Abstract
Freeze–thaw cycles can promote soil N losses as a result of microbial and root cell lysis; however, minimal freeze–thaw effects have typically been observed in studies that have imposed moderate temperature cycles. We conducted laboratory incubations on surface soil (top 3 cm) collected in a temperate old field from late fall through mid-winter to examine how variation in freeze–thaw amplitude, number, timing of collection, and freezing rate altered soil extractable N. We varied freeze–thaw amplitude by imposing minimum cycle temperatures of 0, −1, −2, −5, and −10°C for a series of either one or two cycles and held control samples constant at 3°C. We also examined the effects of freezing rates of 1, 3, and 30°C h−1. We hypothesized that extractable N would be highest for both the maximum freezing amplitudes and rates. While multiple freeze–thaw cycles at −10°C and freeze–thaw cycles associated with artificially high freezing rates increased extractable N, freeze–thaw cycles representative of field conditions at our site had no effect on extractable N in late fall and early winter. By mid-winter there was a significant freeze–thaw cycle effect but, contrary to our prediction, less N was extracted from freeze–thaw treated samples than from the control samples, which remained thawed over the treatment period. Increased extractable N in control samples was driven by increased organic N rather than increased inorganic N. Our results suggest that freeze–thaw damage to soil organisms does not contribute substantially to N release in our system. Instead, soil extractable N may increase during mid-winter thaws as a result of increased soil proteolytic activity above freezing temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Climate warming can influence soil N losses by altering snow pack depth, soil freezing, and snow melt patterns in arctic (Grogan and Jonasson 2003), alpine (Brooks and Williams 1999; Kaste et al. 2008), and northern temperate ecosystems (Groffman et al. 2001; Campbell et al. 2005; Callesen et al. 2007). Warming typically decreases or eliminates snow cover, which exposes soil to diurnal fluctuations in air temperature or solar heating and leads to increased variation in soil temperatures over winter (Isard and Schaetzl 1998; Mellander et al. 2007). Coupled with more extreme and variable weather events (Easterling et al. 2000; IPCC 2007), reduced snow cover resulting from climate warming will likely increase the frequency of soil freeze–thaw cycles over the next century in many systems (Henry 2008). Soil freeze–thaw cycles can lyse soil microorganisms (Skogland et al. 1988; Yanai et al. 2004) and fine roots (Fitzhugh et al. 2001; Weih and Karlsson 2002; Cleavitt et al. 2008), and disrupt soil aggregates (Oztas and Fayetorbay 2003; Sahin and Anapali 2007), potentially increasing soil N export in leachate or as trace gases (Christensen and Christensen 1991; Hobbie and Chapin 1996; Schimel et al. 1996; Ludwig et al. 2004). However, soil microbial activity continues below freezing temperatures (Wynn-Williams 1982; Clein and Schimel 1995; Schimel and Mikan 2005) and plant roots can also remain active over winter (Andresen and Michelsen 2005; Bannister 2005). Consistent with a high tolerance of soil microorganisms and plant roots to freezing, some studies have alternatively reported little to no effect of freeze–thaw cycles on soil N losses (e.g. Herrmann and Witter 2002; Grogan et al. 2004; Koponen et al. 2006).
While variation in the results of freeze–thaw studies could indicate differential tolerances of both soil microorganisms and plants to freeze–thaw among systems (Ludwig et al. 2006; Freppaz et al. 2007; de Gerenyu 2007), Henry (2007) hypothesized that much of this variation could be explained by methodological differences among studies. Freeze–thaw cycle amplitudes and minimum cycle temperatures have varied widely, and in general, studies that have imposed large amplitude freeze–thaw cycles have demonstrated large effects on soil processes (Christensen and Christensen 1991. DeLuca et al. 1992; Wang and Bettany 1994; Bechmann et al. 2005; Sharma et al. 2006), whereas those employing moderate amplitude cycles have reported minimal effects (Lipson et al. 2000; Larsen et al. 2002; Grogan et al. 2004; Sjursen et al. 2005). The few studies that have tested a range of amplitudes within a single soil type also reported the lowest effect sizes for their mildest freeze–thaw cycle amplitude treatments (Sulkava and Huhta 2003; Koponen and Martikainen 2004). In the field, snow cover and plant litter often insulate soils from large diurnal air temperature cycles (Sharratt 1993; Decker et al. 2003), which supports the assertion that moderate freeze–thaw cycles in the laboratory provide a more ecologically relevant treatment. In addition, in many laboratory incubations, surface and sub-surface soils are frozen at the same rate, despite the slower responses of subsurface soils to air temperature changes in the field, which could further exaggerate the effects of large amplitude freeze–thaw cycle treatments (Hu et al. 2006; Henry 2007). Nevertheless, surface soil may endure large amplitude diurnal freeze–thaw cycles over winter during cold, snow-free periods, or in fall or spring, when high exposure of soil to sun during the day is combined with cold air temperatures overnight (Joseph and Henry 2008).
