Abstract
In a series of laboratory experiments, we presented carnivorous Macrobiotus richtersi (Tardigrada, Macrobiotidae) with nematode prey to assess their importance as predator. We investigated consumption rate for (a) different prey densities (10–400 prey individuals), (b) different prey biomasses (22–80 ng), (c) different prey species (Pelodera teres, Rhabditidae, versus Acrobeloides nanus, Cephalobidae) and (d) different environments (2-D agar surface versus 3-D sand fractions of three different textures). M. richtersi consumed up to 4.6 μg nematode prey in 4 h, that is, 43% of the tardigrade’s body mass. Predation rate was positively correlated with prey density. The optimal prey in the present investigation was the biggest prey because it yielded the highest biomass uptake per time. In addition, the size of M. richtersi played an important role in consumption rate. Bacterivorous nematodes reacted differently to attack. Even in a water film on stiff agar where nematode agility was limited, a vigorous undulation reaction of P. teres led to a measurable reduction in consumption rate. A. nanus, in contrast, showed little response to attack. Microcosm experiments with sands of different particle size demonstrated that M. richtersi is able to chase and consume small bacterivorous nematodes in a 3-D soil matrix. However, consumption rate in sand microcosms was significantly reduced compared with pure agar. The sand matrix improved nematode agility and possibly provided small pores as refuge for the nematodes. The lowest consumption rate was observed in fine sand. Effects of predatory tardigrades on nematode numbers in the field are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With densities of over one million individuals per square meter, nematodes are the most numerous metazoan soil inhabitants (Parmelee 1995). Their activities affect microbial activity (Traunspurger et al. 1997), rates of litter decomposition (Sohlenius 1980; Anderson 1995), nutrient cycling (Coleman et al. 1984; de Ruiter et al. 1993), and plant growth (Philips et al. 2003). Many predators exploit the nematode pool. As regulatory forces (Wasilewska 2000), these predators may have a top-down influence on nematode species composition and population dynamics (Mikola and Setälä 1998a; Laakso and Setälä 1999) and thus indirectly affect nutrient cycling (Bouwman et al. 1996) and overall ecosystem performance (Koehler 1997). Terrestrial nematodes are fed upon by predatory nematodes (Yeates 1969; Small and Grootaert 1983; Walter et al. 1986; Small 1987; Bilgrami 1992, 1993; Khan et al. 1995; Yeates and Wardle 1996), earthworms (Hyvönen et al. 1994; Ilieva-Makulec and Makulec 2002), various arthropods (Murphy and Doncaster 1957; Martikainen and Huhta 1990; Hyvönen and Persson 1996; Huhta et al. 1998), and fungi (Bouwman et al. 1996). Even amoeboid and testate protozoa may attack nematodes (Doncaster and Hooper 1961; Yeates and Foissner 1995).
However, despite the suggested abundance of predators of nematodes and their considered importance in ecosystem processes, our knowledge on predator–nematode systems and predation rates is scarce (Traunspurger 2002). Often, accidental observations are all the evidence we have about a predator–prey interaction, and quantitative estimates of the predation intensity are rare (Moens et al. 1999, 2000; Schmid-Araya and Schmid 2000; Beier et al. 2004). This lack of information is particularly evident for the soil food web. Feeding habits of soil inhabitants are highly diverse and non-specific, resulting in remarkably complex food-web entities (Moore and de Ruiter 1997; Gange and Brown 2002). To construct a complete food web and to predict ecosystem processes, it is essential to learn both the types of interactions between food-web components (Bengtsson et al. 1995; Wardle 1995) and their strength (Scheu 2002).
In the present investigation, we focused on the tardigrade Macrobiotus richtersi as a predator of nematodes. The first detailed observations on a tardigrade (Macrobiotus sp. and Hypsibius microps) feeding on nematodes were recorded by Doncaster and Hooper (1961) and Sayre (1969). Later, tardigrade predation of nematodes was documented and described by Hallas and Yeates (1972). However, these studies only include observations that are not experimentally quantified. In the present study, we investigated simple predator–prey interactions in an artificial system where we could directly watch and measure feeding parameters and then tested the validity of our results under more natural conditions in microcosms. A series of laboratory experiments was used to (1) measure per capita feeding rate in relation to prey density (functional response), (2) investigate the impact of the prey species (behaviour) and the influence of the predator–prey size ratio on attack rate, handling time and consumption, and (3) test the effect of different soil textures on feeding rate.
