Abstract
Under semiarid subtropical field conditions, denitrification was measured from the arable soil layer of an irrigated wheat–maize cropping system fertilized with urea at 50 or 100 kg N ha−1 year−1 (U50 and U100, respectively), each applied in combination with 8 or 16 t ha−1 year−1 of farmyard manure (FYM) (F8 and F16, respectively). Denitrification was measured by acetylene inhibition/soil core incubation method, also taking into account the N2O entrapped in soil cores. Denitrification loss ranged from 3.7 to 5.7 kg N ha−1 during the growing season of wheat (150 days) and from 14.0 to 30.3 kg N ha−1 during the maize season (60 days). Most (up to 61%) of the loss occurred in a relatively short spell, after the presowing irrigation to maize, when the soil temperature was high and a considerable NO3−-N had accumulated during the preceding 4-month fallow; during this irrigation cycle, the lowest denitrification rate was observed in the treatment receiving highest N input (U100+F16), mainly because of the lowest soil respiration rate. Data on soil respiration and denitrification potential revealed that by increasing the mineral N application rate, the organic matter decomposition was accelerated during the wheat-growing season, leaving a lower amount of available C during the following maize season. Denitrification was affected by soil moisture and by soil temperature, the influence of which was either direct, or indirect by controlling the NO3− availability and aerobic soil respiration. Results indicated a substantial denitrification loss from the irrigated wheat–maize cropping system under semiarid subtropical conditions, signifying the need of appropriate fertilizer management practices to reduce this loss.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Quantitative estimates of denitrification loss from croplands may vary from 2 kg N ha−1 year−1 (under a rain-fed wheat system; Aulakh et al. 1983) to 233 kg N ha−1 year−1 (under a heavily fertilized irrigated vegetable production unit; Ryden and Lund 1980). Besides differences in the quantification techniques, which often lead to variable denitrification estimates (Ryden and Rolston 1983), the entrapment of gaseous N products in soil is well known to lead to an underestimation of denitrification (Ambus and Christensen 1993; Benckiser 1994; Mahmood et al. 1998a). In flood-irrigated systems, in which the soil structure is disrupted and the gas diffusion retarded (Terry et al. 1986), as much as 77% of the denitrification gaseous N products may remain entrapped within soil (Mahmood et al. 1999). In most denitrification studies, however, the entrapped N2O has not been considered, thus leading to an underestimation of N loss. This is particularly true for studies pertaining to irrigated systems.
Oxygen, NO3−, and organic C are the major factors controlling the denitrification process at cellular level, whereas soil moisture, organic amendments, fertilizer management practices, and edaphoclimatic conditions are important factors affecting the denitrification process by controlling the dynamics of O2, NO3−, and organic C. Although substantial data have been reported on denitrification loss from different agro-ecosystems, only few studies exist to ascertain the significance of denitrification loss from irrigated croplands under semiarid subtropical conditions. In Pakistan, where crop husbandry largely depends on flood irrigation, fertilizer-N recovery is often poor and may range from 58% (in cotton–wheat system; Mahmood et al. 2000) to 67% (in wheat–maize system; Mahmood et al. 1998a). Flood irrigation to wheat–maize system caused a high water-filled porosity only for a limited duration, thus causing a relatively low denitrification loss (up to 14 kg N ha−1 year−1; Mahmood et al. 1998b); although the loss was underestimated due to diffusional constraints (Mahmood et al. 1998a). However, under irrigated cotton fertilized with urea at 173 kg N ha−1, and also exposed to high summer temperatures and heavy monsoon downpours, the denitrification loss (including the N2O entrapped in soil) was as high as 65 kg N ha−1 (Mahmood et al. 2000). High summer temperatures during the cotton-growing period in this region support high soil respiration rates, which stimulate the O2 consumption thus producing high denitrification rates even at relatively low water-filled pore space (WFPS) (Mahmood et al. 2000). Therefore, due to the interplay of soil temperature and organic matter decomposition, the dynamics of denitrification in irrigated systems under semiarid subtropical conditions might be different than those under rain-fed systems in other agro-climatic zones.
