine max L. Merr.), wheat (Triticum aestivum L.), and dairy manure were investigated using ultraviolet (UV)–visible, Fourier transform infrared (FT-IR), solution 31P nuclear magnetic resonance (NMR), and solid state 13C NMR spectroscopies. UV–visible and FT-IR spectra of the plant-derived WEOM samples were typical for natural organic matter, but possessed less humic-like characteristics than dairy manure-derived WEOM. Solution 31P NMR spectra indicated that WEOM samples extracted from alfalfa, corn, and soybean shoots contained both orthophosphate and monoester P. Of the monoester P in WEOM from soybean shoot, 70% was phytate P. WEOM from crimson clover, hairy vetch, lupin, and wheat shoots contained orthophosphate only. The solid-state 13C NMR spectra of the seven plant-derived WEOM samples indicated that they all were primarily composed of sugars, amino acids or peptides, and low molecular mass carboxylic acids. Carbohydrates were dominant components with very few aromatics present in these samples. In addition, WEOM from crimson clover and lupin, but not other three leguminous plant WEOM samples, contained significant asparagine. On the other hand, WEOM from corn and wheat contained less amino acids or peptides. The spectra of WEOM of dairy manure revealed the presence of significant amounts of nonprotonated carbons and lignin residues, suggesting humification of the manure-derived WEOM. Significant carbohydrates as well as aromatics were present in this WEOM. The P and C bonding information for these WEOM samples may be useful for understanding the effects of WEOM on soil nutrient availability to plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Incorporation of plant residues into soils could improve soil quality and nutrient availability by increasing soil organic matter content (Gigliotti et al. 2002; Grandy et al. 2002). The levels of total soil organic matter and the portion of dissolved organic matter (WEOM) are both influenced by the type of organic amendments used (Ohno et al. 2005). Even though the amount of WEOM in soil and organic amendments is small compared to the bulk organic matter content, it plays a significant role in the regulation of nutrients, metals, and microbial activity in soil (Gigliotti et al. 2002; Hunt et al. 2007b).
Previously, WEOM extracted from plant shoot biomass representing commonly utilized US agricultural amendments was used to investigate P sorption inhibition by WEOM (Hunt et al. 2007b). The results demonstrated that the extent of inhibition is closely related to the chemical structure of both the WEOM molecule and the sorbing mineral surface. Furthermore, sorption to goethite (α-FeOOH) of the fresh and decomposed hydrophilic and hydrophobic fractions of these WEOM samples was positively related to apparent molecular weight, aromaticity, and phenolic acid content (Hunt et al. 2007a). Characterization by wet chemical methods demonstrated that in contrast to previously published observations, characteristics of these WEOM samples were wide ranging, varying greatly within fractions from different sources (Hunt et al. 2007a).
Prior research characterized plant-derived WEOM fractions by excitation–emission matrix fluorescence spectroscopy with multiway analyses (Hunt and Ohno 2007). Although the results indicated that the multidimensional fluorescence spectra of these WEOM fractions can be modeled by seven fluorophore components, structural information at the molecular level has not yet been obtained. We characterized these plant-derived WEOM samples with ultraviolet (UV)–visible, Fourier transform infrared (FT-IR), solution 31P nuclear magnetic resonance (NMR), and solid state 13C NMR spectroscopies. Spectral information obtained from this study will provide a better understanding of the relationship between WEOM composition and behavior in soil environments. For comparison, WEOM from dairy manure was also characterized in the same way.
Materials and methods
WEOM preparation
Plant shoot biomass was obtained from field grown alfalfa (Medicago sativa L.), corn (Zea mays L.), crimson clover (Trifolium incarnatum L.), hairy vetch (Vicia villosa L.), lupin (Lupinus albus L.), soybean (Glycine max L. Merr.), and wheat (Triticum aestivum L.), air-dried, and ground to pass through a 1-mm sieve. The alfalfa, crimson clover, hairy vetch, and lupin were field grown and harvested at full flowering stage. The corn, soybean, and wheat were crop residue left after harvest. The dairy cattle (Bos taurus) manure sample was acquired locally, air-dried, and passed through a 2-mm sieve. The dry manure contained 7.9 g N, 7.4 g K, 3.7 g P, 8.8 g Ca, 6.0 g Mg, 6.6 g Al, and 11.9 g Fe per kg of dry matter. The WEOM was extracted with a 40:1 (v/w) water to sample ratio using cold water and refrigerating (4°C) the suspension for 18 h with periodic shaking by hand (Brown and Sposito 1991). The suspensions were then centrifuged (900×g) for 30 min prior to vacuum filtering through 0.4-μm pore size polycarbonate filters (Hunt et al. 2007b). The WEOM extracts were then freeze-dried and kept in a desiccator at room temperature until use.
