Abstract
A number of biological and chemical processes may affect soil phosphorus availability when forest fires occur, partly as a result of heating. We describe here a laboratory experiment to study the effects of soil heating on changes in sorption and desorption of P. Autoclaving was also included as an additional treatment of moist heating under pressure. Five forest soils (two Podzols, one Arenosol, one Luvisol and one Alisol) were heated to 60°C, 120°C and 250°C or autoclaved for 30 min. They were repeatedly extracted with Bray I and analysed for inorganic and organic P fractions. The desorbed P data were fitted to an asymptotic exponential equation to obtain the desorption rate and capacity parameters. Podzol and Arenosol soils showed a quick P desorption after heating, while Luvisol and Alisol soils showed a slow desorption rate. The immediate increase in available P that occurred after heating or autoclaving originated mostly from solubilisation of microbial metabolites and soil organic components. Autoclaving decreased P sorption capacity in all soils, but the effects of heating on P sorption differed among soils. Except for one of the soils, the low P-fixing soils (Podzol and Arenosol) showed a decrease in P sorption when heated to high temperatures, whereas the high P-fixing soils (Luvisol and Alisol) showed little changes after heating. Fire intensity and soil characteristics are important factors determining short-term and long-term soil P dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fire is a common feature of the forests that can occur as wildfire, prescribed fire and slash burning (Walker et al. 1986; Russell-Smith et al. 2007). Changes in nutrient availability are often reported in the literature after fire (Neary et al. 1999; Hart et al. 2005), as effects of soil heating, combustion and ash deposition (Raison et al. 1985; Kauffman et al. 1993, Serrasolsas and Khanna 1995). Many studies found that the availability of P often increased after forest fires (Raison et al. 1985; Romanyà et al. 1994; Cade-Menun et al. 2000), probably as result of the solubilisation of organic P and the death of microorganisms. Giovannini et al. (1990) and Saa et al. (1993) found that soil heating produced an intense mineralising effect on organic P resulting in an increase in inorganic P.
However, the increase in the amount of available P does not last long, and its availability may quickly decrease during the months following heating (Kutiel and Shaviv 1989; Serrasolsas and Khanna 1995) or after several years following clear cutting and burning (Binkley and Christensen, 1992; Cade-Menun et al. 2000). The decline in P availability could be associated to changes in the mechanisms of P adsorption and desorption (Kwari and Batey 1991; Romanyà et al. 1994; Juo and Manu 1996) and also to the changes in microbial population and P mineralisation (Ilstedt et al. 2003). In fact, changes in the microbial community structure and biomass are often reported as a consequence of fire and post-fire recovery (Díaz-Raviña et al. 2006; Liu et al. 2007).
Changes in the P adsorption sites (on the clay surface and Fe and Al oxides) that may occur during soil heating or burning may change the dynamics in the supply of P. Similarly, reductions in microbial populations as a result of soil heating may decrease microbial P immobilisation. Together, interactions between biological and geochemical processes control how much of soil P remains bioavailable (Olander and Vitousek 2004; Barros et al. 2005; Sato and Comerford 2006).
Intense slash and burn often increases P sorption capacity in the soil surface layer (Juo and Manu 1996; Ketterings et al. 2002). Kwari and Batey (1991) observed slight increases in P sorption capacity after heating soils in a furnace (at 250°C for 3 h) and dramatic increase after field burning of straw. They stated that the increase in P sorption reduced P in the soil solution and P availability. Studying fire effects on P sorption of Eucalypt forests, Romanyà et al. (1994) found slight decreases in P sorption in burnt soils with little ash deposition, whereas they found large sorption increases on ashbed areas. Giardina et al. (2000) reported that in a slash-and-burn experiment with a small deposition of ash, soil heating had a greater influence on soil P availability than the P inputs by ash. Both heating conditions and ash deposition may affect the binding of P with soil minerals and organic matter and the composition and activity of soil microflora.
