Abstract
A plot study was conducted to quantify long-term (>12 years) impacts of a single biosolids application, and short-term impacts (<2 years) of a repeated application, on semi-arid rangeland soil chemical and microbial parameters. In 2003 and 2004, plots which had received 0, 2.5, 5, 10, 21, or 30 Mg biosolids ha−1 once in 1991 (long-term plots), or again in 2002 (short-term plots), were sampled and analyzed for soil chemical parameters, microbial biovolumes, C and N mineralization activities, Biolog EcoPlate substrate utilization potential, and plant productivity and tissue quality. Repeated applications temporarily exacerbated differences in soil chemical properties among treatments, but after 2 years, soil chemistry trends were similar between short-term and long-term plots. Soils which received a repeated application of 21 or 30 Mg biosolids ha−1 had greater bacterial biovolumes and C and N mineralization activities. In long-term plots, mineralization activities were stimulated only at the highest rate. Biosolids-amended soil communities also utilized Biolog substrates more quickly compared to communities from control plots. Plant biomass increased, whereas plant diversity and plant C/N ratio decreased with increasing application rate for both short- and long-term plots. Infrequent biosolids application had positive ecosystem effects in terms of site management objectives, with relatively low extractable metal levels in soil and greater plant biomass and tissue quality despite reduced species richness.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biosolids land application often results in significant changes in the structure, diversity, or richness of plant and animal communities at a given site, due directly, and indirectly, to resulting soil improvements and changing environmental conditions (US Environmental Protection Agency 1991). Few studies, however, have examined the soil microbial community in ecologically based studies, particularly in semi-arid grasslands (Dennis and Fresquez 1989; Pascual et al. 1999; Barbarick et al. 2004; García-Gil et al. 2004). Such research is crucial, considering that among other functions, microbial populations control decomposition and nutrient cycling in soils (Coleman and Crossley 1996), which in turn, control aboveground dynamics (Reynolds et al. 2003). Furthermore, a greater understanding of biosolids long-term effects on above- and belowground dynamics will lead to a greater understanding of ecosystem-level effects.
Dennis and Fresquez (1989) found that cultivable fractions of the soil microbial community from a semi-arid grassland increased linearly with the biosolids application rate (22.5, 45, and 90 Mg ha−1), and they noted that improvements in soil fertility and site restoration were reflected in diversity and composition of the soil microbial community. García-Gil et al. (2004) reported increased microbial biomass, basal respiration, metabolic quotient, and enzymatic activities in another semi-arid soil, amended 9 or 36 months previously, with biosolids at approximately 36 Mg ha−1. Barbarick et al. (2004) examined microbial responses to biosolids in both semi-arid grassland (0 and 30 Mg ha−1) and shrubland (0 and 40 Mg ha−1) sites 6 years after biosolids surface application. At both sites, CO2 evolution and actively metabolizing microbial biomass were greater in plots 6 years following biosolids application than in control (nonamended) plots. Pascual et al. (1999) examined microbiological and biochemical parameters of semi-arid soils 8 years after biosolids amendment (65 and 260 Mg ha−1). Compared to control plots, the amended plots exhibited greater total organic C, microbial biomass, basal respiration rate, and enzymatic activity even 8 years after biosolids incorporation.
Relatively high biosolids application rates were employed in most of the studies described above, and thus, their results may not be applicable to many rangeland situations where biosolids may be applied once or a few times at very low rates (e.g., 2 to 5 Mg ha−1) for purposes of beneficial use. Therefore, the objectives of this study were to determine, using low and moderate application rates, the long-term effects of a single biosolids application and the short-term effects of a repeated application on soil chemical properties, microbial biomass and activities, and plant productivity and tissue quality in a semi-arid rangeland ecosystem. The primary hypothesis was that infrequent biosolids amendment events would result in long-term shifts in nutrient cycling activities and thus increased plant productivity via soil–plant feedback loops. The merit of this particular study lies in the relatively low application rates and the length of time since biosolids were applied, both of which are on a realistic scale for assessing impacts of long-term land-based beneficial recycling programs in the semi-arid west rather than with rates and application frequencies used for fertilization practices or simply as a means of disposal.