UV–visible spectroscopy
Freeze-dried WEOM samples were redissolved in deionized water to make stock solutions with 5 mg dry matter per milliliter of water. To reduce the absorbance to the range of minimum relative uncertainty (usually 0.3 to 0.9), these stock solutions were diluted 1/120 to 1/180 with deionized water for UV–visible spectroscopic analysis. UV–visible spectra were recorded with an Agilent 8453 DAA UV–visible spectrophotometer (Agilent Technologies, Wilmington, DE, USA).
FT-IR analysis
The FT-IR spectra were obtained in the 450–4,000 cm−1 range on a PerkinElmer Spectrum One FT-IR Spectrophotometer from discs containing about 1 mg sample and 100 mg KBr. Each sample was scanned 24 times with a resolution of 2 cm−1. All spectra were normalized.
Solution 31P NMR analysis
Another subsample (10 to 79 mg) of freeze-dried WEOM was dissolved in 0.6 mL 10 M NaOH, 1.0 mL D2O, and 0.6 mL water and allowed to stand for 30 min with occasional vortexing. Solution 31P NMR spectra were acquired at 242.75 MHz on a Varian INOVA600 MHz spectrometer equipped with a 10-mm broadband probe, using a 90° pulse, 0.68-s acquisition, 4.32-s pulse delay, 82.4-μs dwell time, 17.9-μs pulse width, and 12-Hz spinning (He et al. 2008).
Solid-state 13C NMR analysis
Solid-state 13C NMR spectroscopic analyses were performed in a Bruker DSX 400 spectrometer at 100 MHz for 13C using 7-mm sample rotors for optimal sensitivity, in a double-resonance probe head. Qualitative composition information was obtained with good sensitivity by 13C cross polarization/total sideband suppression (CP/TOSS) NMR experiments at a spinning speed of 6.5 kHz and a CP time of 1 ms, with a 1H 90° pulse length of 4 μs. Four-pulse total suppression of sidebands (TOSS) was employed before detection, and two-pulse phase-modulated decoupling was applied for optimum resolution. The corresponding subspectrum with signals of nonprotonated carbons and mobile groups such as rotating CH3 was obtained by 13C CP/TOSS combined with 40-μs dipolar dephasing (Mao et al. 2007a, b).
In order to separate the signals of sp3-hybridized anomeric C (O–C–O) from those of sp2-hybridized aromatic C, both of which may resonate between 120 and 90 ppm, the aromatic-C signals were selectively suppressed by a five-pulse 13C chemical-shift anisotropy (CSA) filter with a CSA-filter time of 35 μs. In particular, this technique identifies anomeric O–C–O C around 100 ppm typical of sugar rings (Mao and Schmidt-Rohr 2004).
Results and discussion
UV–visible spectra
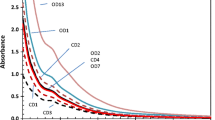
The seven plant extracts contained dissolved organic C (DOC) levels ranging from 1,153 to 3,566 mg L−1 (Table 1). As all extractions were performed with the same water to sample ratio, the DOC levels in the extracts reflected the abundance of DOC in these plant materials. The dairy manure extract contained less DOC (481 mg L−1) than the plant extracts, suggesting that the more-soluble C fraction had been preferentially removed by the animal digestive systems. The UV–visible spectrum of dairy manure shows an absorbance shoulder around 280 nm which was similar to that of swine manure (He et al. 2003). This basically featureless characteristic of the UV–visible spectra is also typical for soil humic substances (Baes and Bloom 1990), which indicates that there were many different chromophores in this sample. In the spectra of plant-derived WEOM fractions, the absorbance shoulder was more obvious, or became an absorbance peak between 260 and 280 nm. An apparent second absorbance shoulder or peak between 300 and 350 nm appeared in the spectra of the WEOM fractions of corn, hairy vetch, and alfalfa (Fig. 1). Therefore, in plant-derived WEOM fractions, some chromophores were apparently more abundant than in soil and manure WEOM. Plant shoot extracts may contain amino acids, nucleic acids, and other phenolic compounds (Hunt and Ohno 2007), and these abundant chromophores could be aromatic and/or phenolic compounds with conjugated C=C and C=O double bonds which have strong absorbance in the range of 200 to 300 nm (Abbt-Braun et al. 2004; Baes and Bloom 1990). The additional peak or shoulder between 300 and 350 nm indicated that there were some ring-fission products of phenolic carboxylic compounds present in some WEOM fractions, because strong absorbance of these ring-fission intermediates has been frequently observed during microbial metabolism of phenolic compounds (He and Spain 2000).
