Abstract
A silty loam soil was incubated with the leaves and stems of two transgenic Bacillus thuringiensis (Bt) cotton varieties and nontransgenic Bt cotton to study the soil persistence of the Bt toxin from the decomposing transgenic Bt cotton tissues and its effect on soil enzyme activities. The results showed that after Bt cotton tissue amendment, Bt toxin was introduced into soil upon decomposition; about 50% of the introduced Bt toxin persisted in soil for at least 56 days. No Bt toxin was detected in the nontransgenic Bt cotton-amended soil; the amount of Bt toxin was the highest in the soil treated with the residue with the higher Bt toxin content. Activities of soil urease, acid phosphomonoesterase, invertase, and cellulase were stimulated by the addition of Bt cotton tissues, whereas activity of soil arylsulfatase was inhibited. Probably cotton tissue stimulated microbial activity in soil, and as a consequence, enzyme activities of soil were generally increased. This effect can mask any negative effect of the Bt toxin on microbial activity and thus on enzyme activities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of genetically modified or transgenic plants has great potential for future agriculture, but asks for a well-defined risk assessment (Bruinsma et al. 2003). The Bacillus thuringiensis (Bt) gene-expressing product of transgenic Bt crops, B. thuringiensis insecticidal crystal protein (Bt toxin), could be introduced into soil through root exudation or through decomposition of the specific crop residues (Palm et al. 1996; Sims and Holden 1996; Saxena et al. 1999; Saxena and Stotzky 2000). Once in the soil, the toxin could be adsorbed or bound on clay particles, humic components, or organic-mineral complexes and then be protected against degradation by soil microorganisms; in this way it could accumulate to a certain concentration that might affect the composition and activity of soil microbial communities (Tapp and Stotzky 1995; Crecchio and Stotzky 1998; Tapp and Stotzky 1998; Stotzky 2000; Crecchio and Stotzky 2001).
The treatment of soil with Bt maize tissues showed that the bioactivity of the Bt toxin decreased quickly after tissue introduction into soil (Sims and Holden 1996), and then the decreasing rate lowered and even became zero when the Bt concentration reached a low value after several weeks (Palm et al. 1996). The free or bound Bt toxin had no effect on soil bacteria, fungi, algae, and protozoa and nematodes (Donegan et al. 1996; Saxena and Stotzky 2001).
Soil urease, acid phosphatase, arylsulfatase, invertase, and cellulase activities play an important role in soil microbial activity, being related to some important N, P, S, and C reactions, respectively (Nannipieri et al. 1990; Deng and Tabatabai 1997; Kandeler et al. 1999; Nannipieri et al. 2002). Jepson et al. (1994) pointed out that soil urease, dehydrogenase, and phosphatase activities could be used as indicators of the impact of toxins on soil microbiological activity. Wu et al. (2004) found that the phosphatase activity of paddy soils was not affected by the incorporation of Bt-transgenic rice straw into soil, whereas the dehydrogenase activity was increased. However, little is known about the effects of Bt toxin of transgenic Bt cotton tissues on soil enzyme activities. Therefore, we have studied the effect of Bt toxin from the decomposing transgenic Bt cotton tissues on soil urease, acid phosphomonoesterase, arylsulfatase, invertase, and cellulase activities.
Materials and methods
Materials and treatments
We used leaves and stems of transgenic Bt cottons Guo-Kang 12 (Bt-GK) and Zhong-Kang 30 (Bt-ZK), which were given by the Chinese Academy of Sciences and the Chinese Academy of Agricultural Sciences, respectively; non-Bt cotton Zhong-Mian 30 (Non Bt-ZM) was used as the control. The tissues were air-dried, ground, and sieved (<2-mm screen). The average Bt toxin contents in ZK and GK tissues were 208.14 and 169.35 ng g−1, respectively.
