Abstract
In a field study, potassium (K) applied as muriate of potash (MOP) significantly reduced methane (CH4) emission from a flooded alluvial soil planted to rice. Cumulative emission was highest in control plots (125.34 kg CH4 ha−1), while the lowest emission was recorded in field plots receiving 30 kg K ha−1 (63.81 kg CH4 ha−1), with a 49% reduction in CH4 emission. Potassium application prevented a drop in the redox potential and reduced the contents of active reducing substances and Fe2+ content in the rhizosphere soil. Potassium amendment also inhibited methanogenic bacteria and stimulated methanotrophic bacterial population. Results suggest that, apart form producing higher plant biomass (both above- and underground) and grain yield, K amendment can effectively reduce CH4 emission from flooded soil and could be developed into an effective mitigation option, especially in K-deficient soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice paddies are considered as one of the major biogenic sources for atmospheric CH4, a major greenhouse gas contributing approximately 10–13% to the global CH4 emission (Neue 1997; Crutzen and Lelieveld 2001). However, there is large uncertainty in the estimated values for total CH4 emission from the world's rice paddies, and estimates on CH4 emission from rice paddies show an average of 20–150 Tg year−1 (Mosier et al. 1998) with an approximate increase of about 0.5% CH4 year−1 in the troposphere (Dlugokencky 2001). Projected increase in rice production during the coming decades (IRRI 1999) is anticipated to result into further increase in CH4 fluxes to the atmosphere if the prevalent cultivation practices are continued (Anastasi et al. 1992). In order to increase the accuracy in the estimation of CH4 emission from rice paddies and to predict the future CH4 emission as well as to develop desired mitigation options, understanding the mechanism of CH4 emission from rice paddy is highly imperative. Methane emission from rice paddies is related to a number of contributing processes such as production, oxidation, and transport of CH4 (Bosse and Frenzel 1998). While the most crucial process for CH4 emission is its production, which is influenced by a number of soil processes as well as common cultural practices associated with rice growing, plant-mediated transport of produced CH4 is important for its release to the atmosphere.
Plant nutrients (N, P, S, etc.), either naturally occurring or added as fertilizer (Lindau et al. 1991; Wang et al. 1992; Adhya et al. 1997; Kruger and Frenzel 2003; Liou et al. 2003) to paddy soil, may affect the emission of CH4 by either influencing the growth of rice plant or methanogenic microbial communities in the soil. Methane emission has been correlated with plant biomass (both above- and underground) (Sass et al. 1990). It is possible that application of plant nutrients affects the total CH4 emission from flooded rice paddies by their direct or indirect effect on plant growth. Ammonium-containing N fertilizers often result in increased CH4 emission either by stimulation of plant growth and methanogenic activity or inhibition of methane-oxidizing activity (Lindau 1994; Kruger and Frenzel 2003). Phosphorus applied as single superphosphate (SSP) distinctly inhibited CH4 emission from a flooded plot planted to rice and the inhibitory effect was related to the sulfate content of the SSP (Adhya et al. 1997). Low phosphate supply to rice plants results in the enhancement of CH4 emission (Lu et al. 1999), and application of P has been found to inhibit acetotrophic methanogenesis (Conrad et al. 2000).
Addition of K has well-known effect on plant growth and yield, and deficiency in available K content can adversely affect crop growth and yield (Dobermann et al. 1998). Potassium application is known to alleviate the extreme reducing conditions and associated imbalances in rice plants including iron toxicity (Chen et al. 1997). Several reports have shown that adequate K nutrition is very important in maintaining oxidizing power of rice roots, inducing iron oxidation and pH change in the rhizosphere, and thereby preventing excess uptake of Fe2+ by the rice plants (Trolldenier 1973; Kirk and Bajita 1995). The development of aerenchyma in rice is particularly sensitive to the K status of the plant (Trolldenier 1977).
Oxygen deficiency and reducing conditions are characteristics of flooded rice soils (Ponnamperuma 1972; Leisack et al. 2000). Such reducing conditions often provide a congenial environment for CH4 production (Kruger et al. 2001). As K amendment is known to decrease the reduction reactions and affect the general redox status of flooded soil (Chen et al. 1997), it was deemed worthwhile to investigate the effect of K nutrition on CH4 emission. In the present study, we investigated the effect of K added as muriate of potash (commercial formulation of K as KCl) on CH4 emission from a flooded field planted to rice and grown under irrigated condition. In addition, the effect of added K on other soil processes and plant parameters were also investigated.