FT-IR spectra
FT-IR spectra of the eight WEOM fractions were typical for natural organic matter (Fig. 2). The broad band around 3,400 cm−1 was due to O–H and N–H bond stretching, and the minor band at 2,936 cm−1 or so was assigned to aliphatic C–H stretching (Agnelli et al. 2000; He et al. 2006). The sharp band at 1,605 cm−1 is generally attributed to aromatic C=C vibrations, symmetric stretching of COO− groups, and H-bonded C=O of conjugated ketones (Agnelli et al. 2000; He et al. 2006; Olk et al. 2000). However, unlike humic and fulvic acids (Agnelli et al. 2000; He et al. 2006; Olk et al. 2000), several other strong peaks associated with COO− groups and conjugated ketones, such as those in the 1,720–1,710, 1,660–1,630, and 1,230–1,210 cm−1 regions, were not apparent in the spectra in Fig. 2. There were two possible explanations on the observation: (1) no significant abundance of COO− groups in these plant-derived WEOM samples and (2) there were COO− groups in these samples, but they were formed bonds with polyvalent ions present in the WEOM extracts. However, the relatively less polyvalent ions in these WEOM samples (Table 1) implied less possibility of the second hypothesis. In other words, even though we could not exclude the second hypothesis, the lack of these strong absorbance bands should be mainly due to the first hypothesis. With the lack of significant carboxylic groups in these WEOM fractions, we attributed the peak at 1,605 cm−1 in Fig. 2 to aromatic compounds in these WEOM fractions. The strong band around 1,380 to 1,430 cm−1 was due to phenolic OH and aliphatic C–H groups (Agnelli et al. 2000). The composite bands around 1,070 cm−1 are usually assigned to alcoholic and polysaccharide C–O stretching or silicate vibrations (Agnelli et al. 2000; Francioso et al. 1996). Recent reports (He et al. 2006) observed that the strength of this FT-IR peak is related to the abundance of P in humic fractions. Based on the selected element contents of these WEOM fractions (Table 1), polysaccharide and P compounds may have been the major contributors to the peaks around 1,070 cm−1.
There were some differences observed among the eight WEOM samples. A relatively strong band at 1,678 cm−1 and a minor band at 1,529 cm−1 appeared in the spectrum of lupin WEOM (Fig. 2). The former one could be assigned to olefine or aromatic compounds (Chang Chien et al. 2007; Francioso et al. 1996) and the latter one to the presence of nitrogen (N) compounds (N–H stretching and NH2 deformation). We assigned the band at 1,678 cm−1 to olefine compounds as the aromatic compounds were identifiable by the peak at 1,605 cm−1 and these two peaks did not always appear simultaneously in these spectra. Based on the strengths of the two bands (Fig. 2), these olefinic and N compounds decreased in the order of lupin > manure > crimson clover > hairy vetch > corn > wheat > alfalfa > soybean. On the contrary, the band at 1,260 cm−1 was relatively strong in the spectra of WEOM fractions of soybean, alfalfa, wheat, and corn (Fig. 2). This band was contributed by C–O stretching of esters, ethers, and phenols (Chang Chien et al. 2007). Combined with the broad bands at 1,400 cm−1 in these spectra, it appears that these WEOM fractions contain more aliphatic and/or phenolic groups than other natural organic matter, such as humic fractions (He et al. 2006).
