Abstract
The succession of cyanobacteria was studied in a usar (alfisol, solonetz, alkaline) soil, located in a tropical region of upper Gangetic plain, following the first rainfall for a period of 10 months (i.e., July–April). A dozen cyanobacteria were identified to grow on the soil surface and their appearance was in the following order: Microcoleus sp., Calothrix brevissima, Scytonema sp., Cylindrosprmum licheniformae, Cylindrosprmum fertilissima, Nostoc calcicola, Nostoc punctiformae, Aphanothece parietina, Nostoc commune, Aulosira fertilissima, Phormidium sp., and Oscillatoria sp. Among these cyanobacteria, N. calcicola was the dominant species. N. calcicola was inoculated on the alkaline soil and incubated under ambient conditions in the light for 2 years in the laboratory. Changes in soil properties were more rapid after the incorporation of pyrite (FeS2). Recovery was monitored by using a filamentous heterocystous cyanobacterium N. calcicola and its bicarbonate-resistant (HCO3−R) mutant. The mutant strain showed better response to modification of soil pH following growth in soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Usar soils (Alfisol, solonetz, alkaline, sodic, saline) extensively distributed in the northern part of India (2.5 million ha) are characterized as alkaline and/or saline depending on the salt content. These soils are unproductive, impermeable, and hard due to the presence of undesirable salts on the surface. Solonetz soils are characterized by exchangeable Na+ (more than 15%), low quantities of Ca2+, and pH values that usually range between 8.5 and 10.5 (Dhar and Mukherji 1936; Singh 1950, 1961).
Physical and chemical methods such as addition of gypsum, sulfur, or excessive irrigation usually used in the reclamation of alkaline soils do not completely remove the soluble salts and exchangeable sodium in soil. Singh (1961) suggested that cyanobacteria could be used to reclaim alkaline soils because they form a thick stratum on the surface of the soil during the rainy season and the winter months. The algal material incorporated in the soil conserves organic C, organic N, and organic P as well as moisture, and converts Na+ clay to Ca2+ clay. Organic matter and N added by cyanobacteria bind the soil particles, and thus improve soil permeability and aeration (Singh 1961). They are capable of solubilizing microbial nutrients (Fritsch 1945) and dissolving insoluble carbonates nodules through the secretion of oxalic acid (Fritsch 1945; Singh 1961). Improvement of soil aggregation by lowering the pH and electrical conductivity and by increasing the hydraulic conductivity of saline and alkali soils by cyanobacteria was documented by Kaushik and Subhashini (1985) and Kaushik (1987). A significant role of cyanobacteria and associated moss community in the soil formation of Schirmacher Oasis, Antarctica, was also described (Pandey et al. 1992).
Although the occurrence of cyanobacteria in saline/alkaline soil has been described (Singh 1950, 1961; Ali and Sandhu 1972), the sequence of appearance of cyanobacteria in an alkaline (saline) (Whitton and Potts 2000) soil having monsoon type of character has not been studied. For this reason, we followed the sequence of appearance of cyanobacteria in a usar soil area and showed that some of them constitute dominant flora. Since the cyanobacterial growth markedly decreased the pH of the alkaline soils under natural condition (Singh 1961) and pyrite has been used to reclaim such soils (Verma and Abrol 1980), we conducted an experiment to study if the cyanobacteria and pyrite could show a cumulative effect in the reclamation of alkaline soils. The other scope of the work was to study the effect of the inoculation of dominant cyanobacterium Nostoc calcicola and its bicarbonate-resistant (HCO3−R) mutant on soil properties, and to compare it with treatment using FeS2 (pyrite).
Materials and methods
Site description
The investigation site is located approximately 4 km south of Banaras Hindu University campus (latitude 25°18′N, longitude 80°1′E, 76 m above sea level) and lies in the upper Gangetic plains. Annual rainfall was approximately 983.4 mm and the soil moisture content of alkaline soil varied between 3.2 and 24.7% throughout the year, with the maximum level recorded during the rainy season (July–September) and the minimum in June. The alkaline soil was not subjected to cultivation for the last 25 years or so and remained fallow during that period.