Materials and methods
Field experiment
Methane emission from paddy fields was estimated during the dry cropping season (January–May) of 2002 at the experimental farm of the Central Rice Research Institute (CRRI) in Cuttack, India (85°55′E, 20°25′N; elevation 24 m). Annual precipitation is about ∼1,500 mm year−1, of which ∼75% occurs during June to September. Mean maximum and minimum temperatures during the wet season of 2001 was 34.4 and 21.9°C, and the mean maximum and minimum temperatures during the dry season of 2002 was 43 and 11.9°C, respectively. The difference between mean summer soil temperature and mean winter soil temperature is more than 5°C, thus qualifying for hyperthermic temperature class. The soil was an Aeric Endoaquept with sandy clay loam texture (25.9% clay, 21.6% silt, 52.5% sand), bulk density 1.40, percolation rate <10 mm day−1, pH (H2O) 6.16, cation exchange capacity 15 mEq 100 g−1, electrical conductivity 0.5 dS m−1, total C 0.66% and total N 0.08%, exchangeable K 120 kg ha−1. The field was initially plowed on December 27, 2001, and after flooding on January 22, 2002 was puddled thoroughly to a depth of 15 cm, and leveled on January 24, 2002. Rice plants (21-day-old seedlings of the hybrid rice cultivar Proagro-6201) were transplanted on January 26, 2002 at a spacing of 15×20 cm in field plots (5×5 m) well separated by levees.
The experiment was carried out in a randomized block design with four treatments each with four replicates. The treatments included: (1) no K control (K0), (2) K at 30 kg ha−1 (K30), (3) K at 60 kg ha−1 (K60), and (4) K at 120 kg ha−1 (K120). In all the K-amended field plots, K was applied in two splits with two thirds of the fertilizer being applied as basal dressing on January 26, 2002, and the remaining one third at panicle initiation stage. Phosphorus (60 kg P2O5 ha−1) as single superphosphate (SSP) was applied uniformly to all the field plots as basal dressing. Nitrogen (120 kg N ha−1 as urea) was applied to all the field plots in three splits with half of the total N being applied at the time of transplantation and the rest divided into two equal halves and applied at maximum tillering (February 28, 2002) and panicle initiation (March 25, 2002) stages, respectively. All the field plots were continuously flooded to a water depth of 10±2 cm during the entire period of crop growth and drained 10 days before harvest. The crop was grown without application of any pesticide and was harvested at maturity on May 02, 2002, at 96 days after transplantation.
Methane emission measurement
Methane emission from flooded rice fields was monitored, as described previously (Adhya et al. 1994), at regular intervals from the day of transplanting till maturity. Samplings for CH4 efflux measurement was done in the morning (0900–0930 hours) and in the afternoon (1500–1530 hours), and the average of morning and afternoon fluxes was used as flux for the day. For measuring CH4 emission, six rice hills were covered with a locally fabricated Perspex chamber (53×37×51 cm, length×width×height). A battery-operated air circulation pump with air displacement of 1.5 l min−1 (M/s Aerovironment Inc., Monrovia, CA, USA), connected to polyethylene tubing was used to mix the air inside the chamber and draw the air samples into Tedlar air sampling bags (M/s Aerovironment Inc.) at fixed intervals of 0, 15, and 30 min. The air samples from the sampling bags were analyzed for CH4.
Methane concentration in the air samples collected from the crop canopy were analyzed by gas chromatography in a Varian 3600 gas chromatograph equipped with FID and Porapak N column (2 m length, 1/8 in. OD, 80/100 mesh, stainless steel column). The injector, column, and detector were maintained at 30 ml min−1. A 1-ml gas sample was injected into the gas chromatograph with a gastight syringe. The gas chromatograph was calibrated before and after each set of measurements using 5.38, 9.03, and 10.8 μl CH4 ml−1 in N2 (Scotty II analyzed gases; M/s Altech Associates Inc., USA) as primary standard and 2.14 μl ml−1 in air as secondary standard to provide a standard curve linear over the concentration range used. Under these conditions, the retention time of CH4 was 0.53 min and the minimum detectable limit was 0.5 μl ml−1. Cumulative CH4 emission for the entire cropping period was computed by plotting the flux values against the days of sampling, calculating the area covered under the plot of such relationship and expressed as kg CH4 ha−1.