Materials and methods
Experimental site description
This study was conducted in the summer of 2003 and 2004 on long-term experimental research plots within the 40,000 ha Meadow Springs Ranch, Larimer County, CO (40 53′46″N, 104 52′28″W). The ranch (1,750 m elevation) is owned by the city of Fort Collins, CO, which uses it for its land-based biosolids recycling program. A detailed description of the ranch and the experimental site are given in Harris-Pierce (1994). In brief, the study site is a semi-arid, shortgrass steppe rangeland community dominated by perennial grasses, including blue grama (Bouteloua gracilis) and western wheatgrass (Pascopyron smithii). The surface soil texture is sandy loam bordering on gravelly, classified as a deep, well-drained Aridic Argiustoll (Soil Conservation Service and Forest Service 1980). Precipitation averages 380 mm annually, with 25% in the form of winter snow (Soil Conservation Service and Forest Service 1980). However, from July 2002 to June 2003, total precipitation for the year exceeded 2,128 mm, with roughly 1,700 mm in the form of snow. Precipitation from July 2003 to June 2004 dropped to 822 mm, with roughly 686 mm in the form of snow (National Oceanic and Atmospheric Administration 2002–2004).
Plots (15×15 m) were originally established in 1991 (Harris-Pierce 1994) and arranged in a randomized, complete block design. In 1991, a single biosolids application (0, 2.5, 5, 10, 21, or 30 Mg ha−1) was surface-applied to each of four replicate plots. In the fall of 2002, the plots were divided in two, and a second application was applied to the eastern half of each of the original plots, resulting in a split-plot block design with rate as the main factor and reapplication (yes or no) as the split factor. The rate applied to each plot (7.5×15 m) in 2002 corresponded to the rate applied to the whole plot in 1991. We defined short-term plots as those that received biosolids application in both 1991 and 2002 and long-term plots as those that only received biosolids application in 1991. The application rates are realistic for semi-arid rangelands, and with large expanses of such native grasslands on site, many years occur between reapplication at a single location. Biosolids originated from the city of Fort Collins' wastewater treatment plant and were surface-applied without incorporation in both 1991 and 2002.
Biosolids chemical properties
Biosolids from the 1991 and 2002 applications were analyzed for pH, electrical conductivity (EC), and total organic N, NH4–N, NO3–N, P, K, Fe, Al, Cu, Zn, Ni, Mo, Cd, Cr, and Pb. Biosolids from 2002 were also analyzed for total As, Se, Hg, and Ag. Elemental composition was determined by HClO4–HNO3–HF–HCl digestion (Soltanpour et al. 1996) followed by elemental analysis with inductively coupled plasma–atomic emission spectrometry (ICP-AES). Total N content of biosolids was determined by a concentrated H2SO4 digestion (Bremner 1996). The materials were very comparable across years, but the 2002 biosolids contained four times the amount of salt as compared to the 1991 biosolids, expressed as EC (Table 1). Chemical constituents fall below the US Environmental and Protection Agency (EPA) 40 code of federal regulations (CFR) Part 503 limits (US Environmental Protection Agency 1993).
Plant and soil sampling
In July 2003 and June 2004, vegetation and soil samples were collected in each plot from ten randomly located 0.5-m2 quadrants. Soil samples (0–15 cm depth) were obtained 24 h before vegetation sampling began to assure as little perturbation to the soil microbial community as possible while maintaining measurements in the same sampling locations. Soil cores (10–30) from a plot were composited in a Ziploc freezer bag to create a composite sample. Composite samples were stored on ice in the dark for transport to the laboratory where soils were sieved through a 2-mm mesh screen. After sieving, gravimetric water content was determined for each sample, and the remaining portion was stored for approximately 24 h at 5°C until microbial analyses could commence. In 2004, samples were allowed to air-dry one full day in opened Ziploc bags before analyses began, due to excessive moisture conditions.
Vegetation was clipped by species within each quadrant, dried at 60°C to a constant mass, and weighed for determination of aboveground biomass and species composition. Bulked vegetation samples were analyzed for total C and N content of the aboveground plant community.