Materials and methods
Predator and prey source
Both the tardigrade (M. richtersi) and the bacterivorous nematodes (Pelodera teres, Acrobeloides nanus) were recovered from young soils of the afforested coal-mining areas at Berzdorf (near Goerlitz, East Germany) in May 1999 (see Hohberg 2003) and cultured in the laboratory. Nematodes were cultured on Escherichia coli in LB agar (0.33%), whereas tardigrades were reared in a water film on pure agar (2%) and were fed on P. teres and A. nanus. For adult tardigrades (M2), females without mature oocytes and with a body length of 550–650 μm were chosen (Table 1). Juvenile tardigrades (M1) with a body length of 300–380 μm were chosen. P. teres (P) and A. nanus (A) were graded into size classes by letting them actively pass through nylon nets (Hydrobios, Kiel, Germany) with mesh sizes of 10 and 5 μm (Table 1). Smallest worms passing through the 5-μm net were graded into size class 1 (P1, A1); individuals passing through 10- but not through 5-μm meshes were assigned to size class 2 (P2, A2). The body lengths and widths of 100 individuals of each prey class were measured under an inverted microscope after killing them by warm formaldehyde. Mean tardigrade and nematode biomasses were calculated using the formula m=l(d/2)2(3.14)(1.04×10−6) (Hallas and Yeates 1972) for tardigrade biomass and m=l[d2/(1.6×106)] (Andrássy 1956) for nematode biomass, where l is body length, d is body width (both in micrometers), and m is individual fresh weight (in micrograms).
Experimental procedures
An arena of 0.79 cm2 was bordered by a piece of silicone hose (Ø, 1 cm; 0.5 cm long), which was lowered into a thin film of hot agar (2%) within a petri dish, forming a ring of silicone with agar at the bottom. Living nematodes were then counted accurately into the arena with a micropipette. Water was cautiously added or removed to achieve a water film approximately 1 mm thick. A single predator that had been starved for 3 days was added to each arena. The experiment started when the predator touched ground (agar) and began to walk or feed. At the end of the experiment, the predator was removed and the remaining nematodes were transferred to a petri dish and recounted. In recounting, we distinguished between uninjured, pierced, and partially sucked prey individuals. The last were treated as 0.5 of nematodes that had been totally consumed.
First experiments on experimental conditions
In order to determine optimal exposure time for functional-response experiments we presented 250 individuals of A1 to a starved predator M1 and continuously observed consumption during 4 h of exposure (N=3). In a second experiment we determined the optimal temperature (resulting in highest feeding rate) for feeding experiments: we presented 50 individuals of A1 to M1 at 10, 15, or 20°C and measured consumption after 4 h of exposure (N=4).
Functional-response experiments
We investigated the consumption rate of adult M. richtersi (M2) in dependence of prey class and density by offering four different prey classes, A1, A2, P1, and P2, and 11 different prey densities, 10, 25, 50, 75, 100, 150, 200, 250, 300, 350, or 400 individuals per arena (N=8 for each combination of prey class and prey density). The experiments were conducted in the dark at 20°C for 4 h. In order to examine the influence of predator age (size) on functional response, juvenile M. richtersi (M1) were also tested with P1 in densities of 10, 25, 50, 75, 100, 150, 200, 250, or 300 individuals (N=8 for each prey density).
Functional responses were fitted to Rogers (1972) random predator equation that considers prey exploitation during an experiment: Ne=N0[1−e−a(T−NeTh)], where Ne is the number of prey consumed, T is the total time prey was exposed to predation (240 min), N0 is the initial prey density, a is the attack coefficient, and Th is the handling time. The equation was transformed to Rogers’ linearisation, ln[(N0−Ne)/N0]=aThNe−aT, and solved for the parameters a and Th by means of linear regression (SPSS) for each prey class separately.
Furthermore, handling time Th was determined in direct observations for adult tardigrades feeding on prey A. nanus of various body lengths. Handling time Th was the time in seconds that the predator was occupied in attacking, overwhelming, and consuming one single prey individual.
Microcosm experiments
To investigate the influence of soil texture on consumption rate, experiments were carried out in microcosms of 0.79 cm2 without substrate (A) and with three different sand fractions: coarse sand (CS), medium sand (MS), and fine sand (FS). A layer (5 mm) of sterilised sand grains (autoclaved at 120°C for 20 min) in three different particle size classes (CS, 0.50–0.63 mm; MS, 0.25–0.32 mm; and FS, 0.10–0.16 mm) was added to three of four microcosms (N=6). We then added 200 individuals of P1 (22 ng) and water until a film of water (approximately 1 mm thick) remained at the sand surface. After 30 min, a single adult tardigrade (M2) was placed into the surface water film. Experiments were conducted at 20°C for 24 h. After 24 h, nematodes, sand, and tardigrade were transferred to a petri dish, the tardigrade was removed, and nematodes were killed by adding hot water (60°C). Dead nematodes were carefully washed off the sand and counted as described above (see functional-response experiments). To evaluate the number of prey individuals lost during washing, controls without predator (N=6) were conducted for each substrate type.