Autoclaving concerns heating of moist soils under pressure, and it is more effective at killing soil microorganisms than dry heat (Choromanska and DeLuca 2002; Hart et al. 2005). For this reason, it is used to sterilise the soil (Endlweber and Scheu 2006). Autoclaving kills soil microorganisms releasing P and other nutrients into the solution and solubilises labile organic matter (López and Barbaro 1988; Xie and MacKenzie 1991). Xie and MacKenzie (1991) found a decrease in P sorption capacity after soil autoclaving. However, very little is known of the effects of autoclaving on P desorption dynamics.
In a previous study, Serrasolsas and Khanna (1995) found that both heating in an oven to temperatures ranging from 60°C to 250°C and autoclaving forest soils increased labile inorganic P content, whereas the P content associated to microorganisms decreased with heat because high temperatures killed the microbial population. The concentration of the immediately released P by heating slowly decreased during laboratory incubations approaching the pre-heating values, partially because it was immobilised by microorganisms. However, it was not clear whether soil P desorption was replacing labile P pools once the labile P released by fire/heating was removed from the soil solution by plants or microorganisms or by sorption and leaching.
This present study was aimed at studying the effect of soil heating or autoclaving on the dynamics of P desorption by monitoring available P by Bray extractions and describing P sorption by P sorption isotherms. Five temperate Australian forest soils with contrasting available P, microbial P and P sorption capacities were studied to show a wide array of responses to the heating and autoclaving effects. The used temperatures ranged from 60°C to 250°C and were similar to those found in a mineral soil during mild and intense fires.
Materials and methods
Experimental sites and soil characteristics
Five soils from different experimental sites in the south-east of Australia were studied (Table 1; Khanna et al. 1986; Serrasolsas and Khanna 1995). Soils from Du, Un and Gn sites were sampled at Cabbage Tree Creek, East Gippsland, Victoria (37°42′ S, 148°43′ E), under a lowland sclerophyll forest of eucalypt, with Eucalyptus sieberi, E. globoidea and E. baxteri being the dominant trees. The mean annual rainfall is about 1,100 mm, evenly distributed throughout the year. Soils from Pc and Biology of Forest Growth Experiment (BFG) sites were collected at Brindabella Range, west of Canberra, ACT (35°21′ S, 148°56′ E). Pc site was located at the Picadilly catchment and under Eucalyptus pauciflora sub-alpine forest, which had a cool temperate climate and annual rainfall of about 1,200 mm occurring throughout the year. The BFG site is a Pinus radiata plantation at Pierces Creek with an annual rainfall of 790 mm. BFG and Du soils are Haplic Podzols (WRB 2006) corresponding to Yellow Podzolic soils of the Australian soil classification (Stace et al. 1968) and are characterised by a duplex profile, with a sandy loam A horizon, a distinct pale A2 horizon, and a yellowish B horizon of much higher clay content; the pH of the A horizon (the one sampled for this study) is moderately acidic, with poor to moderate organic matter content (Table 1). Un soil, an Haplic Arenosol (WRB 2006) or a Siliceous Sand (Stace et al. 1968), is a sandy soil with a uniform profile; it has a low pH and a high content of organic matter (Table 1). Gn is a Haplic Luvisol and Pc a Humic Alisol (WRB 2006), and both correspond to a Yellow Earth and Red Earth, respectively (Stace et al. 1968). Both soils present a Gradational profile form, with a gradual change in texture becoming more clayey with soil depth, and have a high content of sesquioxidic clay minerals. The Luvisol Gn has a sandy–loam texture, is acidic and contains a moderate content of organic matter. The Alisol Pc is a very acid loamy A horizon, with high amounts of exchangeable Al and organic matter contents (Table 1).
Soil sampling and heating treatments
Soil sampling was described in a previous paper (Serrasolsas and Khanna 1995). Bulk samples were taken from the surface (0–5 cm) mineral soil at ten randomly located points. These samples were mixed to form a composite sample and sieved (<0.5 cm). The field moist samples were stored at 4°C until used. All experiments were carried out within a few days after sampling.