Following plant sampling, three soil samples were collected from each experimental plot using a hydraulic Giddings probe to a depth of 30 cm. Soil cores were divided into two depth increments (0–15 cm and 15–30 cm), and cores were composited per plot by depth increment. Samples were transported back to the lab in Ziplock bags, and a 10-g subsample of each was partitioned for determination of gravimetric water content before further processing. Samples were passed through a 2-mm sieve and air-dried; samples analyzed for total C and N were further ground to a fine powder.
Soil chemical and physical analyses
Soil samples collected with the hydraulic probe were analyzed for pH, EC, total C and N, NH4–N, NO3–N, and ammonium bicarbonate–diethylenetriaminepentaacetic acid (AB-DTPA) extractable Al, Ba, Ca, Cd, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Pb, Sr, Ti, V, and Zn. Soil pH was determined by the saturated paste method of Thomas (1996), and EC was measured by the method of Rhoades (1996). Total C and N were measured using a LECO CHN-1000 automated analyzer (LECO, St. Joseph, MI) according to the protocols of Nelson and Sommers (1996). Exchangeable soil NH4–N and NO3–N were extracted in 2 M KCl according to Mulvaney (1996) and analyzed on a Perstorp Enviroflow flow injector (Perstorp Analytical, Inc., Silver Spring, MD). The method of Barbarick and Workman (1987) was used for soil AB-DTPA-extractable metals, followed by a determination of constituent concentrations on an inductively coupled plasma–atomic emission spectrophotometer (Thermo Jarrell Ash Corp., Franklin, MA). Only the 2003 data from the 0–15 cm depth increment are reported here.
Microbial biovolumes
Microbial biovolumes in soil were determined by direct enumerations of soil bacteria and fungi. Soil bacteria were visualized with a SYBR I green fluorescent nucleotide stain solution (1 μL SYBR I ml−1 TE buffer, pH 7.5; Weinbauer et al. 1998) and enumerated according to the method of Bloem et al. (1995). Quantification of soil fungal hyphae was performed with the coverslip–well slide method of Lodge and Ingham (1991). Bacterial and fungal slides were observed at 1000 and 400× resolution, respectively, with a Nikon Eclipse E600 epifluorescent microscope (Nikon Instruments, Inc., Mellville, NY) equipped with a Texas Red/UV/DTAF combination filter set and an ocular grid. Bacteria were counted in a total of 20 fields of view across four vertical transects. Images of bacterial cells and fungal hyphae were captured with a CoolSNAP Pro cf digital camera (A.G. Heinze Precision MicroOptics, Lake Forest, CA) and ImagePro Plus imaging software (Media Cybernetics, Silver Spring, MD). Biovolume conversions of bacteria and fungi were determined based on the average diameter of at least 60 bacterial cells and 20 hyphal fragments per slide (Klein and Paschke 2000).
Microbial C and N mineralization activities
Soil respiration activity was determined during a 28-day incubation which employed the alkali-trap method of Barbarick et al. (2004). One 25.0-g (dry weight) subsample of soil from each plot was placed in a sealed 0.95-l airtight glass Mason jar along with a vial containing 10.0 ml of 0.5 M NaOH, and the jars were maintained in the dark at 25°C for 28 days. At day 10 of incubation, vials of NaOH were removed, and the CO2 respired was measured by titration with 0.5165 M HCl to a phenolphthalein endpoint. A new NaOH vial was added to each jar, and the jars were incubated for another 18 days. On the last day, the vials were removed and titrated for CO2 as described for day 10 vials. For each block of experimental plots, a blank jar without soil was set up to determine background CO2, and all samples were corrected accordingly. For both years, soils were incubated under field-moist conditions, which was very dry in 2003 (2–5%) and met or exceeded 60% field capacity in 2004 (14–35%).
Net N mineralization (NO3–N+NH4–N) was determined by extracting 25 g of freshly sampled and day 28 soils from the above incubation in 50 ml of 2 M KCl on a shaker (200 rpm) for 1 h (Mulvaney 1996). Each sample was filtered through Whatman #2 filter paper and analyzed for NO3 − and NH4 + on a Perstorp Enviroflow flow injector (Perstorp). Net N mineralized was determined by subtracting the NO3–N + NH4–N concentration at the beginning of the incubation period from the concentration at the end of the period for each sample.