The mean predation rate, P200=1−(Rp/Rc), was calculated for each substrate type (A, CS, MS, FS), where Rp is the mean recovery with predator present and Rc is the mean recovery in controls without predator. To examine whether the predator was significantly feeding on nematode prey, a Mann–Whitney U test (SPSS) was performed for each texture. Finally, the influence of texture on consumption rate was tested with a two-way ANOVA (SPSS) after log transformation of the data (R). The two factors were predator (present or absent) and substrate type (FS, MS, or CS). The interaction term then tested whether the influence of the predator presence on recoveries is consistent over the different grain sizes, i.e. whether the consumption rate depends on soil texture.
Results
Direct observation of predator–prey interaction for 4 h revealed that even tardigrades of the same age, size, and history differed in feeding behaviour. Some individuals of M. richtersi started feeding instantly and consumed nematodes rapidly one after the other. Then they had a rest for a variable time. Others were much slower but continued feeding for some hours. Mean consumption increased from 38.2 nematodes after 1 h to 62.3 nematodes after 4 h (initial prey density, 250 A1). At the same time, the standard deviation decreased from 32.5 to 19.6 prey individuals. After 4 h, waste (fragments of dead nematodes and tardigrade faeces) began to accumulate on the agar surface and then stuck to tardigrade legs and claws, inhibiting locomotion. Thus, 4 h was an appropriate exposure time for functional-response experiments. Tests revealed that the temperature had significant influence on feeding rate: mean consumption (±SD) was 9.3±5.7 at 10°C, 14.8±4.9 at 15°C, and 23.5±6.0 at 20°C (initial prey density, 50 A1). Thus, we performed functional response and substrate experiments at 20°C where tardigrade activity and consumption rate were highest.
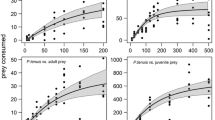
The number of prey consumed increased curvilinearly with prey density. The more prey individuals that were presented to the predator, the shorter was the searching time, until searching time was small compared with handling time and a plateau was reached (Fig. 1). Consumption rates at different prey densities are best described by Holling’s type II functional response (Holling 1959). Consumption rate Ne depended on individual prey size (Fig. 1). The highest one was measured for the smallest prey P1 (Fig. 1a): adult M. richtersi ingested up to 105 individuals of P1 in 4 h (Ne maximum consumption, Table 2). With increasing prey size, handling time increased and, consequently, the number of prey individuals consumed decreased. The maximum uptake of biomass by adult M. richtersi, on the other hand, increased with increasing prey size (Fig. 2). In terms of biomass uptake, the optimal prey in the present investigation was the largest nematode A2 (80 ng): up to 4.64 μg biomass, that is, 43% of its own body mass, was consumed by the predator within 4 h (Fig. 2, Table 2). Even though being much smaller (18% of the adult biomass), juvenile tardigrades consumed up to 56 prey individuals (P1) in 4 h, that is, 1.23 μg or 65% of their own mass (Table 2).
Functional responses of M. richtersi to the densities (N0) of different prey classes (N=8). Ne is the number of prey consumed within 4 h. a–d Adult predator M2 feeding on a P1 (22 ng), b A1 (28 ng), c P2 (63 ng), and d A2 (80 ng). e Juvenile predator M1 feeding on P1. Predicted curves were obtained by separately fitting the data of each prey class to Rogers’ (1972) equation, EquationSource% MathType!Translator!2!1!AMS LaTeX.tdl!TeX -- AMS-LaTeX!% MathType!MTEF!2!1!+-% feaaeaart1ev0aaatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLn% hiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr% 4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq-Jc9% vqaqpepm0xbba9pwe9Q8fs0-yqaqpepae9pg0FirpepeKkFr0xfr-x% fr-xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGaamOtamaaBa% aaleaacaqGLbaabeaakiaab2dacaWGobWaaSbaaSqaaiaabcdaaeqa% aOWaamWaaeaacaaIXaGaeyOeI0IaaeyzamaaCaaaleqabaGaeyOeI0% IaamyyamaabmaabaGaamivaiabgkHiTiaad6eadaWgaaadbaGaaeyz% aaqabaWccaaMc8UaamivamaaBaaameaacaqGObaabeaaaSGaayjkai% aawMcaaaaaaOGaay5waiaaw2faaaaa!49AB!]]</EquationSource> <EquationSource Format="TEX"><![CDATA[$$N_{{\text{e}}} {\text{ = }}N_{{\text{0}}} {\left[ {1 - {\text{e}}^{{ - a{\left( {T - N_{{\text{e}}} \,T_{{\text{h}}} } \right)}}} } \right]}$$, by means of linear regression
Apart from body-size relationships between predator and prey, the type of prey also affected consumption rate and biomass uptake. In contrast to A. nanus that showed little response to attack, P. teres responded with vigorous undulation. Several times we observed that a predator lost its prey in consequence of the active movements of P. teres. This observation was confirmed by actual consumption rates. Firstly, P. teres P2 was consumed at a rather lower rate than the bigger prey A. nanus (A2; Table 2). Secondly, high densities of P. teres (P1) seem to have confused the predator, leading to a decreased consumption rate (Fig. 1a).