Coupled with potentially exaggerated freeze–thaw cycle amplitudes, unrealistic freezing rates have also often been used in freeze–thaw manipulations (Henry 2007). As with high freezing amplitudes, rapid freezing rates are particularly unrealistic when imposed on subsurface soils. One method for simulating realistic freeze–thaw cycles across the soil profile has been to insulate the bottom and sides of intact soil cores during laboratory or field incubations (Lipson et al. 2000; Herrmann and Witter 2002; Hu et al. 2006; Joseph and Henry 2008). However, very few studies have explicitly tested the influence of freezing rate on the magnitudes of freeze–thaw effects. Lipson et al. (2000) reported decreases in soil microbial biomass in response to rapid freezing rates but not in response to slower rates, presumably due to cell lysis in the former. Rapid freezing causes ice crystals to form extracellularly, concentrating solutes and resulting in cell dehydration and consequent membrane damage and protein denaturation (Mazur 1966; Walker et al. 2006).
In this study, we used laboratory incubations to examine how variation in freeze–thaw amplitude (minimum freezing temperature) and freezing rate alter extractable N in soil collected from a temperate old field. Temperate ecosystems often experience multiple melts over winter, and since many feature soils that remain close to the freezing point over this season, their soils may be particularly likely to experience an increased frequency of freeze–thaw cycles with climate warming (Henry 2008). We collected soil from the upper 3 cm, where the most extreme freeze–thaw cycles occur. Based on the hypothesis that freezing lyses microbial and fine root cells, we predicted that beyond a threshold minimum temperature, the soil extractable N pool would increase. Likewise, we predicted that extreme freezing rates would increase soil extractable N. Although we did not explore soil N losses explicitly, we inferred that increased extractable N may facilitate N leaching and trace gas losses from soil over winter when plant N uptake is minimal.
Materials and methods
Site description
We collected soil samples from a former agricultural field (43° 01′46″ N, 81° 12′52″ W), east of London, Ontario, Canada, that has not been ploughed, fertilized, or mowed for over 20 years. This old field system is also the site of a recently initiated winter warming and N addition field experiment and we collected the soils for our experiment in the vicinity of these plots. The soil is classified as a well to imperfect drained silt loam glacial till (Hagerty and Kingston 1992), and pH is approximately 7.5 (T. Bell, unpublished data). The site is dominated by the perennial grasses Poa pratensis L. and Bromus inermis Leyss. and the forb Cirsium arvense L. and the legume Lotus corniculatus L. are also common at lower densities. Mean annual temperature at the site is 8.3°C and the mean annual precipitation is 818.3 mm (Environment Canada, National Climate Data and Information Archive). Ambient air and soil temperature data over the duration of the collection periods are shown in Fig. 1.
Air temperatures (dotted line) and soil temperatures at depths of 1 cm (thin solid line) and 5 cm (thick solid line) at the study site in late 2007 and early 2008. Collection dates for the freeze–thaw cycle amplitude experiments (black arrows), the multiple freeze–thaw cycle experiment (hatched arrow), and the freezing rate experiments (open arrows) are denoted
Freeze–thaw amplitude and number experiments
We collected six soil samples to a depth of 3 cm using a 2-cm diameter cork borer from each of five 50 × 100 cm experimental blocks located at least 25 m apart on 5 November (prior to soil freeze-up) and 5 December 2007 and 7 January and 23 January 2008 (Fig. 1). We bulked and homogenized samples collected from within a block and subdivided each into six 6–7 g sub-samples. We placed the sub-samples into 15-ml glass vials and subjected them to single freeze–thaw cycles using a Proline RP-355 refrigerated circulator (Lauda, Lauda-Königshofen, Germany). The temperature minima treatments were 0, −1, −2, −5, and −10°C (n = 5), and the small soil volume allowed for rapid equilibration of the entire sample with the treatment temperatures. All samples were equilibrated to 3°C prior to the freeze–thaw treatment, and we maintained control samples (n = 5) at 3°C for the duration of the experiment. We froze and thawed the samples at a rate of 1°C h−1, which is consistent with maximum soil freezing rates at our site, and samples were held for 1 h at the minimum temperature. On 7 November, we repeated the freeze–thaw treatments listed above on two consecutive days for a second set of samples.