Biolog EcoPlate substrate utilization activity
To determine the degree to which the cultivable microbial communities from each plot utilize different types of C substrates, Biolog EcoPlates (Biolog, Inc., Hayward, CA) were employed according to the modified protocol of Choi and Dobbs (1999). Each plate contained 31 different carbon substrates and a control replicated three times. Substrates were primarily amino acids (n=6), carbohydrates (n=10), and carboxylic acids (n=7). Each well was inoculated with 150 μl of a 10−3 soil dilution prepared in sterile physiological saline (0.85% NaCl). The plates were maintained at 25°C in the dark, and the optical density (595 nm) was recorded for each well every 24 h until most wells had reached maximum color development using a microtitre plate reader (Model 680, BioRad Laboratories, Hercules, CA). In 2003, the plates were read at 24, 48, 72, 96, 120, and 168 h, and in 2004, the plates were read at 24, 48, 72, 96, 120, and 144 h.
Statistical analyses
All univariate data were analyzed by analysis of variance (α=0.05), specifically with the general linear model (GLM) procedure in SAS (SAS Institute 2002), and mean comparisons were performed using a Fisher-protected least significant difference (LSD).
Results
Soil water content and chemical properties
Despite a wet spring, soil samples taken in July 2003 were dry, and the moisture content was not significantly different among the treatments. Water contents ranged from 22.5 g kg−1 soil in control plots to 28.8 g kg−1 in plots amended with the highest application rate. In 2004, a drier year, plots were sampled shortly after a rain, and thus, soil samples were much wetter. Plots which had received at least 5 Mg biosolids ha−1 were significantly wetter (ranging from 203 to 225 g kg−1 soil) than nonamended control plots, which contained 159 g−1 water kg−1 soil (data not shown).
In 2003, biosolids application significantly affected many of the soil chemical properties in the 0–15 cm depth. The interaction between treatment rate and reapplication was not significant for concentrations of extractable Al, Ca, Cd, Cr, Fe, K, Mg, Na, Ni, P, Pb, Sr, Ti, V, Zn, pH, or total C. The main effects of treatment rate are shown in Table 2. Concentrations of Fe, Ni, P, Pb, Ti, Zn, and total C all increased significantly with increasing biosolids application rate, whereas pH decreased with increasing application rate. However, Mg and V levels peaked at the 5 Mg ha−1 treatment rate then fell to below the control level in the two highest rate treatments. Concentrations of Al, Ca, K, Na, and Sr did not change in response to biosolids application, and Cd and Cr were nondetectable in all plots in 2003. Interactions between rate and reapplication were significant in 2003 for concentrations of extractable Ba, Cu, Mn, Mo, EC, NO3–N, NH4–N, and total N (Table 3). In the short-term plots, Ba concentrations decreased significantly with increasing application rate; a similar trend was observed for the long-term plots, but the differences were not statistically significant. Concentrations of Cu, Mn, Mo, EC, NO3–N, NH4–N, and total N all increased significantly with an increasing application rate on the short-term plots, with similar trends in the long-terms plots. In the second year of this study, fewer soil chemical parameters were significantly affected by treatment level. Only pH, total C, and N, and extractable concentrations of Cd, Cu, Fe, K, Mg, Ni, P, and Pb exhibited significant differences among treatment levels (data not shown). Only soil EC and exchangeable NO3–N and NH4–N exhibited significant interactions between application rate and reapplication in 2004 (data not shown). There was no evidence of an accumulation of soil nutrients and elements at the lower depth (15–30 cm) (data not shown).
Microbial biovolumes
In both sampling years of this study, overall microbial biovolumes, as determined from summed enumerations of bacteria and fungi, yielded no significant differences among treatment rates or between short- and long-term plots (data not shown). Statistically significant differences among treatments were observed in both years only for total bacterial biovolumes as determined from direct microscopic enumerations (Table 4). In the second year of this study, interactions between rate and reapplication treatments were significant for total bacterial biovolumes. Total fungal biovolumes were approximately 100-fold greater than bacterial biovolumes and ranged from 9.52 to 9.67 μm3 g−1 soil in 2003, and from 9.49 to 9.71 μm3 g−1 in 2004, with no significant treatment effects observed.