The handling time Th (Rogers 1972) calculated from functional-response regression curves highly differs from that evaluated in direct observations for different prey sizes of A. nanus (Table 2). In direct observations, we measured only the time the predator was occupied in actually overwhelming prey and feeding. Thus, the difference between the observed and the calculated values is the time the predator is occupied in digestion.
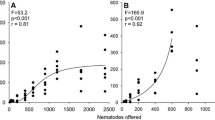
Sand did not hinder tardigrade activity, but allowed tardigrades to intrude into the pore system where they moved quickly between sand grains. We were able to observe their agility through an inverted light microscope when a tardigrade reached the bottom of the sand layer. In all four microcosm systems (no sand, coarse sand, medium sand, and fine sand), tardigrades were able to successfully capture nematodes (Fig. 3). Predation rates over 24 h of exposure, however, were highest on pure agar (P200=89.9%). Considering different sand treatments (coarse, medium, and fine sand), the effect of predation on the number of nematodes recounted significantly depended on grain size (ANOVA: two-way interactions Predator × Substrate, P=0.009, F=5.522). Calculated predation rate (P200) decreased with decreasing sand particle size (Fig. 3). In addition, the recovery rate (Fig. 3, white columns) varied among different sand sizes. It was highest when nematode and sand grain were of equal size (medium sand). Larger sand grains easily covered nematode bodies. Smaller sand grains were, due to their small mass, difficult to separate from nematodes in the washing treatment.
Microcosm experiments. The effect of the soil texture and of the predator M. richtersi on the recounts of prey individuals P1 after 24 h of experimental procedure (N=6, mean±1 SE, initial prey number of 200 individuals). P is the calculated predation rate and p is the probability with which the assumption “there was no significant consumption” is agreed (U test)
Discussion
Predatory tardigrades and nematodes compose the highest trophic level within water-filled soil pores (Wardle 1995). Several field studies (Hallas and Yeates 1972; Sohlenius 1979; Yeates and Wardle 1996) suggested that tardigrades may reduce nematode numbers. Field data, however, are very difficult to interpret because there are many factors influencing the numbers of soil organisms, e.g. climatic or nutritional changes. The present investigations for the first time quantified tardigrade–nematode feeding interactions, using simple functional-response experiments, where the actual feeding was directly observed and counted, as well as more naturally conditioned microcosm experiments. These experiments revealed that a tardigrade, M. richtersi, effectively reduced the number and biomass of nematodes. In our functional-response experiments, the adult tardigrade consumed up to 4.6 μg nematode biomass, corresponding to 43% of the tardigrade body mass, within 4 h.
Encounter rate and consequently consumption rate were positively related to prey density. Like the majority of non-filter feeding predators (Jeschke et al. 2004), the tardigrade’s consumption followed a curvilinear type II functional-response curve (Holling 1959). In addition to prey density, the body-size relationship between predator and prey highly influenced the feeding rate. Small prey led to high encounter rate, high attack rate, high capture success, short handling time, and consequently to a high consumption rate (Pastorok 1981; Manly and Jamieson 1999; Wahlström et al. 2000; Cogni et al. 2002).
McKee et al. (1997) and Beier et al. (2004) demonstrated for a triclad predator (Dugesia) preying on nematodes that there may be a maximum size of prey accessible to the predator where larger prey are not attacked. In the present investigation, all prey types presented were voluntarily consumed by the tardigrade. A limiting prey size was not experienced. The maximum feeding rate, however, decreased with increasing prey size. Still, the optimal prey of the present investigation was the biggest prey (A2) leading to the highest biomass uptake for the tardigrade.