Freezing rate experiments
We collected and bulked soil samples as described above on 18 December and 7 January 2007 and 23 January 2008 (Fig. 1). We subjected the samples to three different freezing and thawing rates (1, 3, and 30°C h−1), crossed factorially with two different amplitude minima (−2 and −5°C) (n = 5 for each treatment combination).
Soil extractions and analyses
Immediately after thaw, we extracted all soil samples in 30 ml of 0.5 M K2S04 (Mulvaney 1996) for 1.5 h on a shaker. We filtered each extract through Whatman no. 1 filter paper (prepared by rinsing with 2 M KCl and deionized water to remove nitrate contamination) and froze them for later analysis. Ammonium concentrations were determined colorimetrically following reaction with phenol-sodium nitroprusside (Solorzano 1969) using a SmartChem 140 discrete autoanalyzer (Westco Scientific, Brookfield, CT, USA). Nitrate was reduced to nitrite prior to analysis and nitrite concentrations determined colorimetrically following reaction with sulphanilamide coupled with N-1-naphthylethylene HCl (Keeney and Nelson 1982) using the discrete autoanalyzer. For total extractable N, samples were oxidized using alkaline persulphate oxidation (Cabrera and Beare 1993) and subsequently analyzed for nitrate. Total extractable soluble organic N was estimated by subtracting extractable ammonium and nitrate from the total extractable N.
Statistical analyses
For the freeze–thaw cycle amplitude experiments, we used a two-factor analysis of variance (ANOVA) with freezing treatment and date as fixed factors to assess treatment effects on extractable N. We tested the treatment effects of multiple freezes using a one-factor ANOVA. For the freezing rate experiment, we conducted a three-factor ANOVA with freezing rate, amplitude, and sampling date as fixed factors. For all analyses, block effects were not significant and were excluded from the analyses. Following analysis of variance, we used Tukey’s post-hoc tests to discriminate significant pairwise differences among treatments. Statistical analyses were conducted using SPSS version 16.0 (SPSS, Chicago, Il, USA).
Results
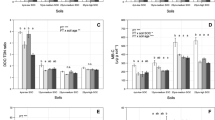
For the freeze–thaw amplitude experiment, there was a significant interaction between treatment and sampling date (P = 0.001) explained by the combination of a lack of a treatment effect over the first two sampling dates and an increase in extractable N in the control plots on 7 January and in both the control and 0°C treatment on 23 January (Fig. 2). Across all treatments, there was also a trend of increased extractable N over time (P < 0.001; Fig. 2). Increased extractable N in the control plots over the last two sampling dates was driven by increases in organic N, the dominant form of extractable N, rather than changes in inorganic N (Table 1). Although soil samples collected in November were insensitive to a single freeze–thaw cycle (above), after two consecutive freeze–thaw cycles there was an increase in extractable N for the most extreme (−10°C minimum temperature) freeze–thaw treatment for samples also collected at this time (P = 0.005; Fig. 3). Increases in extractable organic N, NH4 +, and NO3 − all contributed to increases in the latter (Table 2).
Mean total extractable N from soil samples collected on a 5 November and b 5 December 2007 and c 7 January and d 23 January 2008 and exposed to freeze cycles of varying minimum temperature. Different lower case letters above bars indicate statistically significant differences at P < 0.05 (Tukey’s HSD tests). Error bars denote standard error (n = 5)
Mean total extractable N from soil samples collected on 7 November and exposed to two consecutive freeze cycles of varying minimum temperature. Different lower case letters above bars indicate statistically significant differences at P < 0.05 (Tukey’s HSD test). Error bars denote standard error (n = 5)
For the freezing rate experiment, there were significant interactions between rate and minimum temperature (P < 0.001) and rate and sampling date (P < 0.001). There was no significant effect of freezing rate across either amplitude for samples collected on 18 December, whereas the faster freezing–thawing rates (3 and 30°C h−1) generally resulted in significantly more extractable N than the slowest rate (1°C h−1) across both amplitude minima (−2°C, −5°C) for the later two dates (Fig. 4). These increases in extractable N were driven by increases in organic N (Table 3).