Solution 31P NMR spectra
The solution 31P NMR spectra of the WEOM extracted from corn, alfalfa, and soybean shoots were relatively simple (Fig. 3). A sharp chemical shift peak at appropriately 6 ppm is typical for inorganic orthophosphate. All other minor broad peaks between 6 and 4 ppm were attributed to monoester P. In the orthophosphate monoesters region of the spectrum of the soybean WEOM fraction, peaks were further assigned for phytate (5.95, 5.06, 4.70, and 4.52 ppm with the ratio 1:2:2:1). Based on the integration of these peak areas, the distribution between the two types of P is 86% orthophosphate and 14% monoester P in alfalfa shoot. However, the distribution in soybean shoot WEOM was 68% orthophosphate and 32% monoester P. Within the monoester P of soybean shoot WEOM, 70% is phytate P. WEOM from corn shoot contained less monoester P (8% of total P) and the other five WEOM fractions contained orthophosphate only (data not shown). As these fractions came from the plant shoots, orthophosphate in these fractions might not be truly part of the water extractable organic matter composition. It could be simply the free orthophosphate component (nutrient) present in these plant shoots.
Potassium and Mg salts of phytate are the major storage forms of P in plant seeds (Bohn et al. 2008). Elemental analysis of the NMR samples (Table 1) indicates that both inorganic and organic P species in these plant-derived WEOM fractions were associated with Ca and Mg; K was not determined. Thus, the presence of phytate P in the WEOM derived from soybean shoot might reflect phytate synthesis when this plant shoot was collected.
To our knowledge, there have been no reports identifying P species in plant-derived WEOM by solution 31P NMR analysis. Similar to our analysis of dairy manure WEOM, Gigliotti et al. (2002) reported that the only solution 31P NMR spectral signal of the WEOM extracted from pig waste slurry was for orthophosphate. However, solution 31P NMR analysis demonstrated that orthophosphate (55%), phosphomonoester (33%), and pyrophosphate (12%) were present in hydrophilic WEOM from sewage sludge, and both phosphomonoesters and phosphodiesters were present in hydrophobic WEOM from the same sludge samples (Gigliotti et al. 2002).
Solid-state 13C NMR
Three different techniques were applied: 13C CP/TOSS, 13C CP/TOSS with dipolar dephasing, and 13C chemical-shift anisotropy filter (Fig. 4). 13C CP/TOSS provides the whole qualitative structural information on these samples, dipolar dephasing detects nonprotonated carbons and mobile groups such as CH3 groups, and 13C CSA filter selects sp3-hybridized carbons. The TOSS spectra of all plant-derived WEOM fractions exhibited similarities. Based on the general spectral pattern (Fig. 4), the spectra of plant-derived WEOM could be separated into three groups: those of (1) alfalfa, hairy vetch, and soybean, (2) crimson clover and lupin, and (3) corn and wheat. In contrast, the CP/TOSS spectrum of dairy manure was totally different. The functional group assignments are as follows (Mao et al. 2007b): 0–50 ppm, nonpolar alkyl; 50–60 ppm, NCH and OCH3; 60–96 ppm, carbohydrate C; 96–110 ppm, O–C–O anomerics; 110–145 ppm, aromatic C; 145–162 ppm, aromatic C–O; 162–190 ppm, COO and N–C=O; and 190–220 ppm, ketone, quinone, or aldehyde C.
Spectral editing for identification of functional groups in 13C NMR. Three experiments were conducted: 13C CP/TOSS spectra showing qualitative structural information, corresponding dipolar-dephased (DD) 13C CP/TOSS spectra showing nonprotonated carbons and mobile segments like CH3, acquired after a period of 40 μs without decoupling, and selection of sp3-hybridized carbon signals by a chemical-shift anisotropy filter, which in particular identified OCO carbons, near 100 ppm, typical of sugar rings. a Alfalfa, b hairy vetch, c soybean, d dairy manure, e crimson clover, f lupin, g corn, and h wheat. The number of scans of all the spectra is 3 k with a recycle delay of 5 s