Plant parameters
Mean aerial biomass (fresh and dry weights) was measured by harvesting aboveground portions of rice plant, one hill from each replicated plot, on each day of CH4 sampling as well as at maturity. Root biomass (dry weight) was measured only during the maturity stage of the rice plant. Both the aerial and the underground (root) biomass values were expressed as g m−2 (dry weight basis). The α-naphthylamine oxidase activity of roots (root base and root tip) was measured by the method of Ota (1970) as modified by Satpathy et al. (1997). In brief, rice plants were uprooted and the roots were washed several times in distilled water. All operations were conducted with minimum exposure of roots to the atmosphere. The roots were differentiated into root tip (2–3 cm from root tip) and root base (2–3 cm from root base), and cut into 1-cm segments. The washed and clean root segments were squeezed gently with Whatman filter paper no. 41 to remove excess of moisture and 1-g portions of root tip and root base were incubated separately with 50 ml α-naphthylamine solution. The root oxidase activity was expressed as μg of α-naphthylamine oxidized g−1 dry root h−1. Tiller no., grain weight, and grain and straw yields from individual replicated plots were measured at maturity and the harvest index was calculated (Bharati et al. 2000).
Soil analyses
Redox potential and pH of the soil in the immediate vicinity of the roots (5 cm deep) in the planted field plots was measured with each set of CH4 flux measurement. The redox potential of the field soil was measured by inserting a combined platinum–calomel electrode (Barnant Co., Barrington, IL, USA) to the root region and waiting for the potentiometer reading to be stable for at least 10 s, which usually occurs 2–3 min after insertion of the electrode. The potential difference was measured in mV (Bharati et al. 2000). All the values were corrected to that of a hydrogen electrode by adding +240 mV to the redox readings. pH of the soil was measured with a portable pH meter (Philips Analytical, Cambridge, UK).
Soil chemical components were analyzed from field soils sampled by inserting a tube auger (2 cm diam.) to a depth of 5–7 cm, in between two rice hills. The soil samples, after excess water was drained, were immediately subsampled for the measurement of active reducing substances (ARS), Fe2+, and readily mineralizable C (RMC) contents. ARS content was measured by extracting 5 g of fresh soil sample with 100 ml of 0.1 mol l−1 Al2(SO4)3 (pH=2.5) and titrating with standard KMnO4 solution (Chen et al. 1997). Fe2+ content was measured by agitating soil samples with NH4OAC/HCl (pH 2.8) and determining Fe2+ spectrophotometrically after reaction with orthophenanthroline (Pal et al. 1979). RMC content was measured after extracting soil samples with 0.5 M K2SO4, followed by dichromate digestion of the soil extract (Mishra et al. 1997). In brief, 10 g of soil was extracted with 40 ml 0.5 M K2SO4. Reduced iron (Fe2+) present in the soil extract was coprecipitated with 1 ml of FeCl3 (2.5% solution) and 4 ml of 6 N NaOH. After allowing the precipitate to settle down at 4°C, aliquots from clear supernatant were titrated with ferrous ammonium sulfate after wet digestion with chromic acid (Vance et al. 1987).
The dynamics of select microbial groups from different treatments as represented by total heterotrophs, methanogenic bacteria, and methanotrophic bacteria at tillering, panicle, and maturity stages of the rice crop, was estimated. The population of total aerobic bacteria in the rhizosphere soil was estimated by the standard dilution plate technique using tryptone yeast extract medium (Rand et al. 1975) and expressed as colony-forming units (CFU) g−1 dry soil. Methanogenic bacterial population in the rhizosphere soil was enumerated following the anaerobic culture tube technique (Kaspar and Tiedje, 1982). Methanotrophic bacteria in the rhizosphere soil with soluble methane monooxygenase (sMMO) activity were enumerated as described by Graham et al. (1992).
Statistical analyses
Individual character datasets were statistically analyzed, and the mean comparison between treatments was established by Duncan's multiple range test using statistical package (IRRISTAT, version 3.1, International Rice Research Institute, Philippines). Simple and multiple correlations between CH4 flux and select soil and plant parameters were analyzed using the variation at each time of observation.