Mean total extractable N from soil samples collected on a 18 December 2007 and b 7 January and c 23 January 2008 and exposed to freeze cycles of varying minimum temperature over a range of freezing rates. Different lower case letters above bars indicate statistically significant differences at P < 0.05 (Tukey’s HSD tests). Error bars denote standard error (n = 5)
Discussion
In our experiments, multiple, large amplitude freeze–thaw cycles and those associated with rapid freezing rates increased extractable N, whereas moderate freeze–thaw cycles did not. This finding helps reconcile the contradictory results of previous studies that have employed mild freeze–thaw cycles and failed to detect significant effects with those that have imposed more extreme freeze–thaw treatments and detected strong effects, as described in the “Introduction”. The question of which intensity of freeze–thaw cycle treatments is most representative of realistic soil conditions depends on the context of the system. In our temperate old field study site, it is possible that a thin veneer of surface soil may have experienced diurnal cycles with minimum temperatures as severe as −10°C or freezing rates in excess of 1°C h−1 in the absence of snow cover. However, even as shallow as 1 cm depth, such extreme rates were not encountered (Fig. 1). Combined with the results of our freeze–thaw experiments, these data indicate that increases in extractable N resulting from freezing-induced microbial cell lysis are not likely common in our system. Nevertheless, our site is characterized by a relatively thick grass litter layer that provides insulation from air temperature fluctuations, whereas more extreme and deeper diurnal freeze–thaw cycles may be experienced by more exposed soils.
Contrary to our prediction, over a single freeze–thaw cycle and at a realistic freezing rate, treated samples contained less extractable N than control samples over the last two sampling dates (with the exception of the mildest (0°C) treatment on 23 January). Unlike the earlier sampling points, these mid-winter sampling points both occurred following prolonged periods of snow cover and soil freezing (Fig. 1), and the relatively low extractable N in the freeze–thaw treated plots may have reflected increases in extractable N in the control plots in response to thaw, rather than decreases in extractable N in response to freeze–thaw (extraction of a set of samples immediately after thawing would have been required to confirm this). Although soil microbes remain active below 0°C, there are often abrupt increases in soil respiration above freezing (Mikan et al. 2002; Kato et al. 2005; Sjursen et al. 2005), coinciding with shifts in the profiles of C and N substrate usage (Michaelson and Ping 2003; Schmidt and Lipson 2004; Schimel and Mikan 2005). Therefore, the time soil remains frozen, not only freeze–thaw dynamics, has important implications for C and N cycling (Monson et al. 2006), and the control samples in our experiment remained thawed longer than the treatment samples prior to extraction. While pulses of labile C in the form of root exudates or fine root turnover products are available to soil microorganisms in late fall and early winter (Kielland et al. 2006), increased extractable N in response to mid-winter thaw has been explained by increased N mineralization, driven by microbial C limitation over winter (Giblin et al. 1991; Schimel and Clein 1996; Miller et al. 2007; Buckeridge and Grogan 2008). However, the mid-winter freeze–thaw cycles in our experiment increased extractable organic N, rather than inorganic N, which suggests that increased soil proteolytic activity (Kielland et al. 2007), rather than increased N mineralization, may have been responsible for increased extractable N in the control samples, provided that proteins were the major organic N compound being hydrolysed in the soil.
Ultimately, although freeze–thaw cycle damage and increased N mineralization and proteolytic activity during thawed periods can all potentially increase soil extractable N over winter, these mechanisms may differ with respect to the fraction of this released N that is retained in the system for plant uptake over the following growing season. The form of N and timing of release has clear implications for its susceptibility to leaching or trace gas losses during melt periods (Catt et al. 1998; Phillips 2008). In addition, soil freezing can damage fine roots (Fitzhugh et al. 2001; Weih and Karlsson 2002), and in a review of ecosystem N losses in response to freeze–thaw cycles, Matzner and Borken (2008) concluded that soil NO3 − losses following severe soil frosts likely result from reduced root uptake, as opposed to increased net N mineralization, although this mechanism remains to be tested explicitly. The ability of plants to exploit N pulses during spring melt varies widely among species and systems (Bilbrough et al. 2000; Bowman and Bilbrough 2001; Joseph and Henry 2009), and in some systems, microbial or abiotic immobilization of N in both soil and litter can result in high ecosystem N retention during periods over winter when plant uptake is low (Stetlzer and Bowman 2005; Judd et al. 2007). The sensitivity of these processes to variation in winter freezing dynamics remains largely unexplored, yet this information is critical for further evaluating the influence of soil freeze–thaw cycles on soil fertility.