The 13C CP/TOSS spectra of alfalfa, hairy vetch, and soybean (group 1; Fig. 4a–c) all showed a predominant band of OCH at 72 ppm, COO/N–C=O at ca. 178 ppm, O–C–O around 100 ppm, and OCH2 at 62 ppm. Broad signals at around 55 ppm were assigned to NCH or OCH3 and around 0–50 ppm for CCH, CCH2, and CCH3. Thus, we concluded, in contrast to terrestrial humic acids (Mao et al. 2007a; Schmidt-Rohr et al. 2004), these WEOM fractions contained very few aromatic functional groups. This C13 NMR-based observation was consistent with the FT-IR spectral feature of these WEOM samples discussed above. Further dipolar-dephased spectral analysis indicated that most of carbons in the three WEOM fractions were protonated. This result is similar to what was obtained for algae, which is not unexpected given they are all composed of biomolecules (Mao et al. 2007b). In addition to a dominant COO/N–C=O band, there was a small nonprotonated O–Cq–O around 100 ppm, nonprotonated OCq signals barely above the baseline around 60–90 ppm, and a small CCH3 around 22 ppm. Based on aforementioned assignments, the signals around 50–60 ppm could be from OCH3 and NCH; dipolar-dephased spectra indicate that signals around 50–60 ppm were primarily attributed to NCH since dipolar dephasing only retains nonprotonated carbons and mobile groups such as OCH3 groups and removes protonated carbons such as NCH. The 13C CSA-filtered spectra showed that the peak around 100 ppm was from sp3-hybridized O–C–O. Interestingly, residual O–Cq–O signals around 100 ppm were detected in the dipolar-dephased spectra of alfalfa and hairy vetch, but not of soybean, indicating that all O–C–O’s of soybean were protonated. Anomerics are nonprotonated in all ketoses, e.g., in fructose. The anomeric peak plus OCH at 72 ppm and OCH2 at 62 ppm indicated the presence of sugar rings in these WEOM fractions. The existence of a band at ~178 ppm was from either COO or N–C=O. The N–C=O and the NCH around 55 ppm suggested the existence of amino acids or peptides; the COO and CCH, CCH2, and CCH3 indicated the existence of low molecular mass carboxylic acids. Phenolic acids were not observed in alfalfa and hairy vetch, as found by Hunt et al. (2007a); however, in soybean CP/TOSS spectrum, a tiny peak around 160 ppm could be due to phenolic acids. Nevertheless, phenolic acids were minor constituents of these WEOM fractions.
The spectra of corn and wheat (group 3; Fig. 4g, h) were generally similar to those of alfalfa, hairy vetch, and soybean (group 1): a dominant OCH at ~70 ppm, a OCH2 shoulder at ~62 ppm, and an anomeric peak at 100 ppm which indicated the large quantities of sugars; signals around 50–60 ppm from NCH and N–C=O from 178 ppm which suggested the presence of amino acids and peptides; and COO at ~180 ppm and a broad band from 0–50 ppm assigned to CH, CH2, and CCH3 which indicated the existence of low molecular carboxylic acids. However, several significant differences and features should be noted. First, the peak of anomerics at 100 ppm and the peak of OCH at 72 ppm were very sharp compared with those of group 1, suggesting that some sugars be crystalline. Second, there were fewer signals in their CP/TOSS spectra and more residual peaks in their dipolar-dephased spectra around 50–60 ppm, indicating that less amino acids or peptides existed in wheat and corn than in alfalfa, hairy vetch, and soybean. This result was consistent with the fact that the three group 1 plants are leguminous. Third, their dipolar-dephased spectra indicated that in addition to those nonprotonated and mobile signals, there were low-intensity broad nonprotonated aromatic C–O and aromatic signals around 160–110 ppm for corn but less for wheat. Further, the OCq and OCH3 were more intense in wheat than in corn and group 1. The presence of significant OCH3 could indicate the existence of lignin. However, the tiny amount of aromatic C–O around 145–162 ppm excludes the possibility of significant lignin in these samples.
At first sight, the spectra of crimson clover and lupin (group 2; Fig. 4e, f) seemed to be totally different from those of groups 1 and 3. However, if the three sharp peaks at 36, 51, and 176 ppm were removed, they would be quite similar to the others, especially to those of group 1 since they are all leguminous plants. These three peaks probably arose from asparagine because the 13C chemical shifts of CCH2, NCH, COO, and H2NCO in asparagines are at 37.7, 51.5, 173, and 175.6 ppm in solution state, respectively. The broader linewidths and other mechanisms leading to slight changes in chemical shifts in solid state indicated a good match with asparagine. In addition, the approximate ratio of the peak at 176 ppm to either that of 51 or of 36 ppm was 2:1, indicating that there were two C=O functional groups in this compound compared with the signals at 51 and 36 ppm. Thus, WEOM fractions in this group contained significant asparagine in addition to compounds found in group 1.