Results and discussion
Impact of K amendment on CH4 emission from a flooded field planted to rice
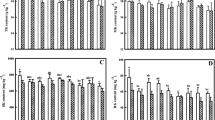
Methane emission from rice plants varied considerably among the treatments with two peaks, one each at vegetative and maturity stages of the rice crop, and it was low in all the plots during the first 2 weeks after transplantation (Fig. 1). During the subsequent stages of rice growth, consistently higher CH4 flux was observed in control plots as compared to K-amended plots and application of K significantly reduced CH4 emission. Thus, mean CH4 emission (mg CH4 m−2 h−1) from transplanting to harvest followed the order: K0 (4.41)>K60 (3.29)>K120 (2.78)>K30 (2.72). Cumulative CH4 emission was highest in the control treatment (125.34 kg ha−1), whereas the lowest emission level was recorded in field plots receiving 30 kg K ha−1 (63.81 kg ha−1). Percentage inhibition in CH4 emission as a result of K amendment was in the order of K30 (49.09)>K120 (48.59)>K60 (34.55). No statistically significant difference in CH4 emission was observed in plots receiving 30 and 120 kg K ha−1. The low CH4 emission rates during the early stages of rice plant growth are considered to be due to the low levels of methanogenesis and poor conductance of CH4 from the bulk of the reduced soil to the atmosphere through the lowly differentiated internal structure of the growing rice plant (Satpathy et al. 1997).
Although the application of K fertilizers improves rice growth, their effectiveness is significantly increased in K-deficient soils. The alluvial soil used in the present study was sufficient in available K content (120 kg ha−1) and does not show any visible symptoms of K deficiency (Padmaja 1975) in spite of regular rice growing for many years. Several reports have shown that adequate K nutrition is very important in maintaining oxidizing power of rice roots, inducing iron oxidation, and maintaining suitable pH in the rice rhizosphere, thereby preventing uptake of excess Fe2+ by rice plants (Trolldenier 1973; Kirk and Bajita 1995). The development of aerenchyma in rice is also particularly sensitive to the status of potassium in rice plants (Trolldenier 1977). Thus, application of K might have induced higher oxidizing conditions in the rhizosphere of the rice plants, thereby adversely affecting the methanogenic environment and inhibiting CH4 formation and its subsequent release through rice plants.
Influence of K nutrition on select plant parameters vis-a-vis CH4 emission
The α-naphthylamine oxidase activity of rice roots, an index of the oxidation status in the rhizosphere region (Ota 1970), was significantly correlated with CH4 efflux from different cultivars (Satpathy et al. 1998), growth stages (Adhya et al. 1994), and even diurnal variation in CH4 flux (Satpathy et al. 1997) from rice plants. Several reports have shown that adequate K nutrition is very important in maintaining the oxidizing power of rice roots (Kirk and Bajita 1995). In the present study, the α-naphthylamine oxidase activity of root tip and root base was higher in control plots compared to K-amended field plots (Table 1). Ando et al. (1983) showed that K deficiency had low O2 availability and higher α-napthylamine oxidation activity. In a pot experiment, Chen et al. (1997) demonstrated that K amendment to K-deficient soils promoted root growth and enhanced α-naphthylamine oxidase activity. The soil used in the present study is not K-deficient (available K content, 120 kg ha−1) and thus the impact of K amendment did not alter the plant biophysical status as drastically as it would have in the case of plants grown in K-deficient soils. However, considering the total underground biomass (Table 2), overall root oxidase activity (μg total root dry weight−1) in the present study was still high with K application. Thus, low CH4 flux from K-amended field plots could be an impact of higher α-naphthylamine oxidase activity, indicating an overall higher oxidation status.
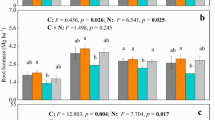
Plant parameters such as aboveground biomass, belowground biomass, and the number of tillers increased with higher K application rates. However, only aboveground biomass and belowground biomass showed statistically significant increase after K application (Table 2). Under K-limiting conditions, rice plants grew poorly and K application promoted root growth and general biomass of rice plants (Chen et al. 1997).
Impact of K amendment on soil parameters
Water culture experiments have demonstrated that mineral nutrition affects the redox processes in the rice rhizosphere (Trolldenier 1973). It has been shown in many studies that the redox potential of a soil solution was higher with K application than without K, and potassium application not only influenced soil redox status but also changed the redox values in and around rice roots (Tanaka and Tadano 1972; Chen et al. 1997). In the present study, significantly higher Eh values were recorded in soils from field plots receiving K amendment (Table 3). After flooding, Eh of the soil decreased in all treatments, reaching its lowest value between 50 and 60 DAT, and increasing again toward maturity. Chiang and Yang (1969) reported that addition of N markedly lowered the redox potential, whereas application of K had the opposite effect. In the present experiment, the application of K prevented a considerable drop in the redox potential, and the seasonal mean Eh (mV) was in the order of K120 (−171)>K60 (−213)>K30 (−232)>control (−286), and was significantly different from each other. A significant negative correlation was found between Eh and CH4 emission (r=−0.554**) (Table 4). It is well established that CH4 emission is highest when redox potential was lowest and vice versa (Masscheleyn et al. 1993; Wang et al. 1993; Hou et al. 2000).