Microbial C and N mineralization activities
Presumably due to extremely dry soils in 2003, both C and N mineralization in year 1 exhibited no statistically significant differences among treatment levels or between short-term and long-term study plots (data not shown). In the second year of this study, when field-moist soils were at least 60% of field capacity, C mineralization and nitrification were significantly affected by biosolids application in the short-term plots (Table 5). In short-term plots, significant increases in carbon mineralization activity were observed as biosolids application rate increased, with rates more than tripling from nonamended control plots to the 30 Mg ha−1 treatment plots. Overall net N mineralization (NH4–N + NO3–N) increased with an increasing application rate for both long-term and short-term plots. In long-term plots, activity nearly doubled in the 30 Mg ha−1 treatment compared to nonamended plots. Within short-term plots, net mineralizable N increased almost tenfold in soils of the two highest treatment rates.
Biolog substrate utilization activity
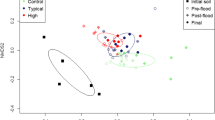
In 2003, all plates inoculated with biosolids-treated soils exhibited faster rates of well color development than the control soils, and the rate of average well color development among Biolog EcoPlates inoculated with soil microbial communities differed significantly by application rate on the fourth and fifth days of incubation (data not shown). Throughout incubation, the rate of substrate utilization increased most rapidly, and at significantly faster rates, for plates inoculated with the 10 Mg ha−1 treatment soils. On the first day of incubation in 2004, the plates inoculated with soil of the highest treatment plots (30 Mg ha−1) exhibited the greatest substrate utilization rates, and plates of the control soils exhibited the lowest substrate utilization rates, with few significant differences among the intermediate rates (Fig. 1). By the second day of incubation, and throughout the remainder of the experiment, the control soil continued to exhibit reduced substrate utilization rates compared to all other treatments except for the 5 Mg ha−1 treatment plates.
Utilization of Biolog substrates over time by microbial communities of nonamended and biosolids-amended soil as measured in 2004. Results are averaged across long-terms plots (biosolids applied once in 1991) and short-term plots (biosolids applied in 1991 and again in 2002). The AWCD is the average well color development (mean absorbance value at 595 nm) for all wells in a Biolog EcoPlate
Plant productivity and C/N ratio
In 2003 and 2004, the number of plant species significantly decreased with increasing biosolids application-rate treatment (Table 6). Plant biomass also increased significantly with increasing treatment rate in 2003; similar trends were observed in 2004, although differences were not statistically significant (Table 6). In 2003, significant increases in plant tissue N concentrations and reductions of the C/N ratio were observed with an increasing application rate (Table 7). Plant tissue N effectively doubled from control to the highest treatment on short-term plots, reducing the C/N ratio by half on those plots. Long-term plots also exhibited significant increases in plant tissue N, reducing the C/N ratio in plots treated with at least 10 Mg ha−1 biosolids. Plant tissue quality in 2004 exhibited similar patterns to those of 2003, with significantly lower C/N ratios in aboveground plant tissues from the 21 and 30 Mg ha−1 plots (17.2 and 15.7, respectively) compared to the control and 2.5 Mg ha−1 treatment (21.7 and 20.8, respectively).
Discussion
The city of Fort Collins currently applies biosolids at a rate of 2.5 Mg ha−1 to land within the Meadow Springs Ranch. Based on this study, this rate has not resulted in soil chemical values significantly greater than those of nonamended soil, suggesting that this rate may not lead to deleterious accumulations of metals in soil. As rates increased, however, levels of some nutrients and elements increased, consistent with other studies (Cuevas et al. 2000; Fresquez et al. 1991; Loftin et al. 1995), which found that concentrations of nutrients, metals, and micronutrients in soil increased as application rates increased. In this study, P concentrations increased more than sixfold from control to the highest application-rate plots in 2003. Excessive P loading in soil due to biosolids land application is one concern regarding land-based recycling programs (Brandt et al. 2004). The soil studied here is naturally enriched in AB-DTPA-extractable P, with levels in the nonamended plots falling into the high-risk category of the Colorado Phosphorus Index Risk Assessment (Sharkoff et al. 2003). The long-term effect of biosolids application was to increase extractable P to very high levels, as measured in 2003. In a study conducted on a similar soil, Ippolito and Barbarick (unpublished data) also measured high levels of AB-DTPA-extractable P, possibly due to chelation of Al from amorphous and crystalline AlPO4 by DTPA, which would increase soil solution P.