Nematode species may react differently to predator attack as we observed in the functional-response experiments. Furthermore, in addition to temperature, prey density, and prey size, prey behaviour did influence the consumption rate. Even swimming in a water film on stiff agar, where nematode locomotion is limited, P. teres (Rhabditidae) responded vigorously to attack. A. nanus (Cephalobidae), on the other hand, showed little response. As a result, P. teres was consumed to a lower rate than expected due to its body size. Above all, at high densities, the active undulation of P. teres seemed to confuse the predator. Consequently, consumption rate of P. teres declined at high prey densities. Also, Grootaert et al. (1977) and Bilgrami (1992, 1993), studying predatory nematodes feeding on several bacterivorous nematode genera, reported of the very pronounced ‘escape response’ of rhabditid nematodes (e.g. Rhabditis and Pelodera spp.). They found that the escape response often provided resistance against predation. We suggest that for the tardigrade the vigorous escape behaviour of P. teres increased handling time rather than reducing success rate. This is confirmed by the fact that the behaviour’s greatest effect was at high prey densities; an influence on the success rate would be noticeable at low prey densities (Jeschke and Tollrian 2000). In our experiments on stiff agar, the prey’s escape seldom saved the prey from dying because individual prey often first detected the predator after being pierced. Doncaster and Hooper (1961) suggested that predatory tardigrades may inject a toxic secretion into the nematode. Recent scanning electron microscopy of the buccopharyngeal apparatus of Echiniscus sigismundi revealed that tardigrade stylets may be hollow, with openings both at the basal part and at the stylet tips. Thus, enzymes or toxins could indeed be injected through the piercing stylets into the food organism (Eibye-Jacobsen 2001). However, a pierced cuticle alone may immobilise and lead to subsequent death of the nematode, as piercing results in a loss of hydrostatic pressure, affects locomotion, and may permit invasion of pathogenic microorganisms (Bilgrami 1992).
Antipredation adaptations, such as vigorous escape movements, toxic or unfavourable secretions, or even a relatively thick, annulated cuticle are found in many bacterial-feeding nematode species (Small and Grootaert 1983). Their presence is presumably attributable to strong top-down effects of top predators, particularly nematodes and tardigrades (Wardle 1995).
Microcosm experiments revealed that M. richtersi is able to detect and consume small nematodes within a 3-D soil matrix. However, the sand matrix clearly reduced predation rates compared with those on stiff agar. We suggest two reasons for this reduction. Firstly, the 3-D soil system allows enhanced nematode agility and an improved ability to escape and defend. Nematode locomotion in soil is directly affected by the water content and the size of soil particles (Freckman 1988; Hunt et al. 2001). The average diameter of soil particles that allows for optimum movement is about one third of the worm’s length (Wallace 1958; Nicholas 1975), 70–100 μm in case of P. teres (P1), which is close to the particle size of fine sand (100–160 μm), where we recorded lowest predation rates. Secondly, soil pores differ in size and smaller pores may serve as places of refuge where an adult predatory tardigrade is not able to intrude. Beier et al. (2004) demonstrated in microcosm experiments with fine sands and gravel that sediment offers a physically complex refuge for nematode prey and may effectively increase predator (Dugesia gonocephala) search and handling time by reducing the opportunity of visual, mechanical, or chemical detection of prey organisms. Spatial heterogeneity (Wardle 1995; Keeling et al. 2000) and prey refuges may have a stabilising effect on predator–prey dynamics (Murdoch and Oaten 1975; Sih 1987; González-Olivares and Ramos-Jiliberto 2003).
The concept of habitable pore space can explain the distribution of predator and prey in soils (Yeates et al. 2002). If a considerably larger predator is present, a part of the prey population may live in soil pores inaccessible to their predators (Mikola and Setälä 1998b; Mikola and Sulkava 2001). The smallest M. richtersi that we observed successfully piercing and sucking on a living nematode was 220 μm long with a body diameter of just 50 μm. However, even this juvenile tardigrade is more than twice the diameter of the smallest nematode in the present investigation. Naturally, there will be soil pores too small even for this juvenile tardigrade to follow its nematode prey. However, Elliott et al. (1980) reported a situation in which a transfer agent (protozoa) made food (bacteria) better available to predators (nematodes) by entering soil pores inaccessible to nematodes, feeding on bacteria, and being later fed upon by nematodes.
From the results of our microcosm experiments where a realistic nematode biomass of about 560 mg m−2 was inoculated, some simple calculations can be made: In the fine sand microcosms, M. richtersi ingested about 19% of the 200 P. teres (P1) individuals in 24 h, i.e. 840 ng or about 8% of the tardigrade’s body mass. For a mine spoil in which the tardigrade (M. richtersi) biomass made up 50% of the nematode biomass (Hohberg in preparation) this would mean that 4% of the nematode biomass could be consumed by tardigrades (M. richtersi) each day. However, the field data did not reveal any correlation between tardigrade and nematode abundances or biomasses (Hohberg in preparation). There are only few correlations of tardigrade and nematode densities published. Small (1987) stated a negative correlation of tardigrades and nematodes (Acrobeloides, Alaimus, and Plectus spp.) in humus and suggested that these nematode species were preyed upon by tardigrades. Hallas and Yeates (1972) demonstrated a significant correlation between the numbers of the tardigrade M. harmsworthi and the total numbers of nematodes in a Danish beech forest where tardigrade predators had a great impact on nematodes and predatory nematodes played a minor role (Yeates and Wardle 1996).
