Abstract
This study was carried out in a semiarid degraded area to assess the effectiveness of mycorrhizal inoculation with a mixture of native arbuscular mycorrhizal (AM) fungi or an allochthonous AM fungus (Glomus claroideum), on the establishment of Olea europaea subsp. sylvestris L. and Retama sphaerocarpa (L.) Boissier in this area. Associated changes in the soil microbiological properties and aggregate stability related to these AM inocula were also recorded. Eighteen months after planting, G. claroideum had increased available P in the rhizosphere of both shrub species. In general, both inoculation treatments increased water-soluble C and water-soluble and total carbohydrates, G. claroideum being the most effective inoculum, particularly in R. sphaerocarpa. The mixture of native AM fungi was the most effective treatment for increasing the aggregate stability of R. sphaerocarpa soil, while that of O. europaea was increased only by G. claroideum. Increased (dehydrogenase, urease, protease-BAA, acid phosphatase and β-glucosidase) enzyme activities, in particular of dehydrogenase and acid phosphatase, were recorded in the rhizosphere of both mycorrhizal shrub species. The mixture of native AM fungi was the most effective treatment for stimulating the growth of O. europaea and R. sphaerocarpa (11.6-fold and 3.3-fold, respectively, greater than control plants). The establishment of mycorrhizal shrub species favoured the reactivation of soil microbial activity, which was linked to an increase in aggregate stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Mediterranean area of southeast Spain is one of those most threatened by desertification processes due to scarce and irregular rainfall and a long, dry and hot summer. Under these environmental conditions, the loss of plant cover can cause a decrease in soil structure (with an increase in soil erosion), microbial activity, contents of available plant nutrients, and organic matter in soils (Caravaca et al. 2003c).
The establishment of autochthonous plant species is a widely used practice for reclaiming degraded lands in Mediterranean semiarid and arid areas, for restoring the biodiversity of these eroded areas and preventing the processes of erosion and desertification, in accordance with the agricultural policies of the European Union (Requena et al. 2001). In this context, we have used Olea europaea L. subsp. sylvestris and Retama sphaerocarpa (L.) Boissier, which are low-growing shrubs, well-adapted to water stress conditions, and which belong to the natural succession in certain plant communities of semiarid Mediterranean ecosystems in the southeast of Spain (Barea et al. 1992). Moreover, R. sphaerocarpa has the additional benefit of being able to fix nitrogen, thus constituting an N input into the ecosystem (Caravaca et al. 2003a).
In reafforestation programmes, inoculation of plants with microsymbionts, such as mycorrhizal fungi, helps plant establishment (Herrera et al. 1993) and can improve the physical, chemical and biological properties of soil (Carrillo-García et al. 1999). There is evidence that mycorrhizae help plants to thrive in arid conditions (Caravaca et al. 2003b) by increasing the supply of nutrients, such as P (Smith and Read 1997), improving soil aggregation in eroded soils (Caravaca et al. 2002) and reducing water stress (Augé 2001).
In these desertification-threatened areas, the native inoculum potential of AM fungi may disappear or, at least, be severely depleted and so it may be necessary to reinforce or replace it by appropriate inoculation (Azcón-Aguilar et al. 2003). The selection of efficient AM fungi is a key prerequisite in inoculation programmes, since there are different levels of compatibility between host plants and AM fungi (Roldán et al. 1992; Smith and Read 1997) and the effectiveness of AM fungi depends on the plant species inoculated (Caravaca et al. 2003b). The use of native mycorrhizal potential may be considered a preferential inoculation strategy to guarantee the successful re-establishment of shrub species in degraded soil (Caravaca et al. 2003b), since native AM fungi are presumably physiologically and genetically adapted to the whole environment of the desertified ecosystems.
There is evidence that mycorrhizas affect the growth, composition and activity of microbial communities by altering root exudation (Wamberg et al. 2003). To date, it is not known whether different strains of AM fungi can produce different effects on the biochemical properties of soil, such as enzyme activities, or which AM fungi, native or allochthonous, are more effective. These facts should be very important when planning reafforestation programmes, since a more effective inoculum to improve the microbial properties of soil may not only be decisive for plant development but also for creating the soil qualities that favour the spontaneous appearance of other autochthonous shrub species.
The objectives of this study were: (1) to determine which type of mycorrhizal inoculation, native or allochthonous AM fungi, is more effective in shrub plant establishment in degraded zones; and (2) to assess the changes in the soil microbiological properties and aggregate stability related to these AM inocula and to ascertain which mycorrhizal treatments may be more effective in improving them.