Fewer chemical properties were significantly affected by the biosolids application rate in 2004 compared to 2003. Within reapplied plots, the effects of reapplication were no longer significant for any soil properties, except EC and concentrations of NO3–N and NH4–N by the second year of the study (data not shown). Based on the data collected, it appears that a second application of biosolids did not exacerbate any long-term effects observed among soil chemical properties 12 years after a single biosolids application event. Furthermore, the effects of a second application within a given treatment rate were short-lived for most chemical parameters in this particular soil, with little effects remaining 2 years after reapplication. The general lack of soil contamination, other than P, may likely be due to the combination of low levels of metals and contaminants present originally in these biosolids, low rates of application, and infrequent application.
With application of biosolids, soil microbial biomass might be expected to increase in response to increased plant productivity and added C, N, and nutrients. On the other hand, a decrease in microbial biomass might be expected if biosolids contain toxic chemical concentrations. No change in total microbial biomass, as observed in this study, may suggest a rapidly acclimating soil microbial community (Porazinska et al. 2003), whose overall biomass was not affected by biosolids inputs, at least in the 0- to 15-cm depth of soil. Similar results were reported by García-Gil et al. (2004), who found no significant change in microbial biomass after 9 months in response to biosolids applied at rates similar to this study. However, we did detect a slight shift within the total biovolume pool toward greater bacterial-to-fungal ratios in plots amended with the highest rates of biosolids, as indicated by enhanced total bacterial biovolumes in plots receiving the highest application rates in 2003 and in the short-term 30 Mg ha−1 plots in 2004. Thus, it appeared that higher biosolids application rates had qualitative, but not quantitative, effects on microbial biovolumes.
In an earlier study conducted on the same plots, Barbarick et al. (2004) concluded that as long as 6 years after biosolids treatment, labile C and N remained in biosolids-treated plots and contributed to enhanced mineralization activity. Thirteen years after a single application, and 2 years after a repeated application, microbial C and N mineralization activities differed among treatments when water was not limiting to soil microorganisms. The Biolog assay also supports that mineralization activity (i.e., oxidation of C substrates) was enhanced in biosolids-amended plots for both 2003 and 2004, as the rates of color development, averaged across all wells of a given plate, generally increased with increasing biosolids application rate. Although the Biolog approach has its drawbacks, such as its cultural bias and requirements for strong growth of inoculum to register a positive signal (Schutter and Dick 2001; Konopka et al. 1998), it suggests, along with the C and N mineralization data, that the metabolism of at least some portion of the soil microbial community remains elevated years after biosolids land application.
In addition to soil moisture, substrate quality is fundamental to decomposition and mineralization activities in soil and is strongly dependent on the N and lignin contents of plant tissue (Porazinska et al. 2003). Microbial community structure and activities can be altered significantly by the quality and quantity of plant material entering the soil (Wardle and Lavelle 1997). Along with soil moisture, the C/N ratio of aboveground plant tissue will also likely affect microbial mineralization activities in biosolids-amended and control soil. In 2003, the plant C/N ratio in the three lowest treatment rates (0, 2.5, and 5 Mg ha−1) were well above the level at which N immobilization would be expected (25:1) (Myrold 2004). Consequently, what very little N that was mineralized (≤2.11 mg kg−1) in these plots in 2003 was likely already in the soil pool or came directly from biosolids additions, not enhanced from plant material that year. As biosolids application rate increased, plant C/N ratios were reduced by half in 2003, thus partially explaining the greater N mineralization activity observed in 2004 in plots which received higher application rates.