Collection of soil samples and determination of physicochemical characteristics
Soil cores (10 cm3) were collected during the driest period of the year, when no growth of cyanobacteria was evident on the soil surface. Sampling was replicated three times at least 50 m apart, and samples were placed in aluminum cans and transported to the laboratory for physicochemical analysis. After the first rain, collection of soil samples was made at 15-day intervals over a period of 3 months.
Texture, water holding capacity, and pH of the soil sample were determined as described by Piper (1966). Soil pH was measured in a soil/water suspension (1:5, w/v) with pH meter. Organic C and total N content (%) of soil were determined by Walkley and Black rapid titration method and micro-Kjeldahl techniques, respectively (Piper 1966). Exchangeable Ca2+ and Na+ (%) were determined after soil leaching as described by Jackson (1968). Total P was estimated by the ignition method (Jackson, 1968).
Collection of cyanobacterial samples and identification of strains
Cyanobacteria growing on alkaline soils and water were collected in sterile polythene bags and specimen tubes using clean implements. They were brought to the laboratory and examined microscopically. The strains were identified as reported by Desikachary (1959) and Rippka et al. (1979).
Cyanobacterial strains, isolation of mutant, culture conditions, and growth measurements
N. calcicola, a heterocystous cyanobacterium and the dominant constituent of alkaline soil, was enriched and grown in Allen–Arnon nutrient agar medium (Allen and Arnon 1955) in Petri dishes. Clones were isolated and an axenic population was grown following standard microbiological techniques.
The exponentially growing culture of N. calcicola was harvested via centrifugation (10,000×g;10 min) and washed with sterilized distilled water. Cells (1×105) were mutagenized with N-methyl-N′-nitro-N-nitrosoguanidine (MNNG; 100 μg ml−1) (Pandey and Singh 1984) in phosphate buffer (0.01 M; pH 8.0) for 1 h in light. The treated population was inoculated on bicarbonate (1 M) containing nutrient agar and incubated under growth conditions. Healthy clones were picked up in 5 ml nutrient broth. One of such clones tolerating 1 M HCO3− in nutrient broth was selected for further study.
The cultures were grown photoautotrophically at 25±1°C and 25 μE m−2 s−1 light intensity. Growth was measured by protein content (Herbert et al. 1971) or by population size (Alexander 1965; Roger and Kulasooriya 1980).
Monitoring cyanobacterial growth in alkaline soil under natural conditions
Alkaline soil patches (10 cm3) with thick growth of cyanobacteria were randomly sampled from three to four places and flooded in glass with double-distilled water and incubated for 2 years under natural conditions.
Alkaline soil (5 cm thick) was heated with distilled water in a 500-ml culture vessel (200 ml), sterilized at 1.05 kg cm−2 pressure for 15 min, and then cooled at room temperature for 24 h. Exponentially growing cyanobacterium N. calcicola was inoculated, flooded with sterilized double-distilled water at 7 days interval, and then incubated under natural conditions for 2 years.
Preparation of soil extract and its effect on the growth of N. calcicola
Dried and sieved alkaline soil (500 g) was treated with 500 ml deionized water in a flask (1 l). The flask was steamed in an autoclave for 30 min and cooled overnight; the procedure was repeated two to three times. The clear supernatant that appeared upon cooling was filtered through Whatman filter paper (No. 42), and soil extract thus obtained was sterilized in an autoclave at 1.05 kg cm−2 pressure for 15 min. This soil extract was supplemented in Allen–Arnon medium at different concentrations (% v/v) while keeping constant the pyrite level 2 mg ml−1. N. calcicola was inoculated at an inoculum density of 20 μg protein ml−1 and the cultures were incubated under growth conditions for 20 days.
Quantitative estimation of cyanobacteria
Soils were taken randomly from the Petri dish and the cyanobacterial mass was scrapped, suspended in the culture medium, and shaken on a gravitory shaker to separate the cyanobacterial growth into individual filaments. Quantitative estimations of the cyanobacteria were made by plating technique (Roger and Kulasooriya 1980).