Methanogenesis in soils is favored at near-neutral to slightly alkaline pH and a near-neutral pH supports higher quantum of CH4 emission (Masscheleyn et al. 1993). The pH of the planted soil increased with plant growth to near neutrality toward maturity (Table 5). Application of K resulted in a statistically significant increase in the pH of the rhizosphere soil of planted plots during the initial stages of plant growth. Differences among the treatments, however, disappeared toward maturity, except at highest K application rate (120 kg K ha−1). The overall impact of K amendment on pH changes was statistically not significant (Table 5).
The amount of ARS (Chen et al. 1997) of soils was monitored throughout the cropping season to find a possible relationship with CH4 emission (Table 6). Potassium application decreased the amount of ARS in the rhizosphere soil. Rice plants with greater dry weight of roots having higher oxidizing power might have drastically reduced the amounts of ARS in the rhizosphere of rice plants in K-amended field plots. The seasonal mean of the contents of ARS (cmol kg−1 soil) was in the order of K0 (7.78)>K30 (6.20)>K60 (5.79)>K120 (5.10). A significant positive relationship was observed between the amount of ARS and CH4 emission (Table 4).
Iron redox system plays an important role in the reduction process of flooded rice soils (Takai and Kamura 1966). Fe2+ content is an indirect measure of the redox status of soil, and highly reduced soils usually exhibit higher Fe2+ content. The Fe2+ content of soils that was initially low in all treatments increased, gradually attaining the peak value at maximum tillering stage of the crop irrespective of treatments (Table 7). It was higher in the control plot than in K-amended ones. However, no significant relationship existed between CH4 flux and Fe2+ content. Potassium application is known to reduce Fe2+ content in the rhizosphere and prevents uptake of excess Fe2+ iron by rice plants (Kirk and Bajita 1995; Saleque and Kirk 1995). Fe2+ content of flooded soil would also contribute to the total ARS pool of the soil and the method employed in the present study for ARS determination also includes Fe2+ content. This is evident from the significant correlation between the ARS and Fe2+ contents (Table 4). The results thus suggest that Fe2+ content is an important indicator of the redox status of the soil. Chen et al. (1997) showed that K application to soils reduced the content of ARS and Fe2+ content of the soil, raised the soil redox potential in the rhizosphere, increased the Eh values of rice roots, and lowered the iron content in rice plants.
Methanogenic population and their activity in flooded soil are influenced by the availability of fermentable substrates, usually expressed as RMC content (Yagi and Minami 1990; Mishra et al. 1997). The RMC content (Table 8) decreased during the reproductive stages of the rice crop with a concomitant increase in CH4 emission. The highest RMC content was recorded in control plots (0.61 mg g−1) at 80 DAT, whereas the lowest amount (0.10 mg g−1) was recorded in the K120 plot at 50 DAT. A simple correlation analysis between RMC and CH4 emission provided a significant positive relationship, indicating a direct effect of RMC content on CH4 emission.
Flooding the soil retards the growth of aerobes and promotes the proliferation of anaerobes (Sethunathan et al. 1983). Populations of aerobic heterotrophs in the rhizosphere soil were high at maximum tillering stage than at panicle initiation and maturity stages in K60 and K120 treatments (Table 9). Field plots receiving 120 kg K ha−1 harbored the highest population of aerobic heterotrophic bacteria and was followed by K60, K30, and control in that order. With increasing K applications, the number of facultative anaerobic fermentative bacteria decreased in solution culture experiments with rice (Trolldenier 1973), and the decrease was related to highly oxidized condition and lower root exudation. The higher population of aerobic heterotrophs in K120, as observed in the present study, may be due to the increase in redox potential that supported their growth in the rice rhizosphere soil. The seasonal mean population of aerobic heterotrophs was 20×106, 23×106, 34×106, and 59×106 in control, K0, K30, K60, and K120, respectively. A simple correlation analysis revealed a negative, but not significant, relation between aerobic heterotrophs and mean CH4 emission (r=−0.553, n=4).