In conclusion, the effects of a single biosolids application on soil chemical properties can persist for many years. In this study, the concentration of some extractable elements in soil were affected even more so with a second application. This exacerbated effect was temporary, and no significant differences were observed between short- and long-term plots by the second sampling year except for soil EC and extractable inorganic N. Biosolids used in this study are low in many elements of environmental concern, and therefore present little ecological danger at low application rates based on the measured parameters here. The city of Fort Collins currently land applies biosolids at 2.5 Mg ha−1 to the majority of the Meadow Springs Ranch. Site management objectives are focused on improved forage quality for livestock grazing, and this study found no detrimental impacts of this rate on soil microbial community biomass or function or plant productivity. At the lower application rates, effects were often insignificant compared to control plots, and at higher applications rates, treatment effects proved to be long-term and somewhat complex. In this study, land application of biosolids to a semi-arid grassland soil enhanced microbial mineralization activities, plant productivity, and plant tissue quality when soil moisture levels were adequate.
References
Barbarick KA, Workman SM (1987) NH4–HCO3–DTPA and DTPA extractions of sludge-amended soils. J Environ Qual 16:125–130
Barbarick KA, Doxtader KG, Redente EF, Brobst RB (2004) Biosolids effects on microbial activity in shrubland and grassland soils. Soil Sci 169:176–187
Bloem J, Bolhuis PR, Veninga MR, Wieringa J (1995) Microscopic methods for counting bacteria and fungi in soil. In: Alef K, Nannipieri P (eds) Methods in applied soil microbiology and biochemistry. Academic, San Diego, CA, pp 162–173
Brandt RC, Elliott HA, O'Connor GA (2004) Water-extractable phosphorus in biosolids: implications for land-based recycling. Water Environ Res 76:121–129
Bremner JM (1996) Nitrogen—total. In: Sparks DL (ed) Methods of soil analysis, part 3. Chemical methods. SSSA, Madison, WI, pp 1085–1121
Choi K, Dobbs FC (1999) Comparison of two kinds of Biolog microplates (GN and ECO) in their ability to distinguish among aquatic microbial communities. J Microbiol Methods 36:203–213
Coleman DC, Crossley DA Jr (1996) Fundamentals of soil ecology. Academic, Amsterdam
Cuevas G, Blázquez R, Martinez F, Walter I (2000) Composted MSW effects on soil properties and native vegetation in a degraded semi-arid shrubland. Compost Sci Util 8:303–309
Dennis GL, Fresquez PR (1989) The soil microbial community in a sewage-sludge-amended semi-arid grassland. Biol Fertil Soils 7:310–317
Fresquez PR, Aguilar R, Francis RE, Aldon EF (1991) Heavy metal uptake by blue grama growing in a degraded semi-arid soil amended with sewage sludge. Water Air Soil Pollut 57/58:903–912
García-Gil JC, Plaza C, Senesi N, Brunetti G (2004) Effects of sewage sludge amendment on humic acids and microbiological properties of a semi-arid Mediterranean soil. Biol Fertil Soils 39:320–328
Harris-Pierce RL (1994) The effects of biosolids application on native rangeland soils and vegetation. MS Thesis, Colorado State University, Fort Collins
Klein DA, Paschke MW (2000) A soil microbial community structural–functional index: the microscopy-based total/active/active fungal/bacterial (TA/AFB) biovolumes ratio. Appl Soil Ecol 14:257–268
Konopka A, Oliver L, Turco RF Jr (1998) The use of carbon substrate utilization patterns in environmental and ecological microbiology. Microb Ecol 35:103–115
Lodge DJ, Ingham ER (1991) A comparison of agar film techniques for estimating fungal biovolumes in litter and soil. Agric Ecosyst Environ 34:131–144
Loftin SR, Aguilar R, Fresquez PR (1995) Rangeland restoration with surface-applied biosolids: effects on soils and vegetation of the Rio Puerco Watershed, Northcentral New Mexico. In: Proceedings: the effect of land application of biosolids in arid and semi-arid environments, Colorado State University, 16–19 May