Four sub-samples from the bulk sample were spread on aluminium foil to form a 2-cm-thick layer and were separately heated for 30 min at 60°C and 120°C in an oven and at 250°C in a muffle furnace. These treatments with the exception of the heating of Pc soil at 250°C were those reported by Serrasolsas and Khanna (1995). Another three sub-samples were placed in plastic containers and autoclaved (121°C and 1.5 MPa pressure) for 30 min. During the heating, the oven and the muffle furnace were opened once, and soils were stirred to ensure homogeneous heating. Heat treatments were selected on the basis that soils affected by wildfire would be subjected to temperatures of around 60°C to higher than 200°C for low and intense burning (Walker et al. 1986).
Soil analyses
The P desorption was studied by extracting repeatedly soils heated at 60°C, 120°C and 250°C, with four replicates per each temperature and with three replicates for the autoclaved soils (121°C and 1.5 MPa). All soils were extracted with a diluted acid-fluoride (Bray-I method; Bray and Kurtz 1945) solution with a soil/extract ratio of 1:5 and 5 min of mild shaking. After the first extraction, the extract was centrifuged at 3,360 × g and filtered; then, the sediment was extracted again using the same centrifuge tubes. This procedure was repeated ten times, except for the BFG soil that was extracted seven times. Other authors have proposed the use of repeated extractions of P as a method for determining soil P-supplying capacity (Stewart et al. 1990; Smethurst 2000) or for studying desorption kinetics of P (Sato and Comerford 2006). Phosphorus of soil extracts was analysed by the molybdate–ascorbic acid procedure of Murphy and Riley (1962) and was operationally defined as inorganic P. Total P of the Bray extract was determined using the same method after a digestion with H2SO4 and H2O2. Organic P in the Bray extracts was calculated from the difference between total P and inorganic P.
To describe the dynamics of P desorption, we fitted the cumulative P released by repeated Bray extractions to the following ion desorption model:
where y refers to the P content (μg g−1 of dry soil), x to the volume of the extracting solution (ml) and A, B and D are constants, representing, respectively, the maximum P desorption, the P desorption rate and the dissolving rate. This equation has two components; the first describes an exponential rise to maximum, which can be desorbed from the soil (the asymptote A) at a desorption rate B, and the second component Dx is the dissolution of P occurring proportionally to the amount of extracting solution. This model was used by Khanna et al. (1986) to distinguish exchangeable ions from other ion sources in burnt soils.
The P sorption isotherms were plotted by the values of two analytical replicates, which were obtained by bulking, the four or the three soil replicates, of the unheated, 250°C heated and autoclaving treatments. We prepared eight 0.01 M CaCl2 solutions containing from 0 to 150 μg P-KH2PO4 ml−1. These solutions were added to soil at 1:10 weight/volume ratio (w/v) and allowed to equilibrate by shaking for 24 h. To separate the solid phase from the liquid phase, samples were centrifuged at 3,360 × g for 10 min and then filtered. We did not add any biocide to kill microorganisms, so P sorption may also include microbial immobilisation. The adsorbed P was calculated as the difference between the initial and the remaining P concentration of the soil solution (Bache and Williams 1971). The initial P was the amount that was added (0–150 μg P) plus the P extracted from soil by the Bray method. Inorganic P in the extracts was determined using the Murphy and Riley (1962) method. The adsorption isotherms were described by fitting the 16 pairs of data for each treatment and soil to the Langmuir equation:
where X is the amount of P adsorbed in the soil (μg P g−1 soil) at an equilibrium P concentration of soil solution, C (μg P ml−1). A plot of C/X against C should gives a straight line of slope 1/X m from which an adsorption maximum X m (μg P g−1 soil) can be calculated. The constant K is related to the P-bonding energy or the affinity coefficient (ml μg−1 P) and can be calculated from the intercept.
Microbial P flush was determined by the fumigation–extraction method using hexanol as a biocide (Serrasolsas and Khanna 1995). Four milliliters of hexanol were added to a 10-g field moist soil sample. After 24 h of fumigation, soil was extracted for 5 min by the Bray-l method. P flush was calculated as the difference in inorganic P between the fumigated and unfumigated soils without multiplying for any coefficient to calculate microbial biomass; thus, we considered microbial P flush as an estimate of the P microbial biomass.