In contrast, the spectra of the dairy manure WEOM were totally different from those of plant-derived WEOM (Fig. 4d). Its 13C CP/TOSS spectrum was very broad and especially the aromatic signals (110–160 ppm) were significant. There were also small carbonyl carbons around 200 ppm, COO/N–C=O at ~178 ppm, sharp OCH at 72 ppm, NCH/OCH3 around 56 ppm, CCH at 40 ppm, and CCH3 around ~25 ppm. Its dipolar-dephased spectrum showed rich environments of nonprotonated carbons and mobile groups and also indicated that the carbonyl groups around 200 ppm were primarily from keto groups. It also showed that the signals around 55 ppm belonged to both NCH and OCH3 since there was a significant OCH3 peak in the dipolar-dephased spectrum. In addition, the dipolar-dephased spectrum had COO/N–C=O around ~178 ppm, aromatic C–O around 150 ppm, nonprotonated aromatics around 120–140 ppm, small nonprotonated anomerics around 100 ppm, OCq from around 65–90 ppm, and mobile CCH3 from 10–25 ppm. The 13C CSA filter technique clearly selected anomeric signals which overlap with aromatics in its CP/TOSS spectrum. Almost all the anomerics were protonated, as suggested by the dipolar-dephased spectrum. The presence of OCH3 and aromatic C–O indicated the existence of significant lignin, the N–C=O and NCH signals showed the existence of peptides, and the O–C–O functional groups were indicative of sugar rings in this manure. In summary, despite certain differences, similar major structures were observed in all plant-derived WEOM samples: sugars, amino acids/peptides, and low molecular mass carboxylic acids. In contrast, WEOM from dairy manure was structurally different, containing significant amounts of nonprotonated carbons and lignin residues.
Conclusions
Dissolved organic matter derived from seven plant shoots and a dairy cattle manure was characterized by UV–visible, FT-IR, solution 31P, and solid-state 13C NMR spectroscopic techniques. The spectral data provided P and C bonding information for these WEOM samples. All plant-derived WEOM sources were primarily composed of significant soluble carbohydrates, as well as some other soluble organic compounds such as organic acids, amino acids/peptides, and phenolic compounds. Carbohydrates are dominant components with very few aromatics present in these samples. In contrast, the spectra of dairy manure-derived WEOM were significantly different. Its spectra were broad and more featureless, indicating humification with significant amounts of nonprotonated carbons and lignin residues. Significant carbohydrates as well as aromatics are present in this manure-derived WEOM. The differential characteristics of WEOM derived from different sources may be useful for investigating the humification process of plant WEOM.
References
Abbt-Braun G, Lankes U, Frimmel FH (2004) Structural characterization of aquatic humic substances—the need for a multiple method approach. Aquat Sci 66:151–170. doi:10.1007/s00027-004-0711-z
Agnelli A, Celi L, Degl'Innocenti A, Corti G, Ugolini FC (2000) Chemical and spectroscopic characterization of the humic substances from sandstone-derived rock fragments. Soil Sci 165:314–327. doi:10.1097/00010694-200004000-00003
Baes AU, Bloom PR (1990) Fulvic acid ultraviolet–visible spectra: influence of solvent and pH. Soil Sci Soc Am J 54:1248–1254
Bohn L, Meyer AS, Rasmussen SK (2008) Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J Zhejiang Univ Sci B 9:165–191. doi:10.1631/jzus.B0710640
Brown AD, Sposito G (1991) Acid–base chemistry of dissolved organic matter in aqueous leaf extracts: application to organic acids in throughfall. J Environ Qual 20:839–845
Chang Chien SW, Wang MC, Huang CC, Seshaiah K (2007) Characterization of humic substances derived from swine manure-based compost and correlation of their characteristics with reactivities with heavy metals. J Agric Food Chem 55:4820–4827. doi:10.1021/jf070021d
Francioso O, Sanchez-Cortes S, Tugnoli V, Ciavatta C, Sitti L, Gessa C (1996) Infrared, Raman, and nuclear magnetic resonance (1H, 13C, and 31P) spectroscopy in the study of fractions of peat humic acids. Appl Spectrosc 50:1165–1174. doi:10.1366/0003702963905169