Manures and crop residues are well known to stimulate the denitrification process by increasing organic C and NO3− availabilities to denitrifiers (van Cleemput et al. 1990; Aulakh et al. 1991). Organic matter decomposition rate, an important factor in controlling the dynamics of denitrification process under semiarid conditions, is strongly influenced by N availability (Conde et al. 2005). Therefore, we speculate that mineral N availability to microbes involved in the decomposition process may also play a role in determining the net effect of organic amendments on denitrification under semiarid conditions. Although literature available on denitrification in systems receiving mineral N fertilizers or manures is extensive, only limited information exists on cropping systems receiving the combined application of mineral N fertilizers and manures; Pakistani farmers possessing small land holdings often follow this practice. The present study reports the seasonal pattern of denitrification under an irrigated wheat–maize cropping system that has been receiving (for the past 20 years) 100–200 kg N ha−1 year−1 applied as different combinations of urea and farmyard manure (FYM).
Materials and methods
The soil at the experimental site (Nuclear Institute for Agriculture and Biology, Faisalabad) belongs to Hafizabad series (Haplic Yermosol; FAO 1966) and is a deep, well-drained sandy–clay loam developed in a mixed calcareous medium-textured alluvium derived from the Himalayas. The site has been under a long-term fertilizer trial with irrigated wheat–maize cropping system that has been receiving different urea and/or FYM treatments for the last 20 years. Twenty experimental plots (7.5×8.5 m) were established for five treatments in a randomized complete block design, each with four replicates. Treatments included an unfertilized control and urea applied at 50 or 100 kg N ha−1 year−1 (U50 and U100, respectively) each in combination with 8 or 16 t ha−1 year−1 of FYM (F8 and F16, respectively). The FYM was stabilized for about 6 months in a pit, and the entire stated dose was applied in late November at land preparation for wheat. One half of the stated dose of urea was applied to wheat and the other half to maize. For each crop, urea-N was again split into two; one half was applied at sowing and the other half with the second irrigation. The FYM contained 0.6% total N (NH4+ and NO 3−, each 149 mg N kg−1) and 0.6% P2O5. The amount of P2O5 in F8 treatments was balanced through the application of single superphosphate. The physicochemical characteristics of the plough layer under different fertilizer regimes are given in Table 1. Wheat (Triticum aestivum L. cv. Pak-81) was seeded on December 1, 2001 and harvested on April 30, 2002, whereas maize (Zea mays L. cv. Akbar) was sown on August 26, 2002 and the fodder harvest on October 27, 2002. In addition to the presowing irrigations (one to each crop), wheat and maize crops received five and four irrigations, respectively. Irrigation and fertilizer applications schedules are indicated in Figs. 1 and 2.
Environmental conditions and denitrification in the arable (0–15 cm) soil layer during the wheat season. Long arrows indicate irrigation (mm); small arrows, rainfall (mm); double-headed arrows, application of urea-N (12.5 kg N ha−1 to U50 and 25 kg N ha−1 to U100); triple-headed arrow, application of farmyard manure (8 t ha-1 to F8 and 16 t ha−1 to F16)
Sampling for the denitrification measurement started about 12 h after irrigation when the field was accessible, and continued until the soil dried to less than 50% WFPS (5–15 days under wheat; 4–7 days under maize); the soil was sampled 18 and 11 times during the growing season of wheat and maize, respectively. An acetylene inhibition/soil core incubation method was used for the direct measurement of denitrification (and soil respiration) including also the N2O (and CO2) entrapped in soil cores during incubation (Mahmood et al. 1999). From each replicate plot, four soil cores [3 cm (diameter) ×15 cm (depth), contained in perforated PVC sleeves] were randomly extracted, placed together in the incubation jar [8.5 cm (i.d.)×20 cm (height); nominal volume 800 ml], and sealed with a silicone rubber stopper provided with a septum port. The head-space of the jars was replaced by 5% acid-washed C2H2, and the jars incubated in holes made within the same field. After 24 h, the atmosphere in the jars was repeatedly mixed using a 50-ml syringe, and the gas sample removed for N2O and CO2 analyses. Nitrous oxide and CO2 entrapped in the soil cores were released by shaking (10 min) the disrupted soil cores with 200 ml of degassed distilled water in a sealed jar (Rice and Smith 1982). Nitrous oxide was analyzed on a Hitachi Model 263-30 gas chromatograph equipped with a 63Ni electron-capture detector; whereas CO2 analysis was carried out on a Gasukuro Kogyo Model 370 gas chromatograph using a thermal conductivity detector (TCD). Nitrous oxide dissolved in the solution phase was calculated by using Bunsen absorption coefficients (Moraghan and Buresh 1977). Data were also corrected for the ambient N2O and CO2 and those entrapped in soil cores before incubation. The total denitrification loss in a given period was calculated from the area of the polygon obtained by plotting denitrification rates against the dates on which these rates were observed.