The 800 to 900 tardigrade species known from freshwater, marine, and terrestrial habitats probably include very different feeding types (Nelson and Marley 2000; Nelson 2002; Nelson and McInnes 2002). Thus, it would be misleading to extrapolate the feeding rates of M. richtersi to overall numbers of tardigrades extracted, for example, from a soil sample. Tardigrades may pierce and suck the contents of living cells (fungi, algae, yeasts) and tissue (animals, plant roots) or swallow whole cells (bacteria, protozoa) or entire animals (nematodes, rotifers). Determination of feeding habit according to the mouth-tube armature of nematodes is not reliable (Yeates et al. 1993; Traunspurger 2002) and is even more limited in tardigrades, which all possess stylets. By this criterion alone, all tardigrades should be able to feed on nematodes. However, we never observed J1 juveniles of M. richtersi feeding on nematode prey. Probably, with their delicate stylet they cannot pierce the nematode cuticle. Instead, we observed J1 juveniles of M. richtersi feeding on detritus, probably consuming bacteria, which, incidentally, makes them competitors of bacterivorous nematodes, their later prey. The same dual-feeding behaviour was reported for ‘predatory’ mononchid nematodes (Yeates 1987). This omnivorous capacity guarantees survival (Diehl and Feißel 2000) and demonstrates that in the soil food web actual feeding depends on life stage and on the type of food available (Bouwman et al. 1996).
Conclusions
This investigation revealed that tardigrades may be very effective predators of bacterivorous nematodes. Feeding rate depends on temperature, prey density, the individual body mass of prey and predator, the behavioural response of prey to attack (which may differ considerably between nematode species), as well as on the structure of the environment. In microcosm experiments, we for the first time demonstrated and quantified that tardigrades actually reduced the numbers of small nematodes in a soil system by their feeding activity. We also found a significant positive correlation between the particle size and the consumption rate. Further investigations on the ecology and distribution of tardigrade species in soils are needed to reveal whether M. richtersi is exceptional or representative for soil tardigrades.
References
Anderson JM (1995) Soil organisms as engineers: microsite modulation of macroscale processes. In: Jones CG, Lawton JK (eds) Linking species and ecosystems. Chapman and Hall, New York, pp 94–106
Andrássy I (1956) Die Rauminhalts- und Gewichtsbestimmung der Fadenwürmer (Nematoden). Acta Zool 2:1–15
Beier S, Bolley M, Traunspurger W (2004) Predator–prey interactions between Dugesia gonocephala and free-living nematodes. Freshw Biol 49:77–86
Bengtsson J, Wei Zheng D, Agren GI, Persson T (1995) Food webs in soil: an interface between population and ecosystem ecology. In: Jones CG, Lawton JK (eds) Linking species and ecosystems. Chapman and Hall, New York, pp 159–165
Bilgrami AL (1992) Resistance and susceptibility of prey nematodes to predation and strike rate of the predators, Mononchus aquaticus, Dorylaimus stagnalis and Aquatides thornei. Fundam Appl Nematol 15:265–270
Bilgrami AL (1993) Analysis of the predation by Aporcelaimellus nivalis on prey nematodes from different prey trophic categories. Nematologica 39:356–365
Bouwman LA, Hoenderboom GHJ, Van der Maas KJ, De Ruiter PC (1996) Effects of nematophagous fungi on numbers and death rates of bacterivorous nematodes in arable soil. J Nematol 28:26–35
Cogni R, Freitas AVL, Amaral Filho BF (2002) Influence of prey size on predation success by Zelus longipes L. (Het., Reduviidae). J Appl Entomol 126:74–78
Coleman DC, Anderson RV, Cole CV, McClellan JF, Woods LE, Trofymow JA, Elliott ET (1984) Roles of protozoa and nematodes in nutrient cycling. In: Giddens SE, Todd RL (eds) Microbial–plant interactions. ASA, Madison, pp 17–28
De Ruiter PC, Van Veen JA, Moore C, Brussaard L, Hunt HW (1993) Calculation of nitrogen mineralization in soil food webs. Plant Soil 157:263–273
Diehl S, Feißel M (2000) Effects of enrichment on three-level food chains with omnivory. Am Nat 155:200–218
Doncaster CC, Hooper DJ (1961) Nematodes attacked by protozoa and tardigrades. Nematologica 6:333–335