Gigliotti G, Kaiser K, Guggenberger G, Haumaier L (2002) Difference in the chemical composition of dissolved organic matter from waste material of different sources. Biol Fertil Soils 36:321–329. doi:10.1007/s00374-002-0551-8
Grandy AS, Porter GA, Erich MS (2002) Organic amendment and rotation crop effects on the recovery of soil organic matter and aggregation in potato cropping systems. Soil Sci Soc Am J 66:1311–1319
He Z, Spain JC (2000) Reactions involved in the lower pathway for degradation of 4-nitrotoluene by Mycobacterium strain HL 4-NT-1. Appl Environ Microbiol 66:3010–3015. doi:10.1128/AEM.66.7.3010-3015.2000
He Z, Honeycutt CW, Griffin TS (2003) Comparative investigation of sequentially extracted P fractions in a sandy loam soil and a swine manure. Commun Soil Sci Plant Anal 34:1729–1742. doi:10.1081/CSS-120021308
He Z, Ohno T, Cade-Menun BJ, Erich MS, Honeycutt CW (2006) Spectral and chemical characterization of phosphates associated with humic substances. Soil Sci Soc Am J 70:1741–1751. doi:10.2136/sssaj2006.0030
He Z, Honeycutt CW, Cade-Menun BJ, Senwo ZN, Tazisong IA (2008) Sequentially-extracted phosphorus from poultry litter and soil: enzymatic and 31P NMR characterization. Soil Sci Soc Am J 72:1425–1433. doi:10.2136/sssaj2007.0407
Hunt JF, Ohno T (2007) Characterization of fresh and decomposed dissolved organic matter using excitation–emission matrix fluorescence spectroscopy and multiway analysis. J Agric Food Chem 55:2121–2128. doi:10.1021/jf063336m
Hunt JF, Ohno T, He Z, Honeycutt CW, Dail DB (2007a) Influence of decomposition on chemical properties of plant- and manure-derived dissolved organic matter and sorption to goethite. J Environ Qual 36:135–143. doi:10.2134/jeq2006.0133
Hunt JF, Ohno T, He Z, Honeycutt CW, Dail DB (2007b) Inhibition of phosphorus sorption to goethite, gibbsite, and kaolin by fresh and decomposed organic matter. Biol Fertil Soils 44:277–288. doi:10.1007/s00374-007-0202-1
Mao JD, Schmidt-Rohr K (2004) Separation of acetal or ketal O–C–O 13C NMR signals from aromatic-carbon bands by a chemical-shift-anisotropy filter. Solid State NMR 26:36–45. doi:10.1016/j.ssnmr.2003.09.003
Mao JD, Fang X, Schmidt-Rohr K, Carmo AM, Hundal LS, Thompson ML (2007a) Molecular-scale heterogeneity of humic acid in particle-size fractions of two Iowa soils. Geoderma 140:17–29. doi:10.1016/j.geoderma.2007.03.014
Mao JD, Cory RM, McKnight DM, Schmidt-Rohr K (2007b) Characterization of a nitrogen-rich fulvic acid and its precursor algae by solid-state NMR. Org Geochem 38:1277–1292. doi:10.1016/j.orggeochem.2007.04.005
Ohno T, Griffin TS, Liebman M, Porter GA (2005) Chemical characterization of soil phosphorus and organic matter in different cropping systems in Maine, USA. Agric Ecosyst Environ 105:625–634. doi:10.1016/j.agee.2004.08.001
Olk DC, Brunetti G, Senesi N (2000) Decrease in humification of organic matter with intensified lowland rice cropping: a wet chemical and spectroscopic investigation. Soil Sci Soc Am J 64:1337–1347
Schmidt-Rohr K, Mao JD, Olk DC (2004) Nitrogen-bonded aromatics in soil organic matter and their implications for a yield decline in intensive rice cropping. Proc Natl Acad Sci U S A 101:6351–6354. doi:10.1073/pnas.0401349101
Acknowledgments
JDM thanks Jeffress Foundation and Petroleum Research Foundation for financial support. We thank Dr. Corey Liu for assistance with 31P-NMR spectroscopy, which was performed at the Stanford Magnetic Resonance Laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trade or manufacturers' names mentioned in the paper are for information only and do not constitute endorsement, recommendation, or exclusion by the USDA-ARS.
Rights and permissions
About this article
Cite this article
He, Z., Mao, J., Honeycutt, C.W. et al. Characterization of plant-derived water extractable organic matter by multiple spectroscopic techniques. Biol Fertil Soils 45, 609–616 (2009). https://doi.org/10.1007/s00374-009-0369-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-009-0369-8