The denitrification potential [an index of the C availability to denitrifiers, often referred in literature as denitrification capacity or denitrification potential-phase II (DNP); Smith and Tiedje 1979] was measured on soil samples collected at different growth stages of wheat and maize crops. Field-moist soil (10 g) in 100-ml serum vials was amended with 10 ml of KNO3 solution so as to obtain 200 mg NO3−-N kg−1. Vials were sealed, made anaerobic by evacuation and flushing with O2-free N2 (three times), and the head space was replaced by 5% acid-washed C2H2. The vials were incubated at 30°C on a rotary shaker, and after 6, 12, 18, and 24 h, the head space was analyzed for N2O using TCD. Data were corrected for N2O dissolved in the aqueous phase. DNP rates were calculated from the slope of accumulated N2O versus time.
During denitrification measurement, soil samples (0–15 cm) were also collected to determine the WFPS (Anonymous 1980) and the mineral N content (Keeney and Nelson 1982). The soil temperature was measured by glass thermometers inserted at a 5-cm depth. Analysis of variance and linear regression analysis were performed according to Gomez and Gomez (1984).
Results
Spatial variability
The spatial variability was highest for the denitrification rate (average %CV=67, range=6–167), followed by the soil NO3−-N (average %CV=46, range=8–165), and the soil respiration rate (average %CV=24, range=5–58); the soil WFPS was spatially uniform (average %CV=6, range=1–11). As such, soil WFPS was best fit with a normal distribution; whereas the data on denitrification rate, soil NO3−-N content, and soil respiration rate were better described by a log-normal distribution and log-transformed before statistical analyses to satisfy the assumption of variance homogeneity.
Environmental conditions and denitrification in the field
Figures 1 and 2 present temporal changes in the denitrification rate in relation to environmental conditions during the wheat and maize seasons, respectively. The soil temperature during the wheat and maize seasons ranged from 11.3 to 27°C and from 20.3 to 33.5°C, respectively. Following irrigations during the wheat season, the soil WFPS increased up to 60–85% depending on the depth of irrigation water (Fig. 1), and then declined to less than 60% WFPS within 4–11 days; the longer time period corresponding to the second irrigation cycle when the evapotranspiration was less as the crop was at an early stage and the temperature was also relatively low. During the maize season, the soil WFPS ranged from 59 to 79% after 12 h of irrigation, and declined to 31–49% in the following 4–7 days (Fig. 2). Under both crops, the soil WFPS always remained higher in unfertilized than in fertilized plots; the latter did not differ with respect to WFPS.