Statistical analyses
Data from the three or four replicates for each treatment and soil were analysed using one-way analysis of variance to compare temperature differences. The Duncan’s multiple-range test was used to define significant differences between heating temperatures and autoclaving at a probability less than 0.05. To fit desorption and adsorption models, we used linear regressions, and for non-linear curves, we used the least-square sum method. Differences between linear curves were tested with analysis of co-variance taking P concentration in the solution as a covariant.
Results
Phosphorus desorption
The podzolic soil BFG showed a high content of inorganic Bray P, 30 μg·g−1, whereas the other soils (Du, Un, Pc and Gn) had very low values, less than 2 μg·g−1. Except for the BFG soil, the amount of P associated with microbial biomass was much higher than that extracted as inorganic P (Table 1). Autoclaving and heating soils at high temperature (250°C) increased the content of inorganic Bray P when compared to the content extracted from unheated soil, except in Pc soil when heated at 250°C (Table 2).
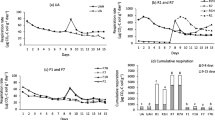
The pattern of P extracted from soil by repeated extractions shows that, after the readily extractable P was removed by the first extraction, soils continued to release P at a lower rate in the subsequent extractions (Fig. 1). On heating and autoclaving, a large increase in the total amount of P released during ten extractions was observed in all soils. However, in the Pc Alisol, very little P was released by the initial extraction of soil heated at 250°C; the extracted P increased by repeated extractions or by the volume of the extracting solution.
The amount of inorganic P released by repeated extraction fitted better to the first than to the second component of the desorption equation 1, indicating that in these soils, the P forms undergoing a continuous P dissolution were not important. There were differences in the pattern of P release between the heated and unheated soil samples. For the Podzols (BFG and Du) and the Arenosol (Un), the P released by repeated extraction followed an asymptotic value (A) for unheated, heated and autoclaved treatments (Table 2). The P data from unheated Luvisol (Gn) and Alisol (Pc) did not fit to this equation as P released increased continuously with the volume of the extracting solution (Fig. 1). However, P desorption data after autoclaving or after some heating treatments (250°C for Gn, and 60°C and 120°C for Pc) successfully fitted to the asymptotic equation (Table 2).
Soils heated at 250°C or autoclaved showed much higher P released, as indicated by A values, than the unheated soils. Parameter B in the equation described the P desorption rate, which was the highest for the 250°C-treated and autoclaved soils than for those unheated or heated to low temperatures (Table 2).
The Luvisol and the Alisol soils (Gn and Pc, respectively) contained considerable amounts of organic P in the Bray extracts; Gn soil had 5 μg·g−1 and Pc soil 1.8 μg·g−1 (Fig. 2a). Bray extracts of the other soils did not show any detectable levels of organic P. Heating the Luvisol (Gn) at 120°C and 250°C decreased the amount of total and organic P in the Bray extraction, while inorganic P increased (Fig. 2a). On the other hand, heating the Alisol (Pc) soil at 120°C solubilised labile organic P and increased the total Bray P in the initial extraction (Fig. 2a). However, heating Pc soil at 250°C decreased both inorganic and organic Bray P forms to almost zero (Fig. 2a). The amount of organic P extracted by repeated extractions of these soils (Fig. 2b) decreased by heating at 250°C, whereas the amount of inorganic P increased in Gn and Pc soils (Fig. 2b).
Inorganic, organic and total Bray P of Luvisol (Gn) and Alisol (Pc) soils heated at different temperatures. In autoclaved soils, only inorganic Bray P was analysed. a Initial Bray extraction by using 5 ml g−1soil. b Amount of P released after ten repeated extractions (50 ml g−1 soil). Mean and SE of three or four sub-samples. Different letters indicate significant differences among temperatures for each P type
Sorption of phosphorus
Phosphorus sorption isotherms of the studied soils are shown in Fig. 3, and the parameters of the Langmuir equation are shown in Table 3. Maximum P sorption values (X m) of unheated Luvisol and Alisol soils (Gn and Pc) were (1,280 μg P·g−1) about 10 to 20 times higher than those of the unheated BFG, Du and Un soils (183, 103 and 54 μg P·g−1, respectively). In contrast, the affinity coefficient K was very high for the sandy Un soil showing a high adsorption of the P added at low concentrations. The lowest K values were for the podozolic Du and BFG soils, whereas these values for Gn and Pc soil were intermediate (Table 3).