The silty loam soil, sampled from the Shenyang Agricultural University of China, had pH 5.72 (1:2.5 water), organic C 14.62 g kg−1, total N 1.22 g kg−1, total P 0.49 g kg−1, total K 20.12 g kg−1, alkali-hydrolyzable N (1 N NaOH, 40°C, 24 h) 106.37 mg kg−1, available P (0.5 N NaHCO3, pH 8.5) 15.92 mg kg−1, and available K (1 N CH3COONH4) 106.37 mg kg−1. All chemical parameters of soil were determined as described by Lu (2000).
The tissues were moistened with distilled water, mixed with soil in a proportion of 1:3 (W/W), and incubated in 10-ml ventilated tubes at 26°C and at a soil moisture content of 20%. Twenty-one replicates were carried out for each treatment, and three tubes of each treatment were sampled after 1, 3, 7, 14, 21, 28, and 56 days, respectively.
Determination of Bt toxin concentration and enzyme activities
Buffer solution (EnviroLogix, Portland, ME, USA) (1 ml) was added to 0.5 g soil sample, then the soil mixture was mixed and centrifuged (13,000×g) at 4°C for 10 min; the Bt toxin concentration in the supernatant was determined by ELISA, using the EnviroLogix Cry1Ab/Cry1Ac Plate Kit (detection limit<0.25 parts per million) (Saxena et al. 1999).
Urease (EC 3.5.1.5), acid phosphomonoesterase (EC 3.1.3.2), and arylsulfatase (EC 3.1.6.1) activities were determined as described by Tabatabai (1994). Briefly, urease activity was determined using urea as substrate, and the soil mixture was incubated at 37°C for 5 h; the residual urea was determined by a colorimetric method. Acid phosphomonoesterase activity was determined with p-nitrophenyl phosphate as substrate, with incubation at pH 6.5 (modified universal buffer) and 37°C. After 1 h, 0.5 M CaCl2 and 0.5 M NaOH were added to precipitate humic molecules responsible for brown coloration and extract p-nitrophenol, respectively. Arylsulfatase activity was determined with p-nitrophenyl sulfate as substrate, with incubation at pH 5.8 (acetate buffer 0.5 M) and 37°C. The enzyme reaction was stopped as reported above.
Both invertase (EC 3.2.1.26) and cellulase (EC 3.2.1.4) activities were determined as described by Schinner et al. (1996). Briefly, invertase activity was determined using sucrose as a substrate and incubation at 50°C for 3 h, before measuring the produced glucose with a colorimetric method. Cellulase activity was determined with carboxymethyl cellulose sodium as a substrate with incubation at 50°C for 24 h, and measuring the produced glucose with a colorimetric method.
Statistical analysis
Comparisons among means were made using the least significant difference test, calculated at P < 0.05. Statistical procedures were carried out with software package SPSS 10.0 for windows (SPSS 2000).
Results and discussion
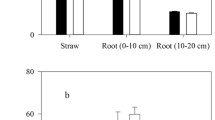
After amendment with cotton tissues, there was no Bt toxin detected in the non-Bt-ZM treatment. The soils treatment with Bt cotton tissues had a significantly higher content of Bt toxin, which rapidly decreased in the first 7 days of incubation, but then the relative decrease rate lowered and the Bt content remained almost unchanged after 28 days (Fig. 1). By the end of incubation (the 56th day), the Bt toxin content was still high (14.19–22.69 ng g−1 soil), representing 40.79% (Bt-ZK) and 59.98% (Bt-GK) of its initial introduced amounts. Throughout the whole period of incubation, the Bt toxin content was higher in Bt-ZK than in Bt-GK treatment (Fig. 1). Our study confirms that by Sims and Holden (1996), who showed that Bt toxin from amended Bt maize biomass decreased quickly after its introduction into soil.
The transgenic Bt cotton tissues and their degradation product had different effects on the tested enzyme activities of soil, with a positive effect on urease, acid phosphomonoesterase, invertase, and cellulase activities, and a negative effect on arylsulfatase activity (Fig. 1). Soil microorganisms are one of the main sources of soil enzymes (Nannipieri et al. 1983). The amendment with Bt-cotton materials supplied a higher content of organic product than the treatment with non-Bt-cotton materials, and this difference was probably responsible for the increase in urease, acid phosphomonoesterase, invertase, and cellulase activities of soil.