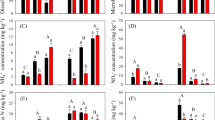
At the time of presowing irrigation before land preparation for wheat, the soil NO3−-N level was low (4–7 mg kg−1; Fig. 1). In December, following FYM and urea applications, the NO3−-N level rose to 24–46 mg kg−1 in the fertilized plots, with highest values recorded in the U100+F16 treatment; at the time of the highest NO3−-N level, the unfertilized plots showed only 10 mg N kg−1. By mid-February, when the crop was at boot stage, the soil NO3− in all treatments declined to a negligible level (<1 mg N kg−1). Due to mineralization/nitrification during the latter crop period, small peaks of NO3− (1–8 mg N kg−1) were observed without showing treatment effect. However, at crop maturity, NO3− concentration ranged from 7 to 24 mg N kg−1, with highest values in the U100+F16 treatment. During the fallow period between wheat harvest and sowing maize (May–mid August), NO3− accumulated in the fertilized (39–49 mg N kg−1) and in the unfertilized (25 mg N kg−1) plots (Fig. 2). After the presowing irrigation to maize, the NO3−-N level declined to 6–16 mg kg−1, most probably due to denitrification and leaching to the subsoil. After land preparation and urea application, the NO3− level in the fertilized plots again increased with a maximum value (44 mg N kg−1) in the U100+F16 treatment. Due to mineralization from the native soil-N pool, the unfertilized plots also showed an increase in the NO3− level (15 mg N kg−1). By mid-October, when crop growth was at a maximum, the soil NO3− level declined to negligible values. However, small peaks of NO3− (4–5 mg N kg−1) were observed near the maize crop maturity without showing treatment effects. Following each urea application, the collective peak of NH4+-N plus NO3−-N (data not shown) was higher than the current amount of fertilizer-N applied, indicating a substantial mineralization from the native soil N pool.
Averaged across treatments and sampling dates, the soil respiration rate during the maize season was higher than that recorded under wheat (14.3 versus 10.7 kg C ha−1 day−1). Under both crops, soil respiration showed a marked temporal trend, the rate being lower during crop sowing, increasing with crop growth and declining toward crop maturity. Furthermore, the rate was strongly influenced by soil moisture content, particularly during the wheat season. Averaged across treatments under wheat, the soil respiration rate during the events of high soil moisture content (i.e., >60% WFPS) was lower (9.1 kg C ha−1 day−1) compared to the rate (13.2 kg C ha−1 day−1) recorded during dry periods (<60% WFPS). During the maize season, however, the average rate under the two moisture regimes was similar. Soil respiration rate was negatively correlated with WFPS (P<0.001), whether utilizing the overall data for regression analysis or grouping the data into two soil moisture classes (rates at >60% WFPS and those at <60% WFPS). Under both crops, unfertilized plots respired at the lowest rates. Averaged across sampling dates during the wheat season, with each FYM level increasing, the urea application rate from 50 to 100 kg N ha−1 had no effect on the soil respiration rate, whereas with each urea level, increasing the FYM application rate from 8 to 16 t ha−1 slightly increased the rate (P<0.05). During the subsequent period of the year (maize season), the average soil respiration rate was highest in U50+F8 (P<0.05) that received the lowest N input among the fertilized treatments.
The DNP during the wheat season was 1.4 times (in unfertilized control) to 1.7–2.2 times (in fertilized treatments) higher than that recorded under maize (P<0.05, Table 2). As observed for soil respiration, DNP also showed marked temporal pattern, i.e., increasing with crop growth, which indicates C availability from the growing plants in the form of rhizodeposits. From stem elongation to flowering stage of wheat, the DNP was the highest in treatments receiving FYM at 16 t ha−1 year−1 (P<0.05). However, during wheat maturity, the values of these treatments were comparable to that of the unfertilized but lower (32%) than F8 treatments. Increasing the urea application rate decreased the DNP, particularly during the later stages of wheat growth (P<0.05). During the 4-month summer fallow between the wheat harvest and sowing maize, the DNP declined; it was, on average, 1.9 times lower at the stem elongation of maize compared to that observed 5 months earlier at the wheat dough stage. Under maize, the DNP increased with crop growth, with the highest values at the flowering stage, and declined (in the fertilized treatments only) at the grain-filling stage.