In rice fields, like any other biogenic source, CH4 is produced by methanogens as one of the terminal products in anaerobic food web (Cicerone and Oremland 1988; Yagi 1997). In paddy soils, acetate utilizers are the predominant methanogenic bacteria (Kruger et al. 2001). The acetate-utilizing methanogenic bacterial population of rhizosphere soil was highest at panicle initiation stage than at maximum tillering and maturity stage of crop (Table 9). Population of methanogens was highest in the rhizosphere soil of K30 and lowest in K120. Trolldenier (1977) reported that when rice is grown under K-limiting condition, the amount of soluble root exudates per root mass increases. In the present investigation, higher methanogenic population in control and K30 treatments could be due to higher root exudation rate and low soil redox potential. Application of high levels of K increased the root biomass that would have increased the O2 release from roots. The high concentration of O2 in the root environment, in turn, might have depressed the growth of methanogenic bacteria in K60 and K120 treatments.
Estimates of methanotrophic bacterial population of rhizosphere soil possessing sMMO activity (Graham et al. 1992) were distinctly stimulated by the application of K (Table 9). Total methanotrophs were higher at tillering and maturity, and lower at panicle initiation stage. Mean methanotrophic population (g−1 soil) was highest in K120 (18×104) followed by K60 (16×104), K30 (12×104), and control (11×104). Methanotrophic bacteria are mostly strictly aerobes, and the less reduced status at K60 and K120 might have favorably influenced the methanotrophic bacterial population at these two levels of K amendment.
Methane emission from irrigated rice fields is regulated by CH4 production, CH4 oxidation, and CH4 transport (Kruger et al. 2001). Potassium amendment is known to affect electrochemical changes in flooded soils and the influences have been repeatedly demonstrated in water culture experiments (Trolldenier 1977). In the present study, K was applied as its chloride salt, while chloride can also be inhibitory to CH4 production (Koyama and Kimura 1998). However, in an incubation study, application of chloride to raise the porewater salinity to 8 dS m−1 did not cause any significant change in the redox status and pH of the soil, despite its inhibitory effect on CH4 production (Mishra et al. 2003). Amendment of chloride caused a significant increase in the RMC content of the soil, which was attributed to osmotic lysis of soil microbiota and associated release of cell contents. On the contrary, K amendment resulted in a change in the redox status of the soil, creating a relatively less reduced condition and a significant reduction in the RMC content especially during the initial days. These influences ultimately inhibited the activity of methanogenic bacteria and stimulated functioning of methanotrophic microorganisms, thereby causing a reduction of CH4 emission from rice fields.
Influence of K amendment on grain yield and yield attributes of rice
Potassium amendment resulted in higher grain and straw yields compared to that of unamended control (Table 2). Application of K at all levels resulted in a significant increase in grain yield over that of control but remained statistically at par among the different amendments. Straw yield, however, was significantly different only at the highest level of K amendment (K120). Computation of CH4 emission (kg) per mg of grain yield indicated that K amendment at 120 kg ha−1 led to the minimum efflux of CH4, whereas the highest efflux values were recorded from unamended control plots.
Conclusion
Addition of K has well-known effects on plant growth and yield, and a deficiency in available K content can adversely affect the growth and yield. In the study site, where K is not limiting and does not exhibit any symptom of K deficiency in the growing rice crop, application of K resulted in a decrease in CH4 emission as well as an increase in grain yield. The results indicate that, apart form producing higher plant biomass (both above- and belowground) and grain yield, K amendment effectively reduced CH4 emission by preventing a drop in soil redox potential and inhibiting methanogenic bacteria and simultaneously stimulating methanotrophic bacterial population. Potassium balance in rainfed rice are precarious because (1) resource-poor, rainfed farmers generally apply little K fertilizer; (2) rainfed rice does not receive K in irrigation water, which is an important component in the K balance in irrigated rice; and (3) farmers generally remove all the straw from their fields for fuel and animal fodder. In light-textured soils of the tropics, additional K may be lost through leaching, resulting into K-deficient conditions. Thus, apart from correcting nutritional imbalance especially in K-deficient soils, K amendment can act as an effective mitigation option for CH4 emission.