Eibye-Jacobsen J (2001) A new method for making SEM preparations of the tardigrade buccopharyngeal apparatus. Zool Anz 240:309–319
Elliott ET, Anderson RV, Coleman DC, Cole CV (1980) Habitable pore space and microbial trophic interactions. Oikos 35:327–335
Freckman DW (1988) Bacterivorous nematodes and organic-matter decomposition. Agric Ecosyst Environ 24:195–217
Gange AC, Brown VK (2002) Soil food web components affect plant community structure during early succession. Ecol Res 17:217–227
González-Olivares E, Ramos-Jiliberto R (2003) Dynamic consequences of prey refuges in a simple model system: more prey, fewer predators and enhanced stability. Ecol Model 166:135–146
Grootaert P, Jaques A, Small RW (1977) Prey selection in Butlerius sp. (Rhabditida, Diplogasteridae). Meded Fac Landbouwwet Rijksuniv Gent 24:1559–1563
Hallas TE, Yeates GW (1972) Tardigrada of the soil and litter of a Danish beach forest. Pedobiologia 12:287–304
Hohberg K (2003) Soil nematode fauna of afforested mine sites: genera distribution, trophic structure and functional guilds. Appl Soil Ecol 22:113–126
Holling CS (1959) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320
Huhta V, Sulkava P, Viberg K (1998) Interactions between enchytraeid (Cognettia spagnetorum), microarthropod and nematode populations in forest soil at different moistures. Appl Soil Ecol 9:53–58
Hunt HW, Wall DH, DeCrapeo NM, Brenner JS (2001) A model for nematode locomotion in soil. Nematology 3:705–716
Hyvönen R, Persson T (1996) Effects of fungivorous and predatory arthropods on nematodes and tardigrades in microcosms with coniferous forest soil. Biol Fertil Soils 21:121–127
Hyvönen R, Andersson S, Clarholm M, Persson T (1994) Effects of lumbricids and enchytraeids on nematodes in limed and unlimed coniferous mor humus. Biol Fertil Soils 17:201–205
Ilieva-Makulec K, Makulec G (2002) Effect of the earthworm Lumbricus rubellus on the nematode community in a peat meadow soil. Eur J Soil Biol 38:59–62
Jeschke JM, Tollrian R (2000) Density-dependent effects of prey defences. Oecologia 123:391–396
Jeschke JM, Kopp M, Tollrian R (2004) Consumer-food systems: why type I functional responses are exclusive to filter feeders. Biol Rev 79:337–349
Keeling MJ, Wilson BH, Pacala SW (2000) Reinterpreting space, time lags, and functional responses in ecological models. Science 290:1758–1764
Khan Z, Bilgrami AL, Jairajpuri MS (1995) A comparative study on the predation by Allodorylaimus americanus and Discolaimus silvicolus (Nematoda: Dorylaimida) on different species of plant parasitic nematodes. Fundam Appl Nematol 18:99–108
Koehler HH (1997) Mesostigmata (Gamasina, Uropodina), efficient predators in agroecosystems. Agric Ecosyst Environ 62:105–117
Laakso J, Setälä H (1999) Population- and ecosystem-level effects of predation on microbial-feeding nematodes. Oecologia 120:279–286
Manly BFJ, Jamieson CD (1999) Functional response and parallel curve analysis. Oikos 85:523–528
Martikainen E, Huhta V (1990) Interactions between nematodes and predatory mites in raw humus soil: a microcosm experiment. Rev Ecol Biol Sol 27:13–20
McKee MH, Wrona FJ, Scrimgeour GJ, Culp JM (1997) Importance of consumptive and non-consumptive prey mortality in a coupled predator–prey system. Freshw Biol 38:193–201
Mikola J, Setälä H (1998a) No evidence of trophic cascades in an experimental microbial-based soil food web. Ecology 79:153–164
Mikola J, Setälä H (1998b) Productivity and trophic-level biomasses in a microbial-based soil food web. Oikos 82:158–168
Mikola J, Sulkava P (2001) Responses of microbial-feeding nematodes to organic matter distribution and predation in experimental soil habitat. Soil Biol Biochem 33:811–817
Moens T, Verbeeck L, Vines M (1999) Feeding biology of a predatory and a facultatively predatory nematode (Enoploides longispiculosus and Adoncholaimus fuscus). Mar Biol 134:585–593
Moens T, Herman P, Verbeeck L, Steyaert M, Vincx M (2000) Predation rates and prey selectivity in two predacious estuarine nematode species. Mar Ecol Prog Ser 205:185–193
Moore JC, De Ruiter PC (1997) Compartmentalisation of resource utilisation within soil ecosystems. In: Gange AC, Brown VK (eds) Multitrophic interactions in terrestrial systems. Blackwell Science, Oxford, pp 375–393
Murdoch WW, Oaten A (1975) Predation and population stability. Adv Ecol Res 9:1–131