Of the total 145 observations, 41 showed denitrification rates exceeding 100 g N ha−1 day−1; the rate during the wheat and maize season ranged from 0 to1,181 g N ha−1 day−1 and from 0 to 4,741 g N ha−1 day−1, respectively (Figs. 1, 2). Under both crops, peaks were recorded 24 h after irrigation when the WFPS was in the range of 60–85%. The denitrification peaks during the first two irrigation cycles under wheat ranged from 166 to 1,181 g N ha−1 day−1, the highest being for the U100+F16 treatment and the lowest for the unfertilized; peaks during the later irrigation cycles were much lower (5–83 g N ha−1 day−1). Presowing irrigation to maize caused the highest denitrification rates during the study period, with peaks ranging from 1,585 to 4,741 g N ha−1 day−1. At this event, the unfertilized plots showed the lowest rate, whereas among the fertilized treatments, the U50+F16 treatment denitrified at the highest rate (4,741 g N ha−1 day−1) and U100+F16 at the lowest (1,868 g N ha−1 day−1). The rates during the next four irrigations to maize were also relatively high and ranged from 351 to 3,346 g N ha−1 day−1; the lowest rates were observed in the unfertilized control and the highest in the U100+F16 treatment (Fig. 2). Averaged across sampling dates during the moist period (>60% soil WFPS), denitrification rate was 5–18 times (under wheat) and 99–178 times (under maize) higher than the rate recorded during the dry period (<60% soil WFPS).
Factors controlling denitrification
The denitrification rate during the wheat-growing season (n=90) was positively correlated (P<0.001) with the soil WFPS (r=0.493) and the soil NO3−-N content (r=0.527), whereas its correlation with the soil respiration rate was negative (r=−0.598; P<0.001). Under maize (n=55), however, the rate was significantly correlated only with the WFPS (r=0.835; P<0.001). When the data of both cropping seasons were combined (n=145), the denitrification rate was significantly correlated with the WFPS (r=0.535; P<0.001), the soil NO3−-N content (r=0.487; P<0.001), the soil respiration rate (r=−0.201; P<0.05), and the soil temperature (r=0.241; P<0.05). The best multiple regression models obtained by the test of significance technique were:
-
For the wheat season: \(\log D = 0.042{\left( W \right)} + 0.809{\left( {\log N} \right)} - 1.909{\left( {R^{2} = 0.454;P < 0.01} \right)}.\)
-
For the maize season: \(\log D = 0.093{\left( W \right)} + 2.636{\left( {\log R} \right)} - 6.197{\left( {R^{2} = 0.768;P < 0.01} \right)}.\)
-
For both cropping seasons: \(\log D = 0.066{\left( W \right)} + 0.879{\left( {\log N} \right)} + 1.685{\left( {\log R} \right)} - 4.682{\left( {R^{2} = 0.509;P < 0.01} \right)}.\)
where D is the denitrification rate (g N ha−1 day−1), W is WFPS (%), N is soil NO3− content (mg N kg−1), and R is soil respiration rate (kg C ha−1 day−1).
Denitrification loss
The denitrification loss integrated over different irrigation cycles varied from 3.7 to 5.7 kg N ha−1 during the wheat season, and from 14.0 to 30.3 kg N ha−1 during the maize season (Table 3). Under both crops, the major loss occurred in relatively short spells. Under wheat, which received a total of six irrigations, the first two irrigation cycles accounted for most (89–94%) of the denitrification loss. Among the five irrigations applied during the maize season, the contribution of the presowing irrigation was the highest; it ranged from 34 to 75%, with the lowest figure corresponding to the U100+F16 treatment. The second and third cycles collectively represented 25–65% of the total denitrification loss under maize, with U100+F16 treatment showing the highest figure. By and large, the U100+F16 treatment showed the lowest denitrification loss among the fertilized treatments because of the lowest denitrification rate during the presowing irrigation to maize; this irrigation event had the highest contribution to the overall denitrification loss under the cropping system investigated. However, excluding the loss during this irrigation cycle, the overall denitrification loss under the wheat–maize system was higher under F16 treatments (14–15 kg N ha−1) than F8 treatments (8–11 kg N ha−1).
Discussion
The higher soil WFPS observed in the unfertilized (than in the fertilized) soil is attributable to the lower crop water requirement in the unfertilized plots, which produced the least biomass (yield data not shown). However, despite having the highest WFPS, the unfertilized plots denitrified at the lowest rates, indicating substrate limitations. Also, in the fertilized plots, denitrification rates were considerably reduced during the later stages of crop growth when the crop uptake had exhausted the soil NO3−-N content.