Inoculation of cyanobacterial strains and pyrite
Powdered alkaline soil (40 g) was spread into Petri dish (diameter 95 cm; height 2 cm), to obtain a 1-cm soil layer and was mixed with pyrite (25–250 mg 100 g−1 soil). Two experiments were performed: in the first one, the Petri dish with soil and pyrite was sterilized, whereas in the second it was kept unsterilized. After flooding with sterilized distilled water for 15 days, the Petri dishes were inoculated with exponentially growing cyanobacterium and incubated at 30±2°C in an incubator for 24 months to observe their growth on alkaline soil. The soil in each Petri dish was flooded with sterilized distilled water at 7 days interval.
Statistical analysis
Cyanobacterial samples were collected from ten to 15 places to study succession, while soils and soil blocks were sampled in three to four replicates randomly. Each experiment was performed in triplicate and the mean values (with standard deviation) of the data are presented in tables and figures.
Analysis of variance (ANOVA) and t test were performed with the help of a computer and using Sigma stat, version 2.0.
Results
Succession of cyanobacteria in alkaline soils under natural conditions
During summer (April–June), alkaline soil in the field becomes dry and cyanobacterial flakes are easily blown away by the wind. Following the first rain shower (July), small patches of Microcoleus sp., along with Calothrix brevissima and Scytonema sp., appeared on the soil surface. Some low-lying areas of the alkaline soil became waterlogged due to heavy rains that lasted for 10–15 days. After about a month, Nostoc punctiforme grew as small patches on the soil and water surface. Nearly 2 months after the appearance of Microcoleus sp., colonization by Cylindrospermum licheniforme, C. fertilissima, Nostoc calcicala, N. punctiforme, and Aphanothece parietina was observed. Nevertheless, growth of Microcoleus and C. brevissima still persisted. Among them, N. calcicola appeared as a dominant N2 fixer, although Microcoleus remained throughout the rainy and winter seasons. After this stage, it was observed that the growth of Nostoc commune formed small flakes on the soil surface with plastic-type stiffness, and Aulosira fertilissima formed a thick mat on shallow water and moist soils. During the later part of cyanobacteria growth, some non-N2 fixers such as Phormidium and Oscillatoria were intermingled with the diazotrophic forms (Table 1).
The succession of cyanobacteria population on the same alkaline soil, but under controlled laboratory conditions, was similar to that observed in situ. However, under laboratory conditions, N. calcicola, N. punctiforme, N. commune, Phormidium, and Oscillatoria were the dominant flora. Indeed, even if Microcoleus appeared on the soil surface, the frequency of its occurrence was low.
Soil properties
The color of alkaline soil was dirty white to grayish, and was of clay-type soil texture. The pH of soil ranged from 10 to 10.5 and the water holding capacity was 45%. The soil with no apparent growth of cyanobacteria or algae contained 0.036% Na2CO3 and the exchangeable Ca2+ and Na+ were 0.35 and 0.78%, respectively. The soil was poor in organic C, N, and P contents (Table 2).
Effect of cyanobacterial growth on the physicochemical properties of alkaline soil
Since tropical climate is characterized by strong seasonal variations, the visible growth of cyanobacteria can be observed during the rainy season. The soil is apparently dry during rest of the year and cyanobacteria undergo perennation. The soil moisture seems to regulate the appearance and disappearance of cyanobacterial flora. Because N. calcicola was the dominant species, it was used in various experiments conducted for 2 years (Table 2). The experiments were performed in glass with soil under natural conditions with naturally occurring cyanobacteria inoculated with N. calcicola; it was observed that soil pH decreased from 10.5 to 8.8 and 8.5, respectively (Table 3). Increase in total N as well as organic C and P contents were also observed, whereas Na+ content declined following cyanobacterial growth either in mixed population or in monoculture.