Murphy IW, Doncaster CC (1957) A culture method for soil meiofauna and its application to the study of nematode predators. Nematologica 2:202–214
Nelson DR (2002) Current status of the Tardigrada: evolution and ecology. Integr Comp Biol 42:652–659
Nelson DR, Marley NJ (2000) The biology and ecology of lotic Tardigrada. Freshw Biol 44:93–108
Nelson DR, McInnes SJ (2002) Tardigrada. In: Rundle SD, Robertson AL, Schmid-Araya JM (eds) Freshwater meiofauna: biology and ecology. Backhuys Publishers, Leiden, Netherlands, pp 177–215
Nicholas WL (1975) The biology of free-living nematodes. Clarendon Press, Oxford
Parmelee RW (1995) Soil fauna: linking different levels of the ecological hierarchy. In: Jones CG, Lawton JK (eds) Linking species and ecosystems. Chapman and Hall, New York, pp 107–117
Pastorok RA (1981) Prey vulnerability and size selection by Chaoborus larvae. Ecology 62:1311–1324
Philips DA, Ferris H, Cook DR, Strong DR (2003) Molecular control points in rhizosphere food webs. Ecology 84:816–826
Rogers D (1972) Random search and insect population models. J Anim Ecol 41:369–383
Sayre RM (1969) A method of culturing a predacious tardigrade on the nematode Panagrellus redivivus. Trans Am Microsc Soc 88:266–274
Scheu S (2002) The soil food web: structure and perspectives. Eur J Soil Biol 38:11–20
Schmid-Araya JM, Schmid PE (2000) Trophic relationships: integrating meiofauna into a realistic benthic food web. Freshw Biol 44:149–163
Sih A (1987) Prey refuges and predator–prey stability. Theor Popul Biol 31:1–12
Small RW (1987) A review of the prey of predatory soil nematodes. Pedobiologia 30:179–206
Small RW, Grootaert P (1983) Observations on the predation abilities of some soil dwelling predatory nematodes. Nematol 29:109–118
Sohlenius B (1979) A carbon budget for nematodes, rotifers and tardigrades in a Swedish coniferous forest soil. Holarct Ecol 2:30–40
Sohlenius B (1980) Abundance, biomass and contribution to energy flow by soil nematodes in terrestrial ecosystems. Oikos 34:186–194
Traunspurger W (2002) Nematoda. In: Rundle SD, Robertson AL, Schmid-Araya JM (eds) Freshwater meiofauna: biology and ecology. Backhuys Publishers, Leiden, Netherlands, pp 63–104
Traunspurger W, Bergtold M, Goedkopp W (1997) The effects of nematodes on bacterial activity and abundance in a freshwater sediment. Oecologia 112:118–122
Wahlström E, Persson L, Diehl S, Byström P (2000) Size-dependent foraging efficiency, cannibalism and zooplankton community structure. Oecologia 123:138–148
Wallace HR (1958) Movement of eelworms. Ann Appl Biol 46:662–668
Walter DE, Hudgens RA, Freckman DW (1986) Consumption of nematodes by fungivorous mites, Tyrophagus spp (Acarina: Astigmata: Acaridae). Oecologia 70:357–361
Wardle DA (1995) Impacts of disturbance on detritus food webs in agro-ecosystems of contrasting tillage and weed management practices. Adv Ecol Res 26:105–185
Wasilewska L (2000) The effect of macroarthropods patrolling soil surface on soil nematodes: a field experiment in a mown meadow. Pol J Ecol 48:327–338
Yeates GW (1969) Predation by Mononchoides potohikus (Nematoda: Diplogateridae) in laboratory culture. Nematologica 15:1–9
Yeates GW (1987) Nematode feeding and activity: the importance of development stages. Biol Fertil Soils 3:143–146
Yeates GW, Foissner W (1995) Testate amoebae as predators of nematodes. Biol Fertil Soils 20:1–7
Yeates GW, Wardle DA (1996) Nematodes as predators and prey: relationships to biological control and soil processes. Pedobiologia 40:43–50
Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS (1993) Feeding habits in soil nematode families and genera—an outline for soil ecologists. J Nematol 25:315–331
Yeates GW, Dando JL, Shepherd TG (2002) Pressure plate studies to determine how moisture affects access of bacterial-feeding nematodes to food in soil. Eur J Soil Sci 53:355–365
Acknowledgements
We thank Hartmut Greven and Jonathan M. Jeschke who commented on earlier versions of this paper. The experiments comply with the current laws of Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hohberg, K., Traunspurger, W. Predator–prey interaction in soil food web: functional response, size-dependent foraging efficiency, and the influence of soil texture. Biol Fertil Soils 41, 419–427 (2005). https://doi.org/10.1007/s00374-005-0852-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-005-0852-9