References
Adhya TK, Rath AK, Gupta PK, Rao VR, Das SN, Parida K, Parashar DC, Sethunathan N (1994) Methane emission from flooded rice paddy fields under irrigated condition. Biol Fertil Soils 18:245–248
Adhya TK, Pattanaik P, Sathpathy SN, Kumaraswamy S, Sethunathan N (1997) Influence of phosphorus application on methane emission and production in flooded paddy soils. Soil Biol Biochem 30:177–181
Anastasi C, Dowding M, Simpson VJ (1992) Future CH4 emissions from rice production. J Geophys Res 97:7521–7525
Ando T, Yoshida S, Nishiyama I (1983) Nature of oxidizing power of rice roots. Plant Soil 72:57–71
Bharati K, Mohanty SR, Singh DP, Rao VR, Adhya TK (2000) Influence of incorporation or dual cropping of Azolla on methane emission from a flooded alluvial soil planted to rice in eastern India. Agric Ecosyst Environ 79:73–83
Bosse U, Frenzel P (1998) Methane emissions from rice microcosms : the balance of production, accumulation and oxidation. Biogeochem 41:199–214
Chen J, Xuan J, Du C, Xie J (1997) Effect of potassium nutrition of rice on rhizosphere redox status. Plant Soil 188:131–137
Chiang CT, Yang CT (1969) Roles of nitrogen, phosphorus and potassium in the metabolism of flooded paddy soils. Taiwan Fest Co Ltd Res Bull 34:54–61
Cicerone RJ Oremland RS (1988) Biogeochemical aspects of atmospheric methane. Global Biogeochem Cycles 2:299–327
Conrad R, Klose M, Claus P (2000) Phosphate inhibits acetotrophic methanogenesis on rice roots. Appl Environ Microbiol 66:828–833
Crutzen P, Lelieveld J (2001) Human impacts on atmospheric chemistry. Annu Rev Earth Planet Sciences 29:17–45
Dlugokencky E (2001) NOAA CMDL Carbon cycle greenhouse gases, global average atmospheric methane mixing ratios. NOAA CMDL cooperative air sampling network. http://www.cmdl.noaa.gov/ccg/figures/chtrend.global.gif
Dobermann A, Cassman KG, Mamaril CP, Sheehy JE (1998) Management of phosphorus, potassium and sulfur in intensive, irrigated lowland rice. Field Crops Res 56:113–118
Graham DW, Korich DG, Leblanc RP, Sinclair NA, Arnold RG (1992) Application of colorimetric plate assay for soluble methane monooxygenase activity. Appl Environ Microbiol 58:2231–2236
Hou AX, Chen GX, Wang ZP, Van Cleemput O, Patrick WH Jr (2000) Methane and nitrous oxide emission from a rice field in relation to soil redox and microbiological processes. Soil Sci Soc Am J 64:2180–2186
IRRI [International Rice Research Institute] (1999) Sustaining food security beyond the year 2000—a global partnership for rice research: medium-term plan 2000–2002. International Rice Research Institute, Los Baños, Philippines
Kaspar HF, Tiedje JM (1982) Anaerobic bacteria and processes. In: Page AL, Miller RM, Keeney R (eds) Methods of soil analysis, Part 2. American Society of Agronomy, Madison, pp 989–1009
Kirk GJD, Bajita JB (1995) Root induced iron oxidation, pH change and zinc solubilization in the rhizosphere of low land rice. New Phytol 131:129–137
Koyama T, Kimura M (1998) Inhibition of methane production in paddy soils. Soil Sci Plant Nutr 44:667–674
Kruger M, Frenzel P (2003) Effects of N-fertilisation on CH4 oxidation and production, and consequences for CH4 emissions from microcosms and rice fields. Glob Chang Biol 9:773–784
Kruger M, Frenzel P, Conrad R (2001) Microbial processes influencing methane emission from rice fields. Glob Chang Biol 7:49–63
Leisack W, Schnell S, Revsbech NP (2000) Microbiology of flooded rice paddies. FEMS Microbiol Rev 24:625–645
Lindau CW (1994) Methane emission from Louisiana rice fields amended with nitrogen fertilizers. Soil Biol Biochem 26:353–359
Lindau CW, Bollich PK, Delaune RD, Patrick WH Jr, Law VJ (1991) Effect of urea fertilizer and environmental factors on CH4 emissions from a Louisiana, USA rice field. Plant Soil 136:195–203
Liou RM, Huang SN, Lin CE (2003) Methane emission from fields with differences in nitrogen fertilizers and rice varieties in Taiwan paddy soils. Chemosphere 50:237–246
Lu Y, Wassmann R, Neue HU, Huang C (1999) Impact of phosphorus supply on root exudation, arenchyma formation and methane emission of rice plants. Biogeochemistry 47:203–218