Soils with the lowest P sorption capacities (Du and Un soils) when heated at 250°C showed a strong decrease in the number of P sorption sites (as indicated by maximum P sorption, X m), especially in the Arenosol (Un), and in the value of the affinity coefficient (K). On the other hand, the BFG soil and the soils (Gn and Pc) with a high P sorption capacity did not show any statistical change in their sorption capacity (X m) or in their affinity coefficient after heating.
On autoclaving, the P sorption capacity decreased significantly in all soils, except in the BFG soil (Table 3). The Arenosol Un showed the highest decrease (88%) in X m after autoclaving, followed by Du (32%), Pc (25%), and Gn (17%; P < 0.05). Autoclaving decreased K values slightly in most of the cases, indicating a decrease in P retention strength by soil.
Discussion
In all the studied soils, heating to high temperature (250°C) or autoclaving increased the amount of Bray P of the first extraction and the successive extractions. Heating soils at temperatures lower than 120°C generally did not affect the amount of available P (Table 2). The increase in the amount of available P after fire has been already reported (Raison et al. 1985; Saa et al. 1993; Cade-Menun et al. 2000) and supposed to be due to soil heating and ash deposition. Heating alone has also produced an increase in available P (Serrasolsas and Khanna 1995; Endlweber and Scheu 2006).
The different soils behaved differently in the dynamics of P sorption and P release after heating or autoclaving due to different soil characteristics. The soils with the lowest P fixing capacity, the Arenosol (Un) and the Podzol (Du) soils, showed an increase in P desorption on heating and autoclaving (Table 2), and this increase was mainly detected by the first extraction following the heat treatment. This was probably related to the release of microbial P (Table 1; Serrasolsas and Khanna 1995). Similarly, Turner and Haygarth (2001) and Styles and Coxon (2006) have reported a microbial origin of high amounts of water-soluble inorganic P released after drying and re-wetting cycles of soils. Sorption of P in low P-fixing soils was affected by heating at 250°C or autoclaving, especially in the Arenosol (Un) soil. Probably, the strong decrease in P sorption depended partially on the suppression of microbial P uptake after heating, as reported by Serrasolsas and Khanna (1995), because microbial P flush decreased to almost zero by heating of soils at 250°C or autoclaving. In these low P-fixing soils, the microbiological cycle seems to control P availability and sorption and desorption processes. Indeed, when the microbial population is killed by heating or autoclaving, P sorption is strongly decreased and a short increase in labile P is observed. The recovery of the microbial population would re-establish the dynamics of available P in these low P-fixing soils.
It is important to underline that any forest fire has a spatially heterogeneous nature. Romanyà et al. (1994) observed that after slash burns of our podzol Du soil, P sorption capacity (X m) decreased only in areas that had low-intensity burns and had received little or no ash deposition, whereas on ashbed areas, a large increase in P sorption was observed. They attributed this increase to the addition of ashes, which contained sesquioxide complexes from burnt organic matter. Thus, P availability after fire probably increased over the long term only in the ashbed areas, while it quickly decreased in low-intensity burnt areas.
Changes in P desorption and sorption after heating soils at high temperatures or autoclaving were less important for the low P-fixing BFG soil, probably because it contained a high amount of available P and any change in P retention would not likely limit P availability (Table 3). The increase in the amount of P desorbed after heating was related to neither microbial P nor organic P, which were at undetectable levels in this soil, but it depended on the solubilisation of other mineral P sources. Serrasolsas and Khanna (1995) reported that the P released in this soil by heating was not immobilised under laboratory conditions but remained in its extractable form.