Acid phosphomonoesterase activity was significantly stimulated by Bt cotton tissue amendment, probably because these enzymes originate from bacteria, fungi, yeasts, and plant (Nannipieri et al. 1983; Nakas et al. 1987). Wu et al. (2004) showed that there were no significant differences in the soil neutral phosphatase activity between non-Bt-straw and Bt-straw amended soils. The difference between our results and those by Wu et al. (2004) might depend on the fact that the amount of Bt cotton tissues (1: 3 w/w) added to our soil was 10-fold higher than that (3% w/w) of Bt rice straw added to soil by Wu et al. (2004).
The inhibition of arylsulfatase activity by Bt toxin may be partly attributed to the increase in the soil phosphate due to the stimulation of soil acid phosphomonoesterase activity. Lou and Warman (1992) showed that phosphate inhibited the enzymatic hydrolysis of ester sulfate.
According to Falih and Wainwright (1996), soil urease activity was stimulated by plant material amendment probably because of the increase in microbial activity. The same probably occurred in this work where soil urease activity was stimulated by the Bt cotton tissue amendment.
The addition of plant straw to soil strongly increased invertase and cellulase activities (Stemmer et al. 1999; Glissmann and Conrad 2002), probably because this addition increase microbial activity. The same explanation can be suggested to explain the increase in invertase and cellulase activities observed here by the addition of Bt toxin in soil. Therefore, the stimulation of some soil enzyme activities after amendment with Bt cotton tissues depended on the stimulation by the addition of cotton tissue on microbial activity of soil. This effect can mask any negative direct effect of the Bt toxin on enzyme activities.
References
Bruinsma M, Kowalchuk GA, van Veen JA (2003) Effects of genetically modified plants on microbial communities and processes in soil. Biol Fertil Soils 37:329–337
Crecchio C, Stotzky G (1998) Insecticidal activity and biodegradation of the toxin from Bacillus thuringiensis subsp. kurstaki bound to humic acids from soil. Soil Biol Biochem 30:463–470
Crecchio C, Stotzky G (2001) Biodegradation and insecticidal activity of the toxin from Bacillus thuringiensis subsp. kurstaki bound to complexes of montmorillonite-humic acids-Al hydroxypolymers. Soil Biol Biochem 33:573–581
Deng SP, Tabatabai MA (1997) Effect of tillage and residue management on enzyme activities in soils. ш phosphatases and arylsulfatase. Biol Fertil Soils 24:141–146
Donegan KK, Schaller DL, Stone JK, Ganio LM, Reed G, Hamm PB, Seidler RJ (1996) Microbial populations, fungal species diversity and plant pathogen levels in field plots of potato plants expressing the Bacillus thuringiensis var. tenbrionls endotoxin. Transgenic Res 5:25–35
Falih AMK, Wainwright M (1996) Microbial and enzyme activity in soils amended with a natural source of easily available carbon. Biol Fertil Soils 21:177–183
Glissmann K, Conrad R (2002) Saccharolytic activity and its role as a limiting step in methane formation during the anaerobic degradation of rice straw in rice paddy soil. Biol Fertil Soils 35:62–67
Jepson PC, Crof BA, Pratt GE (1994) Test systems to determine the ecological risks posed by toxin release for Bacillus thuringiensis genes in crop plants. Mol Ecol 3:81–89
Kandeler E, Luxhoi J, Magid J (1999) Xylanase, invertase and protease at the soil-litter interface of a loamy sand. Soil Biol Biochem 31:1171–1179
Lou G, Warman PR (1992) Enzymatic hydrolysis of ester sulphate in soil organic matter extracts. Biol Fertil Soils 14:112–115
Lu RK (ed) (2000) Methods of soil and agro-chemistry analysis. Chinese Agricultural Science and Technology Press, Beijing (in Chinese)