Effect of alkaline soil extract on the growth of N. calcicola
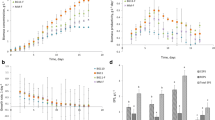
We focused on N. calcicola, isolated from the solonetz alkaline soil, to study the effect of alkaline soil extract on cyanobacterial growth in the presence and absence of pyrite. The preliminary experiment showed that pyrite in culture medium supported the growth of cyanobacterium up to 200 μg ml−1 as growth was nearly 20% higher in the presence than in the absence of FeS2. The increasing percentage of soil extract in the medium enhanced the growth measured as protein content up to 40%. Further increase in soil extract in the medium inhibited growth, and in the presence of 100% soil extract with or without pyrite, it accounted for nearly 60% of control (Fig. 1). Two-way ANOVA indicated that concentration of soil extract had a significant effect on growth (F10,21=28.53; p<0.001) and pyrite addition (F1,21=17.695; p<0.002).
Effect of alkaline soil extract on the growth of Nostoc calcicola in the absence (open bar) and presence (hedged bar) of pyrite (2 mg ml−1) in the medium when soil extract was added before the inoculation of cyanobacterium (10 μg protein ml−1). The cultures were incubated under photoautotrophic condition. Growth was monitored at the end of 20 days of incubation
Results revealed that N. calcicola could tolerate alkaline soil salt stress; therefore, this cyanobacterium was used in further experiments to remediate the alkaline soil.
Effect of pyrite and N. calcicola on alkaline soil
Pyrite increased the cyanobacterial population, expressed as number of filaments per square centimeter, to a level of 200 mg 100 g−1 sterilized soil and 175 mg 100 g−1 unsterilized soil (Fig. 2). Two-way ANOVA indicated that pyrite addition had a significant effect on population size (F10,21=15.95; p<0.001) and the effect of sterilization was also statistically significant (F1,21=28.54; p<0.001).
Role of N. calcicola and HCO3−R mutant in the reclamation of alkaline soil
The previous experiment conducted with N. calcicola clearly indicated that soil properties were modified by the growth of cyanobacteria on alkaline soil. To test whether a bicarbonate-resistant (HCO3−R) mutant of cyanobacterium also has the ability to grow and lower the pH of alkaline soil, we used N. calcicola (wild type) and its mutant (HCO3−R). Results of this experiment are presented in Table 4. Inoculation with N. calcicola decreased the pH value, and the decrease was more pronounced in sterilized soil. The decrease in pH of alkaline soil inoculated with HCO3−R mutant was higher than that with wild-type cyanobacterium. No growth of any cyanobacteria was observed in the uninoculated alkaline soil. The cyanobacterial strains amended with pyrite were more effective in lowering the pH of alkaline soil compared to cyanobacterium inoculated on soil without pyrite. Also, pyrite enhanced the population size of strains (Table 4).
Discussion
The reclamation of solonetz soils (alkaline soils), which are extensively distributed in India, is important so as to make these soils fertile. Depending on the degree of soil alkalinity, these soils can be reclaimed by water leaching and by adding chemical correctives such as gypsum (raw CaSO4) to replace Na+ with Ca2+ (Singh 1961).
Soil pH is an important factor which determines the biodiversity and dominance of cyanobacteria on a given soil surface. Fogg (1956) reported that, under natural conditions, most of the cyanobacteria grow in neutral to alkaline conditions and sometimes the growth of diazotrophic cyanobacteria in rice fields is limited by low pH (Whitton and Potts 2000). Alkaline soil with high pH values and Na+ content favor the growth of diazotrophic cyanobacteria with a consequent decrease in pH. Indeed, cyanobacteria can decrease soil pH from 9.2 to 7.5 (Singh 1961). The role of Na+ in cell uptake of different nutrients and in N2 fixation is well documented (Thomas and Apte 1984).
Pyrite used as a chemical corrective to reclaim sodic soil lacks complete oxidation and may cause toxicity by releasing ferrous and sulfide ions. However, cyanobacteria growing in soils utilize iron and sulfide for their growth. Nitrogenase induction by sulfide in nonheterocystous cyanobacteria is optimal at high pH (Villbrandt and Stal 1996). The sulfide acts as a reducing agent and its addition to the diazotrophic Plectonema boryanum enhanced nitrogenase activity (Kashyap et al. 1996). Cyanobacterial inoculation significantly reduced the oxidizable matter, sulfide, phosphorous, and iron in soils (Aiyer et al. 1972).