Mulvaney RL (1996) Nitrogen-inorganic forms. In: Sparks DL (ed) Methods of soil analysis, part 3. Chemical methods. SSSA, Madison, WI, pp 1123–1184
Myrold D (2004) Transformations of nitrogen. In: Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DA (eds) Principles and applications of soil microbiology. Prentice Hall, Upper Saddle River, NJ, pp 333–372
National Oceanic and Atmospheric Administration (2002–2004) Annual climatological summary, station 053006/99999. NOAA, Asheville, NC. Available at http://www ncdc.noaa.gov/onlinestore.html. Cited 20 Dec 2004
Nelson DW, Sommers LE (1996) Total carbon, organic carbon, and organic matter. In: Sparks DL (ed) Methods of soil analysis, part 3. Chemical methods. SSSA, Madison, WI, pp 975–977
Pascual JA, García C, Hernandez T (1999) Lasting microbiological and biochemical effects of the addition of municipal solid waste to an arid soil. Biol Fertil Soils 30:1–6
Porazinska DL, Bardett RD, Blaauw MB, Hunt HW, Parsons AN, Seastedt TR, Wall DH (2003) Relationships at the aboveground–belowground interface: plants, soil biota, and soil processes. Ecol Monogr 73:377–395
Reynolds HL, Packer A, Bever JD, Clay K (2003) Grassroots ecology: plant–microbe–soil interactions as drivers of plant community structure and dynamics. Ecology 84:2281–2291
Rhoades JD (1996) Salinity: electrical conductivity and total dissolved solids. In: Sparks DL (ed) Methods of soil analysis, part 3. Chemical methods. SSSA, Madison, WI, pp 417–435
SAS Institute, Inc. (2002) SAS/STAT user's guide, version 9.1. The SAS Institute, Cary, NC
Schutter M, Dick R (2001) Shifts in substrate utilization potential and structure of soil microbial communities in response to carbon substrates. Soil Biol Biochem 33:1481–1491
Sharkoff JL, Waskom RM, Davis JG (2003) Colorado phosphorus index risk assessment. Colorado agronomy technical note no. 95. USDA-NRCS, Lakewood, CO
Soil Conservation Service and Forest Service (1980) Soil survey of Larimer country area, CO. USDA, Lakewood, CO
Soltanpour PN, Johnson GW, Workman SM, Jones JB Jr, Miller RO (1996) Inductively coupled plasma emission spectrometry and inductively coupled plasma-mass spectrometry. In: Sparks DL (ed) Methods of soil analysis, part 3. Chemical methods. SSSA, Madison, WI, pp 91–139
Thomas GW (1996) Soil pH and soil acidity. In: Sparks DL (ed) Methods of soil analysis, part 3. Chemical methods. SSSA, Madison, WI, pp 475–490
US Environmental Protection Agency (1991) Interagency policy on beneficial use of municipal sewage sludge on federal land; notice. Fed Regist 56(127):30448–30450
US Environmental Protection Agency (1993) 40 CFR-Parts 257 and 503. Standards for the use and disposal of sewage sludge; final rule. Fed Regist 58(32):9248–9415
US Environmental Protection Agency Region 8 (1999) Biosolids management handbook, section 2. Available at http://www.epa.gov/region8/water/wastewater/biohome/biosolidsdown/handbook/handbook.html. Cited 11 May 2005
Wardle DA, Lavelle P (1997) Linkages between soil biota, plant litter quality and decomposition. In: Cadisch G, Giller KE (eds) Driven by nature—plant litter quality and decomposition. CAB International, Wallingford, UK, pp 107–124
Weinbauer MG, Backmann C, Höfle MG (1998) Utility of green fluorescent nucleic acid dyes and aluminum oxide membrane filters for rapid epifluorescence enumeration of soil and sediment bacteria. Appl Environ Microbiol 64:5000–5003
Acknowledgements
This research was supported by grant no. CP988640-01 from the Environmental Protection Agency, Region 8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sullivan, T.S., Stromberger, M.E., Paschke, M.W. et al. Long-term impacts of infrequent biosolids applications on chemical and microbial properties of a semi-arid rangeland soil. Biol Fertil Soils 42, 258–266 (2006). https://doi.org/10.1007/s00374-005-0023-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-005-0023-z