Masscheleyn PH, DeLaune RD, Pattrick WH Jr (1993) Methane and nitrous oxide emissions from laboratory measurements of rice soil suspensions: effect of soil oxidation reduction status. Chemosphere 26:251–260
Mishra S, Pattnaik P, Sethunathan N, Adhya TK (2003) Anion-mediated salinity affecting methane production in a flooded alluvial soil. Geomicrobiol J 20:579–586
Mishra S, Rath AK, Adhya TK, Rao VR, Sethunathan N (1997) Effect of continuous and alternate water regimes on methane efflux from rice under greenhouse conditions. Biol Fertil Soils 24:399–405
Mosier AR, Duxbury JM, Freney JR, Heinemeyer O, Minami K, Johnson DE (1998) Mitigating agricultural emissions of methane. Climatic Change 40:39–80
Neue HU (1997) Fluxes of methane from rice fields and potential for mitigation. Soil Use Manage. 13:258–267
Ota Y (1970) Diagonostic method for measurement of root activity in rice plant. Jpn Agric Res Q 5:1–6
Padmaja PC (1975) Studies on potassium in rice (Oryza sativa) and rice soils. Ph.D. thesis, Orissa University of Agriculture and Technology, Bhubaneswar, India
Pal SS, Barik S, Sethunathan N (1979) Effects of benomyl on iron and manganese reduction and redox potential in flooded soil. J Soil Sci 30:155–159
Ponnamperuma FN (1972) The chemistry of submerged soils. Adv Agron 24:29–96
Rand MC, Greenberg AE, Taras MJ, Franson MA (1975) Standard methods for the examination of water and waste water. American Public Health Association, Washington
Saleque MA, Kirk GJD (1995) Root induced solubilization of phosphate in the rhizosphere of lowland rice. New Phytol 129:325–336
Sass RL, Fisher FM, Harcombe PA, Turner FT (1990) Methane production and emission in Texas rice fields. Glob Biogeochem Cycles 4:47–68
Satpathy SN, Rath AK, Ramakrishnan B, Rao VR, Adhya TK, Sethunathan N (1997) Diurnal variation in methane efflux at different growth stages of tropical rice. Plant Soil 195:267–27
Satpathy SN, Mishra S, Adhya TK, Ramakrishnan B, Rao VR, Sethunathan N (1998) Cultivar variation in methane efflux from tropical rice. Plant Soil 202:223–229
Sethunathan N, Rao VR, Adhya TK, Raghu K (1983) Microbiology of rice soils. CRC Crit Rev Microbiol 10:125–172
Takai Y, Kamura T (1966) The mechanism of reduction in water logged paddy soils. Folia Microbiol 11:304–313
Tanaka A, Tadano T (1972) Potassium in relation to toxicity of the rice plant. Potash Review Subject 9, 21st suite, pp 1–12
Trolldenier G (1973) Secondary effects of potassium and nitrogen nutrition of rice. Change in microbial activity and iron reduction in the rhizosphere. Plant Soil 38:267–279
Trolldenier G (1977) Mineral nutrition and reduction processes in the rhizosphere of rice. Plant Soil 47:193–202
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass. Soil Biol Biochem 19:703–707
Wang ZP, Delaune RD, Masscheleyn PH, Patrick WH Jr (1993) Soil redox and pH effect on methane production in a flooded rice soil. Soil Sci Soc Am J 57:382–385
Wang ZP, Lindau CW, Delaune RD, Patrick WH Jr (1992) Methane production from anaerobic soil amended with rice straw and nitrogen fertilizers. Fertil Res 33:115–121
Yagi K (1997) Methane emissions from paddy fields. Bull Natl Inst Agroenviron Sci 14:96–210
Yagi K, Minami K (1990) Effect of organic matter application on methane emission from some Japanese paddy fields. Soil Sci Plant Nutr 36:599–610
Acknowledgement
This work was supported, in part by the National Agricultural Technology Project entitled, “Greenhouse Gas Emission from Rice-based Cropping systems” (Grant No. 26(4)/97-NATP) by the Indian Council of Agricultural research, New Delhi. We thank the Director, Central Rice Research Institute, Cuttack, for permission to publish this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jagadeesh Babu, Y., Nayak, D.R. & Adhya, T.K. Potassium application reduces methane emission from a flooded field planted to rice. Biol Fertil Soils 42, 532–541 (2006). https://doi.org/10.1007/s00374-005-0048-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-005-0048-3