In soils of high P-fixing capacity (Gn and Pc soils), P continued to be extracted by repeated extractions with the Bray method without reaching at any asymptotic value. These soils contained labile organic P, which was also extracted by repeated extractions and was 3.3 to 5.5 times greater than microbial P flush (Table 1) indicating that most of the extracted organic P was not of microbial origin. The Gn and Pc soils showed a decrease in total labile P (organic and inorganic) at high temperature (250°C) in the initial single extraction. As P volatilisation does not occur below 360°C (Raison et al. 1985, Kauffman et al. 1993), this P decrease after heating may be attributed to a quick re-adsorption of extracted P by soil particles (Fig. 2b). The Gn and Pc soil did not show any significant change in P sorption after heating at 250°C, suggesting that heating these soils would not have any substantial effect on soil P supply. Changes in P availability occurring in these high P-fixing soils as a result of heating probably were due to solubilisation of organic P with its quick re-adsorption by the soil particles. However, these changes are very small compared to the high amount of P sorbed in these soils, due to the high content of sesquioxidic clay minerals, organic matter and, in the case of the Alisol (Pc), high exchangeable Al content (Table 1).
In contrast to others (Kwari and Batey 1991; Juo and Manu 1996; Giardina et al. 2000; Ketterings et al. 2002), we have found a decrease or no change in the P sorption after heating at 250°C. Probably, in our soils, the changes occurring at temperatures not higher than 250°C mostly involved soil microbiota and organic components and not the mineral components. Indeed, the above mentioned authors found more intense effects than ours heating soils at 300°C, 450°C and 600°C and heating soil for longer times. These effects included combustion of organic matter, production of ashes, changes in the mineralogy fractions and changes in the specific surface area, with generally higher P sorption than those observed in our soils. For instance, Ketterings et al. (2002) found an increase of the maximum sorption capacity (X m) of a high P-fixing oxisol after heating in an oven at temperatures greater than or equal to 450°C, and this increase was attributed to the increase in specific surface area of the mineral fraction.
After autoclaving (121°C), similar or even larger amounts of P were released than by heating at 250°C, suggesting that a number of chemical and/or biological processes can cause P desorption on heating moist soils under pressure. Indeed, Otabbong and Barbolina (1998) and Anderson and Magdoff (2005) found that autoclaving different soils increased the amount of soluble orthophosphate monoesters and diesters and the amount of inorganic P. Hart et al. (2005) stated that moist heat is more effective at killing soil microorganisms than dry heat; lethal temperatures (50–210°C) for specific microbial groups may be reduced by as much as one half under moist conditions. In fact, except for BFG soil, the amount of P extracted by the single initial Bray P extraction after autoclaving was similar to the amount of microbial P of the unheated soils (Table 1), and both parameters showed a significant correlation (r = 0.989, P < 0.012), suggesting that the microbial P was a source of labile P after autoclaving. Other authors have reported similar results (López and Barbaro 1988; Seeling and Zasoski 1993; Serrasolsas and Khanna 1995). In addition, autoclaving soils decreased the P sorption of all studied soils, except in the BFG soil (Table 3). Similarly, Xie and MacKenzie (1991) found a decrease in sorption capacity of the autoclaved soils and an increase in P desorption that were related to a decrease in extractable Fe and Al and an increase in cation exchange capacity and surface tension. The main treatment difference between autoclaving and (air or oven) drying was moisture vapour, so, according to these authors, water molecules could be adsorbed on the soil surface particles blocking or occupying adsorption sites and, as a result, reducing P sorption.
Conclusions
Heating soils at high temperatures or autoclaving increased available P content, probably due to the death of soil microorganisms, soil organic matter solubilisation and chemical changes in soil components. Soil heating and ash deposition can explain the increase in available P during wildfires. In low P-fixing Podzolic soils (BFG and Du) and in the Arenosol (Un), heat-released P was easily desorbed with a single Bray extraction, whereas in the high P-fixing soils (Gn and Pc), P released by heat was transiently re-adsorbed and could be only recovered by repeated Bray extractions.