Pulses of denitrification following irrigation, and a strong correlation between denitrification rate and soil moisture, are well documented (Maag and Vinther 1999; Mahmood et al. 2000). However, other factors such as soil NO3−-N, soil temperature, and soil respiration rate also showed a strong influence on denitrification. The higher denitrification rates during the maize season might be the direct effect of soil temperature on denitrification; the two parameters were significantly correlated (r=0.533; P<0.001). However, the effect of soil temperature might also be indirect, i.e., through accelerating N mineralization and nitrification rates (Sierra 2002), and thus the NO3− availability to denitrifiers. Our hypothesis is supported by the highly significant correlation between the soil temperature and the soil NO3−-N content (r=0.426; P<0.001), and between the denitrification rate and the soil NO3−-N content (r=0.724; P<0.001). Averaged across sampling dates, the soil NO3− (7.4–19.3 mg N kg−1) and the denitrification rate (28–46 g N ha−1 day−1) during the maize season were higher than those during the wheat-growing season (soil NO3−, 3.8–8.7 mg N kg−1; denitrification rate, 6–15 g N ha−1 day−1), although the total fertilizer-N input was higher in wheat crop than in maize. Assuming that >60% soil WFPS provided sufficient anaerobic conditions for denitrification (Linn and Doran 1984), soil NO3−-N and soil temperature were the next important factors influencing denitrification in the system studied. Considering the data of high soil moisture events (i.e., >60% soil WFPS, producing the peak rates), denitrification showed a strong correlation with the soil NO3−-N content both under wheat (r=0.649; P<0.001) and under maize (r=0.655; P<0.001). Thus, in confirmation of earlier reports (Bronson et al. 1992; Estavillo et al. 1994), the present results suggest that the denitrification process may be limited under field conditions by the availability of soil NO3−.
Apparently, denitrification was negatively correlated with soil respiration; this might be an indirect effect resulting from the negative correlation of soil respiration with WFPS and the positive influence of WFPS on denitrification. On some occasions, however, we observed a positive effect of soil respiration on denitrification, most probably due to the interaction of soil temperature. For example, on December 22, 2002, when the soil temperature was 15°C, soil WFPS was 82%, and soil NO3− content was 46.2 mg N kg−1, the soil respiration and denitrification rates were 3.8 kg C ha−1 day−1 and 1,181 g N ha−1 day−1, respectively; whereas on August 15, with a similar soil NO3− level (46.5 mg N kg−1) but with a higher soil respiration rate (9.5 kg C ha−1 day−1) due to a higher soil temperature (33.5°C), the denitrification rate was higher (4,741 g N ha−1 day−1) even if the WFPS was lower (66%). Denitrification and soil respiration are known to be sensitive to soil temperature (Maag and Vinther 1999).
Although all FYM was applied before sowing wheat, the lower soil temperatures during the wheat-growing season reduced the overall decomposition of organic matter (lower soil respiration rates under wheat than under maize), conserved C to be available for denitrifiers, and thus, caused much higher DNP under wheat than under maize. However, under both crops, the DNP was consistently higher than the actual denitrification rates measured in the field, indicating that in the present study, the availability of electron donors to denitrifiers was not a limiting factor. The much higher denitrification loss during the maize season, in contrast to the higher DNP under wheat, also indicates that C availability did not directly control the denitrification process under field conditions. Presumably, the overall C availability in soil might have indirectly influenced the denitrification process by affecting the substrate availability for decomposers, and thus affecting the development of denitrification “hot spots” within otherwise aerobic soil. Following presowing irrigation to maize, the lower denitrification rate observed in the U100+F16 treatment (compared to other fertilized treatments) was obviously related to the lower soil respiration rate. However, the average soil respiration during the wheat-growing season (at WFPS <60%; 11 out of 18 sampling dates) was significantly higher in U100+F16 (12.3 kg C ha−1 day−1) than in other fertilizer treatments (11.0–11.3 kg C ha−1 day−1, P<0.05), whereas during the maize season, the rate was similar among fertilized treatments. This indicates that increasing the mineral N input accelerated the decomposition of C during the wheat-growing period, leaving relatively less substrate available during the subsequent summer fallow. This difference in C availability at the event of presowing irrigation to maize strongly influenced the overall pattern of denitrification loss in the present study.