References
Andresen LC, Michelsen A (2005) Off-season uptake of nitrogen in temperate heath vegetation. Oecologia 144:585–597 doi:10.1007/s00442-005-0044-1
Bannister P (2005) Frost resistance of the New Zealand narrow-leaved snow tussock grass, Chionochloa rigida. NZ J Bot 43:425–430
Bechmann ME, Kleinman PJA, Sharpley AN, Saporito LS (2005) Freeze–thaw effects on phosphorus loss in runoff from manured and catch-cropped soils. J Environ Qual 34:2301–2309 doi:10.2134/jeq2004.0415
Bilbrough CJ, Welker JM, Bowman WD (2000) Early spring nitrogen uptake by snow-covered plants: a comparison of arctic and alpine plant function under the snowpack. Arct Antarct Alp Res 32:404–411 doi:10.2307/1552389
Bowman WD, Bilbrough CJ (2001) Influence of a pulsed nitrogen supply on growth and nitrogen uptake in alpine graminoids. Plant Soil 233:283–290 doi:10.1023/A:1010571920890
Brooks PD, Williams MW (1999) Snowpack controls on nitrogen cycling and export in seasonally snow-covered catchments. Hydrol Process 13:2177–2190 doi:10.1002/(SICI)1099-1085(199910)13:14/15<2177::AID-HYP850>3.0.CO;2-V
Buckeridge KM, Grogan P (2008) Deepened snow alters soil microbial nutrient limitations in arctic birch hummock tundra. Appl Soil Ecol 39:210–222 doi:10.1016/j.apsoil.2007.12.010
Cabrera ML, Beare MH (1993) Alkaline persulfate oxidation for determining total nitrogen in microbial biomass extracts. Soil Sci Soc Am J 57:1007–1017
Callesen I, Borken W, Kalbitz K, Matzner E (2007) Long-term development of nitrogen fluxes in a coniferous ecosystem: does soil freezing trigger nitrate leaching? J Plant Nutr Soil Sci 170:189–196 doi:10.1002/jpln.200622034
Campbell JL, Mitchell MJ, Groffman PM, Christenson LM, Hardy JP (2005) Winter in northeastern North America: a critical period for ecological processes. Front Ecol Environ 3:314–322
Catt JA, Howse KR, Christian DG, Lane PW, Harris GL, Goss MJ (1998) Strategies to decrease nitrate leaching in the Brimstone Farm Experiment, Oxfordshire, UK, 1988–93: the effect of straw incorporation. Plant Soil 203:57–69 doi:10.1023/A:1004389426718
Christensen S, Christensen BT (1991) Organic-matter available for denitrification in different soil fractions—effect of freeze thaw cycles and straw disposal. J Soil Sci 42:637–647 doi:10.1111/j.1365-2389.1991.tb00110.x
Cleavitt NL, Fahey TJ, Groffman PM, Hardy JP, Henry KS, Driscoll CT (2008) Effects of soil freezing on fine roots in a northern hardwood forest. Can J For Res 38:82–91 doi:10.1139/X07-133
Clein JS, Schimel JP (1995) Microbial activity of tundra and taiga soils at subzero temperatures. Soil Biol Biochem 27:1231–1234 doi:10.1016/0038-0717(95)00044-F
de Gerenyu VOL, Kurganova IN, Teepe R, Loftfild N, Flessa H (2007) Effect of contrasting changes in hydrothermic conditions on the N2O emission from forest and tundra soils. Agric Chem Soil Fertil 7:885–889
Decker KLM, Wang D, Waite C, Scherbatskoy T (2003) Snow removal and ambient air temperature effects on forest soil temperatures in northern Vermont. Soil Sci Soc Am J 67:1234–1243
DeLuca TH, Keeney DR, McCarty GW (1992) Effect of freeze–thaw events on mineralization of soil-nitrogen. Biol Fertil Soils 14:116–120 doi:10.1007/BF00336260
Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO (2000) Climate extremes: observations, modelling, and impacts. Science 289:2068–2074 doi:10.1126/science.289.5487.2068
Fitzhugh RD, Driscoll CT, Groffman PM, Tierney GL, Fahey TJ, Hardy JP (2001) Effects of soil freezing, disturbance on soil solution nitrogen, phosphorus, and carbon chemistry in a northern hardwood ecosystem. Biogeochemistry 56:215–238 doi:10.1023/A:1013076609950