Iron is seldom limiting for cyanobacterial growth and its deficiency may be due to the chelating properties of cyanobacteria extracellular products (Whitton 1965). In microbial mats, iron may participate as iron sulfide (FeS) or pyrite (FeS2). Iron reacts with oxygen and keeps the partial pressure of oxygen sufficiently low to allow efficient photosynthesis and N2 fixation by cyanobacteria (Whitton and Potts 2000). Changes in the properties of the upper 0.7 cm of the brown earth silt loom were attributed to an increase in the cyanobacterial population (Rao and Burns 1990). The organic metabolites produced by cyanobacterial growth and released in the extracellular soil environment can be decomposed. However, some metabolites and their degradation products can accumulate and increase the organic N content, an improvement which subsequently maintains the fertility of soil year after year (Roger and Kulasooriya 1980; Ladha and Reddy 1995). Growth of cyanobacteria in soil decreases the C/N ratio due to N2 fixation (Watanabe et al. 1977). An increase in C/N ratio in the experiment conducted under natural conditions with natural inoculum compared to N. calcicola inoculation may be due to the growth of many non-N2− fixers. Soil properties such as soil structure can be improved via the production of extracellular substances by cyanobacteria (Whitton and Potts 2000).
The presence and succession of diazotrophic cyanobacteria in alkaline soil was reported by Singh (1961). The low nutrient content in our soil may have supported the growth of diazotrophic cyanobacteria, which can fix both inorganic C and N. The nutrient-depleted soil and aquatic environment of Schirmacher Oasis, Antarctica, also supported the growth and diversity of diazotrophic cyanobacteria (Pandey et al. 1995). The occurrence of Microcoleus sp. and N. commune on alkaline soil supported the view of Whitton (1990), who reported the widely spread Microcoleus on saline surface. The above discussions revealed that chemical correctives like pyrite may be used with cyanobacteria to prevent iron or sulfur pollution in alkaline soil. A long-term fertility experiment indicated that the use of chemical fertilizers is not sufficient to restore soil fertility—organic matter supplementation is also required. Under such conditions, biofertilizers like cyanobacteria could provide essential nutrient and organic matter to the soil (Singh and Bisoyi 1993).
The solonetz soil contains huge amounts of carbonate. Addition of pyrite in such soils may result in the formation of sulfurous and sulfuric acid, which may react with carbonate and eventually (and predictably) produce CO2. The growth of cyanobacteria in such soils increased soluble CO2 from 0.23×10−4 to 8.1×10−4 (Singh 1961). The inoculation of HCO3−R mutants of N. calcicola in pyrite-treated alkaline soil led to better reclamation results as determined by pH coupled with growth (population) compared to the same treated soil inoculated with the wild-type. Since the HCO3−R mutant strain grew optimally at 250 μM bicarbonate, the presence of high HCO3− concentration in alkaline soil can facilitate its growth and thus its photosynthesis (data not shown) with the release of greater amounts of organic C to soil than at low HCO3− concentration. In conclusion, these experiments were conducted with cyanobacteria only, and the role of other microbes in the reclamation and fertility of solonetz alkaline soil cannot be ignored. For these reasons, further investigation on the subject is required.