Due to high summer temperatures and dry–wet cycles, an appreciable amount of N had mineralized and accumulated as NO3− during the fallow period between wheat harvest and sowing maize. However, most of this NO3− disappeared from the arable soil layer after the presowing irrigation to maize—which may be attributed to the fact that not only denitrification, but also NO3− leaching to the deeper soil layers, occurred (as shown by Mahmood et al. 1998a). However, microbial immobilization in the presence of readily oxidizable C (accumulated during the summer fallow) might also have contributed to the observed reduction in the soil NO3− level. Considerable NO3− immobilization by soil microbes may occur in the presence of easily oxidizable C compounds (Azam et al. 1988), or when exchangeable NH4+ is limited (Rice and Tiedje 1989). It is important to emphasize that NO3−-N accumulated during the summer fallow (May–August) might also be susceptible to denitrification following the heavy monsoon rainfalls. Therefore, under the agro-climatic conditions prevailing in this region, appropriate management practices such as growing cover crops (Isse et al. 1999) would be desirable to conserve N during the monsoon fallow.
Different combinations of factors governing denitrification during different seasons, as observed in the present study, have also been reported earlier for arable soils (Mahmood et al. 1998b) and for forest ecosystems (Vermes and Myrold 1992). Moreover, the amount of variation explained by the multiple regression models in the present study (45–77%) compares favorably with the range (25–70%) reported in other field studies (Vermes and Myrold, 1992; Estavillo et al. 1994; Mahmood et al. 1998b).
Considering the denitrification loss from the unfertilized control (14 kg N ha−1 year−1), N loss ranged from 3.4 to 11.2% of the applied fertilizer-N, the lowest figure obtained from the U100+F16 treatment, which received the highest fertilizer-N input and produced the maximum dry matter and N yields (yield data not shown). The present study was aimed at comparing denitrification loss from the arable soil layer under different fertilizer regimes, without taking into account the processes in the deeper soil layers. At the same site, the deeper soil layers could also significantly contribute to the overall denitrification loss (Mahmood et al. 1998a). Therefore, the figures of total denitrification loss under the cropping system investigated might be higher than those reported in this study. Results of the present study suggest that substantial denitrification loss may occur from irrigated croplands under semiarid subtropical conditions, indicating the need for appropriate management practices to minimize this loss.
References
Ambus P, Christensen S (1993) Denitrification variability and control in a riparian fen irrigated with agricultural drainage water. Soil Biol Biochem 25:915–923
Anonymous (1980) Standard methods of analysis for soil, plant tissue, water and fertilizer. Philippine Council for Agriculture and Resource Research, Los Baños, Laguna, Philippines
Aulakh MS, Rennie DA, Paul EA (1983) Field studies on gaseous nitrogen losses from soils under continuous wheat versus a wheat fallow rotation. Plant Soil 75:15–27
Aulakh MS, Doran WJ, Walters DT, Mosier AR, Francis DD (1991) Crop residue type and placement effects on denitrification and mineralization. Soil Sci Soc Am J 55:1020–1025
Azam F, Mahmood T, Malik KA (1988) Immobilization–remineralization of NO3−-N and total N balance during the decomposition of glucose, sucrose and cellulose in soil incubated at different moisture regimes. Plant Soil 107:159–163
Benckiser G (1994) Relationships between field-measured denitrification losses, CO2 formation and diffusional constraints. Soil Biol Biochem 26:891–899
Bronson F, Mosier AR, Bishnoi SR (1992) Nitrous oxide emissions in irrigated corn as affected by nitrification inhibitors. Soil Sci Soc Am J 56:161–165