Freppaz M, Williams BL, Edwards AC, Scalenghe R, Zanini E (2007) Simulating soil freeze/thaw cycles typical of winter alpine conditions: implications for N and P availability. Appl Soil Ecol 35:247–255 doi:10.1016/j.apsoil.2006.03.012
Giblin AE, Nadelhoffer KJ, Shaver GR, Laundre JA, McKerrow AJ (1991) Biogeochemical diversity along a riverside toposequence in arctic Alaska. Ecol Monogr 61:415–435 doi:10.2307/2937049
Groffman PM, Driscoll CT, Fahey TJ, Hardy JP, Fitzhugh RD, Tierney GL (2001) Colder soils in a warmer world: a snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry 56:135–150 doi:10.1023/A:1013039830323
Grogan P, Jonasson S (2003) Controls on annual nitrogen cycling in the understory of a subarctic birch forest. Ecology 84:202–218 doi:10.1890/0012-9658(2003)084[0202:COANCI]2.0.CO;2
Grogan P, Michelsen A, Ambus P, Jonasson S (2004) Freeze–thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocosms. Soil Biol Biochem 36:641–654 doi:10.1016/j.soilbio.2003.12.007
Hagerty TP, Kingston MS (1992) The soils of Middlesex County. Ontario Ministry of Agriculture and Food
Henry HAL (2007) Soil freeze–thaw cycle experiments: trends, methodological weaknesses and suggested improvements. Soil Biol Biochem 39:977–986 doi:10.1016/j.soilbio.2006.11.017
Henry HAL (2008) Climate change and soil freezing dynamics: historical trends and projected changes. Clim Change 87:421–434 doi:10.1007/s10584-007-9322-8
Herrmann A, Witter E (2002) Sources of C and N contributing to the flush in mineralization upon freeze–thaw cycles in soils. Soil Biol Biochem 34:1495–1505 doi:10.1016/S0038-0717(02)00121-9
Hobbie SE, Chapin FS (1996) Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochemistry 35:327–338 doi:10.1007/BF02179958
Hu QC, Van Bochove E, Warland J, Kay B, Wagner-Riddle C (2006) New method to simulate soil freezing and thawing cycles for studying nitrous oxide flux. Soil Sci Soc Am J 70:2106–2113 doi:10.2136/sssaj2006.0085
IPCC (2007) Summary for policymakers. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Isard SA, Schaetzl RJ (1998) Effects of winter weather conditions on soil freezing in southern Michigan. Phys Geogr 19:71–94
Joseph G, Henry HAL (2008) Soil nitrogen leaching losses in response to freeze–thaw cycles and pulsed warming in a temperate old field. Soil Biol Biochem 40:1947–1953 doi:10.1016/j.soilbio.2008.04.007
Joseph G, Henry HAL (2009) Retention of surface nitrate additions in a temperate old field: implications for atmospheric nitrogen deposition over winter. Plant Soil doi:10.1007/s11104-008-9862-3
Judd KE, Likens GE, Groffman PM (2007) High nitrate retention during winter in soils of the hubbard brook experimental forest. Ecosystems (N Y, Print) 10:217–225 doi:10.1007/s10021-007-9027-x
Kaste O, Austnes K, Vestgarden LS, Wright RF (2008) Manipulation of snow in small headwater catchments at Storgama, Norway: effects on leaching of inorganic nitrogen. Ambio 37:29–37 doi:10.1579/0044-7447(2008)37[29:MOSISH]2.0.CO;2
Kato T, Hirota M, Tang Y, Cui X, Li Y, Zhao X, Oikawa T (2005) Strong temperature dependence and no moss photosynthesis in winter CO2 flux for a Kobresia meadow on the Qinghai-Tibetan plateau. Soil Biol Biochem 37:1966–1969 doi:10.1016/j.soilbio.2005.02.018
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL (ed) Methods of soil analysis. Part 2, Agronomy Monograph 9. 2nd edn. American Society of Agronomy and Soil Science Society of America, Madison, pp 643–698