Autoclaving decreased P sorption of all soils, but the effect of heating depended on the soil. High P-fixing soils (Pc and Gn) showed no or little changes in P sorption when heated at high temperature. In contrast, low P-fixing soils (Un and Du) showed a strong decrease in P sorption or no changes when labile P was high and organic matter and microbial P were low (BFG soil). During forest fires, the reduction in soil P sorption capacity that may occur, in some cases, as a result of heating may be counter-balanced by the increase in P sorption capacity due to ash deposition.
References
Anderson BH, Magdoff FR (2005) Autoclaving soil samples affects algal-available phosphorus. J Environ Qual 34:1958–1963
Bache BW, Williams EG (1971) A phosphate sorption index for soils. J. Soil Sci 22:289–301
Barros Filho NF, Comerford NB, Barros NF (2005) Phosphorus sorption, desorption and resorption by soils of the Brazilian Cerrado supporting eucalypt. Biomass Bioenergy 28:229–236
Binkley D, Christensen N (1992) The effects of canopy fire on nutrient cycles and plant productivity. In: Lavin R, Omi P (eds) Pattern and process in crown fire ecosystems. Princeton University Press, Princeton, NJ
Bray RH, Kurtz LT (1945) Determination of total, organic and available forms of phosphorus in soils. Soil Sci 59:39–45
Cade-Menun BJ, Berch SM, Preston CM, Lavkulich LM (2000) Phosphorus forms and related soil chemistry of Podzolic soils of northern Vancouver Island. II The effects of clear-cutting and burning. Can J For Res 30:1726–1741
Choromanska U, DeLuca TH (2002) Microbial activity and nitrogen mineralization in forest mineral soils following heating: evaluation of post-fire effects. Soil Biol Biochem 34:263–271
Díaz-Raviña M, Baath E, Martin A, Carballas T (2006) Microbial community structure in forest soils treated with a fire retardant. Biol Fertil Soils 42:465–471
Endlweber K, Scheu S (2006) Establishing arbuscular mycorrhiza-free soil: a comparison of six methods and their effects on nutrient mobilization. Appl. Soil Ecol 34:276–279
Giardina CP, Sanford RL, Døckersmith IC (2000) Changes in soil phosphorus and nitrogen during slash and burn clearing of a dry tropical forest. Soil Sci Soc Am J 64:399–405
Giovannini G, Lucchesi S, Giachetti M (1990) Effects of heating on some chemical parameters related to soil fertility and plant growth. Soil Sci 149:344–350
Hart SC, DeLuca TH, Newman GS, MacKenzie MD, Boyle SI (2005) Post-fire vegetative dynamics as drivers of microbial community structure and function in forest soils. For Ecol Manag 220:166–184
Ilstedt U, Giesler R, Nordgren A, Malmer A (2003) Changes in soil chemical and microbial properties after a wildfire in a tropical rainforest in Sabah, Malaysia. Soil Biol Biochem 35:1071–1078
Juo ASR, Manu A (1996) Chemical dynamics in slash-and-burn agriculture. Agric Ecosyst Environ 58:49–60
Kauffman JB, Sanford RL, Cummings DL, Salcedo IH, Sampaio EVSB (1993) Biomass and nutrient dynamics associated with slash fires in neotropical dry forests. Ecology 74:140–151
Ketterings QM, Noordwijk M, Bigham JM (2002) Soil phosphorus availability after slash-and-burn fires of different intensities in rubber agroforests in Sumatra, Indonesia. Agric Ecosyst Environ 92:37–48
Khanna PK, Raison RJ, Falkiner RA (1986) Exchange characteristics of some acid organic-rich forest soils. Aust J Soil Res 24:423–434
Kutiel P, Shaviv A (1989) Effect of a simulated forest fire on the availability of N and P in Mediterranean soils. Plant Soil 120:57–63
Kwari JD, Batey T (1991) Effect of heating on phosphate sorption and availability in some north east Nigerian soils. J Soil Sci 42:381–388
Liu W, Xu W, Han Y, Wang C, Wan S (2007) Responses of microbial biomass and respiration of soil to topography, burning, and nitrogen fertilization in a temperate steppe. Biol Fertil Soils 44:259–268