References
Aiyer RS, Salahudeen S, Venkataraman GS (1972) Long term algalization field trial with high yielding varieties of rice (Oryza sativa L.). Indian J Agric Sci 42:380–383
Alexander M (1965) Most probable number method for microbial populations. In: Black CA (ed) Methods of soil analysis, Am Soc Agron Inc Madison, WI, USA, pp 1467–1472
Ali S, Sandhu GR (1972) Blue green algae of the saline soils of the Punjab. Oikos 23:268–272
Allen MB, Arnon DI (1955) Studies on nitrogen fixing blue green algae. I. Growth and nitrogen fixation by Anabaena cylindrica Lemm. Plant Physiol 30:366–372
Desikachary TV (1959) Cyanophyta. Indian Council of Agricultural Research, New Delhi, India
Dhar NR, Mukherji SK (1936) Alkali soils and their reclamation. Proc Natl Acad Sci India 6:136–148
Fogg GE (1956) Nitrogen fixation by photosynthetic organism. Annu Rev Plant Physiol 7:51–70
Fritsch, FE (1945) The structure and reproduction of algae—II. Cambridge University Press, London pp 658–767
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbon DW (eds) Methods in microbiology. Academic, London, pp 209–234
Jackson ML (1968) Soil chemical analyses. Prentice-Hall, NJ
Kashyap AK, Pandey KD, Sarkar S (1996) Enhanced hydrogen photoproduction by non-heterocystous cyanobacterium Plectonema boryanum. Int J Hydrogen Energy 21:107–109
Kaushik BD (1987) Blue-green algae: their role in Indian agriculture. In: Srivastava HC, Bhaskaran S, Menon KKG (eds) Crop productivity. Oxford IBH Publ., Delhi, pp 307–326
Kaushik BD, Subhashini D (1985) Amelioration of salt affected soils with blue-green algae: improvements in soil properties. Proc Ind Natl Sci Acad 51:380–389
Ladha JK, Reddy PM (1995) Extension of nitrogen fixation to rice: necessity and possibilities. Geol J 35:363–372
Pandey KD, Singh PK (1984) Isolation and characterization of nitrate reductase mutants and regulation of nitrate reductase and nitrogenase in cyanobacterium Nostoc muscorum. Mol Gen Genet 195:180–185
Pandey KD, Kashyap AK, Gupta RK (1992) Nitrogen fixation by cyanobacteria associated with moss communities in Schirmacher Oasis, Antarctica. Isr J Bot 41:187–198
Pandey KD, Kashyap AK, Gupta RK (1995) Nutrient status, algal and cyanobacterial flora of six fresh water streams of Schirmacher Oasis, Antarctica. Hydrobiologia 299:83–91
Piper CS (1966) Soil and plant analyses. Inter Science Publ. Inc., New York
Rao DLN, Burns RG (1990) The effect of surface growth of blue green algae and bryophytes on some microbiological, biochemical and physical soil properties. Biol Fertil Soils 9:239–244
Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 11:1–61
Roger PA, Kulasooriya SA (1980) Blue green algae and rice. The International Rice Res. Inst., Manila, Philippines
Singh RN (1950) Reclamation of ‘Usar’ lands in India through blue-green algae. Nature 165:325–326
Singh RN (1961) Role of blue-green algae in nitrogen economy of Indian agriculture. Indian Council of Agricultural Research, New Delhi
Singh PK, Bisoyi RN (1993) Biofertilizers for restoration of soil fertility. In: Singh JS (ed) Restoration of degraded land: concept and strategies. Rastogi Publ., Meerut, pp 25–47
Thomas J, Apte SK (1984) Sodium requirement and metabolism in nitrogen fixing cyanobacteria. J Biosci 6:771–794
Verma KS, Abrol IP (1980) Effect of gypsum and pyrite on soil properties in a highly sodic soil. Indian J Agric Sci 50:844–851
Villbrandt M, Stal LJ (1996) The effect of sulfide on nitrogen fixation in heterocystous and non-heterocystous cyanobacterial mat communities. Algol Stud 83:549–563
Watanabe I, Lee KK, Alimagno BV, Sato M, Del Rosario DC, De Guzman MR (1977) Biological N2-fixation in paddy field studied by in-situ acetylene reduction assay. Int Rice Res Inst Res Pap Ser 3:1–16
Whitton BA (1965) Extracellular products of blue-green algae. J. Gen Microbiol 40:1–11
Whitton BA (1990) Microcoleus In: Kumar HD (ed) Phycotalk. Rastogi Publ, Meerut, India, pp 173–182
Whitton BA, Potts M (2000) The ecology of cyanobacteria, their diversity in time and space. Kluwer Academic Publ., The Netherlands
Acknowledgements
The authors thank the Head of Department for providing infrastructure facility and the University Grants Commission and Council of Scientific and Industrial Research, New Delhi, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, K.D., Shukla, P.N., Giri, D.D. et al. Cyanobacteria in alkaline soil and the effect of cyanobacteria inoculation with pyrite amendments on their reclamation. Biol Fertil Soils 41, 451–457 (2005). https://doi.org/10.1007/s00374-005-0846-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-005-0846-7