Kielland K, Olson K, Ruess RW, Boone RD (2006) Contribution of winter processes to soil nitrogen flux in taiga forest ecosystems. Biogeochemistry 81:349–360 doi:10.1007/s10533-006-9045-3
Kielland K, McFarland JW, Ruess RW, Olson K (2007) Rapid cycling of organic nitrogen in taiga forest ecosystems. Ecosystems (N Y, Print) 10:360–368 doi:10.1007/s10021-007-9037-8
Koponen HT, Martikainen PJ (2004) Soil water content and freezing temperature affect freeze–thaw related N2O production in organic soil. Nutr Cycl Agroecosyst 69:213–219 doi:10.1023/B:FRES.0000035172.37839.24
Koponen HT, Jaakkola T, Keinanen-Toivola MM, Kaipainen S, Tuomainen J, Servomaa K, Martikainen PJ (2006) Microbial communities, biomass, and activities in soils as affected by freeze thaw cycles. Soil Biol Biochem 38:1861–1871 doi:10.1016/j.soilbio.2005.12.010
Larsen KS, Jonasson S, Michelsen A (2002) Repeated freeze–thaw cycles and their effects on biological processes in two arctic ecosystem types. Appl Soil Ecol 21:187–195 doi:10.1016/S0929-1393(02)00093-8
Lipson DA, Schmidt SK, Monson RK (2000) Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biol Biochem 32:441–448 doi:10.1016/S0038-0717(99)00068-1
Ludwig B, Wolf I, Teepe R (2004) Contribution of nitrification and denitrification to the emission of N2O in a freeze–thaw event in an agricultural soil. J Plant Nutr Soil Sci 167:678–684 doi:10.1002/jpln.200421462
Ludwig B, Teepe R, de Gerenyu VL, Flessa H (2006) CO2 and N2O emissions from gleyic soils in the Russian tundra and a German forest during freeze–thaw periods—a microcosm study. Soil Biol Biochem 38:3516–3519 doi:10.1016/j.soilbio.2006.06.006
Matzner E, Borken W (2008) Do freeze–thaw events enhance C and N losses from soils of different ecosystems? A review. Eur J Soil Sci 59:274–284 doi:10.1111/j.1365-2389.2007.00992.x
Mazur P (1966) Physical and chemical basis of injury in single-celled microorganisms subjected to freezing and thawing. In: Merman HT (ed) Cryobiology. Academic, New York, pp 214–315
Mellander PE, Löfvenius MO, Laudon H (2007) Climate change impact on snow and soil temperature in boreal Scots pine stands. Clim Change 85:179–193 doi:10.1007/s10584-007-9254-3
Michaelson GJ, Ping CL (2003) Soil organic carbon and CO2 respiration at subzero temperature in soils of Arctic Alaska. J Geophys Res-Atmos 108:8164 doi:10.1029/2001JD000920
Mikan CJ, Schimel JP, Doyle AP (2002) Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biol Biochem 34:1785–1795 doi:10.1016/S0038-0717(02)00168-2
Miller AE, Schimel JP, Sickman JO, Meixner T, Doyle AP, Melack JM (2007) Mineralization responses at near-zero temperatures in three alpine soils. Biogeochemistry 84:233–245 doi:10.1007/s10533-007-9112-4
Monson RK, Lipson DA, Burns SP, Turnipseed AA, Delany AC, Williams MW, Schmidt SK (2006) Winter forest soil respiration controlled by climate and microbial community composition. Nature 439:711–714 doi:10.1038/nature04555
Mulvaney RL (1996) Nitrogen-inorganic forms. In: Bigham JM (ed) Methods of soil analysis. Part 3. Chemical methods. Soil Science Society of America, Madison, pp 1123–1200
Oztas T, Fayetorbay F (2003) Effect of freezing and thawing processes on soil aggregate stability. Catena 52:1–8 doi:10.1016/S0341-8162(02)00177-7
Phillips RL (2008) Denitrification in cropping systems at sub-zero soil temperatures. A review. Agron Sustain Dev 28:87–93 doi:10.1051/agro:2007045
Sahin U, Anapali O (2007) Short communication. The effect of freeze–thaw cycles on soil aggregate stability in different salinity and sodicity conditions. Span J Agric Res 5:431–434