Conde E, Cardenas M, Ponce-Mendoza A, Luna-Guido ML, Cruz-Mondragón C, Dendooven L (2005) The impacts of inorganic nitrogen application on mineralization of 14C-labelled maize and glucose, and on priming effect in saline alkaline soil. Soil Biol Biochem 37:681–691
Estavillo JM, Rodriguez M, Domingo M, Munoz-Rueda A, Gonzales-Murua C (1994) Denitrification losses from a natural grassland in the Basque Country under organic and inorganic fertilization. Plant Soil 162:19–22
FAO (1966) Guidelines for soil profile description. Soil Survey and Fertility Branch, Land and Water Development Division, Food and Agricultural Organization of the United Nations, Rome
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. Wiley, Singapore
Isse AA, MacKenzie AF, Stewart K, Cloutier DC, Smith DL (1999) Cover crops and nutrient retention for subsequent sweet corn production. Agron J 91:934–939
Keeney DR, Nelson DW (1982) Nitrogen—inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, Monogr 9, Vol. 2, 2nd edn. American Society of Agronomy, Madison, WI, pp 643–698
Linn DM, Doran JW (1984) Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils. Soil Sci Soc Am J 48:1267–1272
Maag M, Vinther FP (1999) Effect of temperature and water on gaseous emissions from soils treated with animal slurry. Soil Sci Soc Am J 63:858–865
Mahmood T, Malik KA, Shamsi SRA, Sajjad MI (1998a) Denitrification and total N losses from an irrigated sandy-clay loam under wheat–maize cropping system. Plant Soil 199:239–250
Mahmood T, Tahir GR, Malik KA, Shamsi SRA (1998b) Denitrification losses from an irrigated sandy-clay loam under a wheat–maize cropping system receiving different fertilizer treatments. Biol Fertil Soils 26:35–42
Mahmood T, Ali R, Azam F, Malik KA (1999) Comparison of two versions of acetylene inhibition/soil core method for measuring denitrification loss from an irrigated wheat field. Biol Fertil Soils 29:328–331
Mahmood T, Ali R, Sajjad MI, Chaudhri MB, Tahir GR, Azam F (2000) Denitrification and total fertilizer-N losses from an irrigated cotton field. Biol Fertil Soils 31:270–278
Moraghan JT, Buresh R (1977) Correction for dissolved N2O in nitrogen studies. Soil Sci Soc Am J 41:1201–1202
Rice CW, Smith MS (1982) Denitrification in no-till and plowed soils. Soil Sci Soc Am J 50:344–348
Rice CW, Tiedje JM (1989) Regulation of nitrate assimilation by ammonium in soils and in isolated soil microorganisms. Soil Biol Biochem 21:597–602
Ryden JC, Lund LJ (1980) Nature and extent of directly measured denitrification losses from some irrigated vegetable crop production units. Soil Sci Soc Am J 44:505–511
Ryden JC, Rolston DE (1983) The measurement of denitrification. In: Freney JR, Simpson JR (eds) Gaseous loss of nitrogen from plant–soil system. Nijhoff/Junk, The Hague, pp 91–132
Sierra J (2002) Nitrogen mineralization and nitrification in a tropical soil: effects of fluctuating temperature conditions. Soil Biol Biochem 34:1219–1226
Smith MS, Tiedje JM (1979) Phases of denitrification following oxygen depletion in soil. Soil Biol Biochem 11:261–267
Terry RE, Jellen EN, Breakwell DP (1986) Effect of irrigation method and acetylene exposure on field denitrification measurements. Soil Sci Soc Am J 50:115–120
van Cleemput O, Malkanti RM, d’Ydewalle Y, Baert L (1990) Denitrification influenced by incorporated harvest residues. Mitt Dtsch Bodenkdl Ges 60:177–182
Vermes JF, Myrold DD (1992) Denitrification in forest soils of Oregon. Can J For Res 22:504–512
Acknowledgements
The authors thank Faqir Hussain and Anwar-ul-Haq for maintaining the experimental plots, and Muhammad Asad Mahmood for valuable technical assistance. Equipment grant by the Alexander-von-Humboldt Foundation (Germany) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahmood, T., Ali, R., Malik, K.A. et al. Seasonal pattern of denitrification under an irrigated wheat-maize cropping system fertilized with urea and farmyard manure in different combinations. Biol Fertil Soils 42, 1–9 (2005). https://doi.org/10.1007/s00374-005-0869-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-005-0869-0