López SC, Barbaro NC (1988) Efecto de la irradiación y el autoclavado sobre el fósforo extractable intercambiable de los suelos. Cienc Suelo 6:159–161
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Neary DG, Klopateck CC, DeBano LF, Ffolliott PP (1999) Fire effects on belowground sustainability: a review and synthesis. For Ecol Manag 122:51–71
Olander LP, Vitousek PM (2004) Biological and geochemical sinks for phosphorus in soil from a wet tropical forest. Ecosystems 7:404–419
Otabbong E, Barbolina I (1998) Changes in solubility of various phosphorus fractions promoted by autoclaving soil and sewage sludge. Swed J Agric Res 28:129–135
Raison RJ, Khanna PK, Woods PV (1985) Mechanisms of element transfer to the atmosphere during vegetation fires. Can J Forest Res 15:132–140
Romanyà J, Khanna PK, Raison RJ (1994) Effects of slash burning on soil phosphorus fractions and sorption and desorption of phosphorus. For Ecol Manag 65:89–103
Russell-Smith J, Yates CP, Whitehead PJ, Smith R, Craig R, Allan GE, Thackway R, Frakes I, Cridland S, Meyer MCP, Gill AM (2007) Bushfires ‘down under’: patterns and implications of contemporary Australian landscape burning. Int J Wildland Fire 16:361–377
Saa A, Trasar-Cepeda C, Gil-Sotres F, Carballas T (1993) Changes in soil phosphorus and acid phosphatase activity immediately following forest fires. Soil Biol Biochem 25:1223–1230
Sato S, Comerford NB (2006) Assessing methods for developing phosphorus desorption isotherms from soils using anion exchange membranes. Plant Soil 279:107–117
Seeling B, Zasoski RJ (1993) Microbial effects in maintaining organic and inorganic solution phosphorus concentrations in a grassland topsoils. Plant Soil 148:277–284
Serrasolsas I, Khanna PK (1995) Changes in heated and autoclaved forest soils of S.E. Australia. II. Phosphorus and phosphatase activity. Biogeochemistry 29:25–41
Smethurst PJ (2000) Soil solution and other soil analyses as indicators of nutrient supply: a review. For Ecol Manag 138:397–411
Stace HCT, Hubble GD, Brewer R, Northcote KM, Slemman RJ, Mulcahy MJ, Hallswoth EJ (1968) A handbook of Australian soils. Rellim Technical, Glenside, South Australia
Stewart HTL, Hopmans P, Flinn DW, Croatto G (1990) Harvesting effects on phosphorus availability in a mixed eucalypts ecosystem of south-eastern Australia. For Ecol Manag 36:149–162
Styles D, Coxon C (2006) Laboratory drying of organic-matter rich soils: phosphorus solubility effects, influence of soil characteristics, and consequences for environmental interpretation. Geoderma 136:120–135
Turner BL, Haygarth PM (2001) Phosphorus solubilisation in rewetted soils. Nature 411:258
Walker J, Raison RJ, Khanna PK (1986) Fire. In: Russell JS, Isbell JS (eds) Australian soils: the human impact. University of Queensland Press, St. Lucia, Australia, pp 185–216
WRB (2006) World Reference Base for soil resources. World Soil Resources Reports, 103. FAO, Rome
Xie RJ, MacKenzie AF (1991) Effects of autoclaving on surface-properties and sorption of phosphate and zinc in phosphate-treated soils. Soil Sci 152:250–258
Acknowledgements
This study was conducted at C.S.I.R.O. Division of Forestry, Canberra, Australia. We would like to thank Mr. K.L. Jacobsen, Mr. S.J. Smith, Ms. J.S. Sweeney, Dr. AKMA Hossain and Mr. J.S. Holtzapffel for their help during field and laboratory work and to the Editor-in-Chief and anonymous reviewers for constructive comments on the manuscript. CEAM is supported by the Generalitat Valenciana and Bancaixa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serrasolses, I., Romanyà, J. & Khanna, P.K. Effects of heating and autoclaving on sorption and desorption of phosphorus in some forest soils. Biol Fertil Soils 44, 1063–1072 (2008). https://doi.org/10.1007/s00374-008-0301-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-008-0301-7