Schimel JP, Clein JS (1996) Microbial response to freeze–thaw cycles in tundra and taiga soils. Soil Biol Biochem 28:1061–1066 doi:10.1016/0038-0717(96)00083-1
Schimel JP, Mikan C (2005) Changing microbial substrate use in Arctic tundra soils through a freeze–thaw cycle. Soil Biol Biochem 37:1411–1418 doi:10.1016/j.soilbio.2004.12.011
Schimel JP, Kielland K, Chapin FS (1996) Nutrient availability and uptake by tundra plants. In: Reynolds JF, Tenhunen JD (eds) Landscape function: implications for ecosystem response to disturbance. A case study of Arctic Tundra. Springer, Berlin, pp 203–221
Schmidt SK, Lipson DA (2004) Microbial growth under the snow: implications for nutrient and allelochemical availability in temperate soils. Plant Soil 259:1–7 doi:10.1023/B:PLSO.0000020933.32473.7e
Sharma S, Szele Z, Schilling R, Munch JC, Schloter M (2006) Influence of freeze–thaw stress on the structure and function of microbial communities and denitrifying populations in soil. Appl Environ Microbiol 72:2148–2154 doi:10.1128/AEM.72.3.2148-2154.2006
Sharratt BS (1993) Freeze–thaw and winter temperature of agricultural soils in interior Alaska. Cold Reg Sci Technol 22:105–111 doi:10.1016/0165-232X(93)90049-E
Sjursen H, Michelsen A, Holmstrup M (2005) Effects of freeze–thaw cycles on microarthropods and nutrient availability in a sub-Arctic soil. Appl Soil Ecol 28:79–93 doi:10.1016/j.apsoil.2004.06.003
Skogland T, Lomeland S, Goksøyr J (1988) Respiratory burst after freezing and thawing of soil: experiments with soil bacteria. Soil Biol Biochem 20:851–856 doi:10.1016/0038-0717(88)90092-2
Solorzano L (1969) Determination of ammonia in natural waters by the phenol-hypochlorite method. Limnol Oceanogr 14:799–801
Steltzer H, Bowman WD (2005) Litter N retention over winter for a low and a high phenolic species in the alpine tundra. Plant Soil 275:361–370 doi:10.1007/s11104-005-3100-z
Sulkava P, Huhta V (2003) Effects of hard frost and freeze–thaw cycles on decomposer communities and N mineralisation in boreal forest soil. Appl Soil Ecol 22:225–239 doi:10.1016/S0929-1393(02)00155-5
Walker VK, Palmer GR, Voordouw G (2006) Freeze–thaw tolerance and clues to the winter survival of a soil community. Appl Environ Microbiol 72:1784–1792 doi:10.1128/AEM.72.3.1784-1792.2006
Wang FL, Bettany JR (1994) Organic and inorganic nitrogen leaching from incubated soils subjected to freeze–thaw and flooding conditions. Can J Soil Sci 74:201–206
Weih M, Karlsson PS (2002) Low winter soil temperature affects summertime nutrient uptake capacity and growth rate of mountain birch seedlings in the subarctic, Swedish lapland. Arct Antarct Alp Res 34:434–439 doi:10.2307/1552201
Wynn-Williams DD (1982) Simulation of seasonal changes in microbial activity of maritime Antarctic peat. Soil Biol Biochem 14:1–12 doi:10.1016/0038-0717(82)90069-4
Yanai Y, Toyota K, Okazaki M (2004) Effects of successive soil freeze–thaw cycles on soil microbial biomass and organic matter decomposition potential of soils. Soil Sci Plant Nutr 50:821–829
Acknowledgements
This work was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant to HALH. We thank Dr. Brent Sinclair for assistance with soil incubation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elliott, A.C., Henry, H.A.L. Freeze–thaw cycle amplitude and freezing rate effects on extractable nitrogen in a temperate old field soil. Biol Fertil Soils 45, 469–476 (2009). https://doi.org/10.1007/s00374-009-0356-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-009-0356-0