Nakas JP, Gould WD, Klein DA (1987) Origin and expression of phosphatase activity in a semi-arid grassland soil. Soil Biol Biochem 19:13–18
Nannipieri P, Muccini L, Ciardi C (1983) Microbial biomass and enzyme activities: production and persistence. Soil Biol Biochem 15:679–685
Nannipieri P, Grego S, Ceccanti B (1990) Ecological significance of the biological activity in soil. In: Bollag JM, Stotzky G (eds) Soil biochemistry, vol 6. Marcel Dekker, New York, pp 293–355
Nannipieri P, Kandler E, Ruggiero P (2002) Enzyme activities and microbiological and biochemical processes in soil. In: Burns RM, Dick RP (eds) Enzymes in the environment: activity, ecology and applications. Marcel Dekker, New York, pp 1–33
Palm CJ, Schaller DL, Donegan KK, Seidler RJ (1996) Persistence in soil of transgenic plant produced Bacillus thuringiensis var. kurstaki δ-endotoxin. Can J Microbiol 42:1258–1262
Saxena D, Stotzky G (2000) Insecticidal toxin from Bacillus thuringiensis is released from roots of transgenic Bt corn in vitro and in situ. FEMS Microbiol Ecol 33:35–39
Saxena D, Stotzky G (2001) Bacillus thuringiensis toxin released from root exudates and biomass of Bt corn has no apparent effect on earthworms, nematodes, protozoa, bacteria, and fungi in soil. Soil Biol Biochem 33:1225–1230
Saxena D, Flores S, Stotzky G (1999) Insecticidal toxin in root exudates from Bt corn. Nature 402:480
Schinner F, Ohlinger R, Kandeler E, Margesin R (eds) (1996) Methods in soil biology. Springer, Berlin Heidelberg New York
Sims SR, Holden LR (1996) Insect bioassay for determining soil degradation of Bacillus thuringiensis var. kurstaki CryIA (b) protein in corn tissues. Environ Entomol 25:659–664
SPSS (2000) SPSS 10.0 for windows. SPSS, Chicago, IL
Stemmer M, Gerzabek MH, Kandeler E (1999) Invertase and xylanase activity of bulk soil and particle-size fraction during maize straw decomposition. Soil Biol Biochem 31:9–18
Stotzky G (2000) Persistence and biological activity in soil of insecticidal proteins from Bacillus thuringiensis and of bacterial DNA bound on clays and humic acids. J Environ Qual 29:691
Tabatabai MA (1994) Soil enzymes. In: Weaver RW, Angle JR, Bottomley PS (eds) Methods of soil analysis: microbiological and biochemical properties. Part 2. SSSA book series 5. Soil Science Society of America, Madison, WI, pp 775–833
Tapp H, Stotzky G (1995) Insecticidal activity of the toxins from Bacillus thuringiensis subspecies kurstaki and tenebrionis adsorbed and bound on pure and soil clays. Appl Environ Microbiol 61:1786–1790
Tapp H, Stotzky G (1998) Persistence of the insecticidal toxin from Bacillus thuringiensis subsp. kurstaki in soil. Soil Biol Biochem 30:471–476
Wu WX, Ye QF, Min H, Duan XJ, Jin WM (2004) Bt-trangenic rice straw affects the culturable microbiota and dehydrogenase and phosphatase activities in a flooded paddy soil. Soil Biol Biochem 36:289–295
Acknowledgements
This research was funded by the Knowledge Innovation Project, CAS (KZCX3-SW-445) and National Natural Sciences Foundation of China (no. 40101016); this work was also financially supported in part by the Global Environment Research Fund of Ministry of the Environment, Japan. The authors thank Prof. G. Stotzky in the Laboratory of Microbial Ecology, Department of Biology, New York University, for providing information about Bt toxin measurement, and the Editor-in-Chief of Biology and Fertility of Soils, Prof. Nannipieri, for his very important suggestions and detailed revision in the improvement of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, C.X., Chen, L.J., Wu, Z.J. et al. Soil persistence of Bacillus thuringiensis (Bt) toxin from transgenic Bt cotton tissues and its effect on soil enzyme activities. Biol Fertil Soils 43, 617–620 (2007). https://doi.org/10.1007/s00374-006-0158-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-006-0158-6