Materials and methods
Study sites
The experimental area was located on the El Picarcho range in the Province of Murcia (southeast Spain: 1°10′W and 38°23′N). The climate is semiarid Mediterranean, with an annual rainfall of 315 mm and a mean annual temperature of 20°C during the experiment. The topography of the area is mainly flat and slopes do not exceed 6%. The climax vegetation was dominated by shrubs of O. europaea subsp. sylvestris and R. sphaerocarpa, which were selected as target species. The plant cover is sparse (less than 20% canopy cover) and degraded due to ancient grazing and logging. Nowadays, dwarf shrubs (<1 m high) such as Rosmarinus officinalis and Stipa tenacissima grass are very common, constituting more than 98% of plant cover. Bare soil surfaces are abundant between the patches of plants. The soil is a Petrocalcic Xerosol (FAO 1998), developed from limestones, with a silt loam texture. Some characteristics of the soil are shown in Table 1.
Plants and mycorrhizal treatments
The plants used, O. europaea subsp. sylvestris and R. sphaerocarpa, are two representative shrub species from semiarid scrublands in southeast Spain. They are also well-adapted to water stress conditions and, therefore, frequently used in the revegetation of semiarid disturbed lands.
The mycorrhizal fungi used were either Glomus claroideum Schenck & Smith (EEZ 24) or a mixture of endophytes isolated from Cieza (SE Spain), a semiarid area where the target plants naturally grow: the fungal mixture consisted of Glomus geosporum (Nicol. & Gerd.) Walker (EEZ 31), Glomus albidum Walker & Rhodes (EEZ 39), Glomus microaggregatum Koske, Gemma & Olexia (EEZ 40), Glomus constrictum Trappe (EEZ 42), Glomus mosseae (Nicol. & Gerd.) Gerd. & Trappe (EEZ 43), Glomus coronatum Giovannetti (EEZ 44), Glomus intraradices Schenck & Smith (EEZ 45) and a Glomus sp. (EEZ 46). The acronym EEZ refers to Estación Experimental Zaidín, Granada (Spain).
Arbuscular mycorrhizal fungal inoculum consisted of a mixture of rhizospheric soil from trap cultures (Sorghum sp.) containing spores, hyphae and mycorrhizal root fragments. Once germinated, seedlings were transplanted into the growth substrate, consisting of peat and cocopeat (1:1, v/v). The corresponding arbuscular mycorrhizal inoculum was applied at a rate of 5% (v/v). The same amount of an autoclaved mixture of the inocula was added to control plants, supplemented with a filtrate (<20 μm) of culture to provide the microbial populations accompanying the mycorrhizal fungi. Inoculated and non-inoculated seedlings were grown for 8 months under nursery conditions without any fertiliser treatment.
Experimental design and layout
The experiment was conducted as two independent one-factor factorials (one per plant species) with five replication blocks. The factor had three levels: non-inoculation, inoculation with G. claroideum and inoculation with the mixture of native AM fungi. In early November 2000, an area of 1,200 m2 was mechanically prepared with a subsoiler. Three rows (1 m wide, 25 m long, 3 m apart) were established. Seedlings of the two selected shrub species (inoculated and non-inoculated) were planted in individual holes, at least 1 m apart in a single row and with 3 m between blocks. At least 15 seedlings per factor level per replication block of each shrub species were planted (225 plants per shrub species). The experiment was carried out under strictly natural conditions, without any watering or fertiliser treatments.
Sampling procedures
Every 6 months after planting, five rhizosphere soil samples (defined as soil strongly adhering to roots and collected at 0–4 mm from the root surface) of each treatment were collected (1 per block, 15 soil samples in total per plant species). Each sample consisted of five bulked subsamples (200 cm3 soil cores), randomly collected at 0- to 20-cm depth in the rhizospheres of five individual plants. Each root system was extracted by excavating manually a hole 40 cm wide, 40 cm long and 20 cm deep. To collect the rhizosphere soil the root system with rhizosphere soil adhered was introduced into a plastic bag, shaken and the rhizosphere soil separated from the root system. Every 3 months after planting, five plants (one per block) of each treatment were also harvested.
Soil physical-chemical, chemical and biochemical analyses
Total N was determined by the Kjeldahl method, and the total organic C according to Yeomans and Bremner (1988). Available P, extracted with sodium bicarbonate, was determined by colorimetry, according to Murphy and Riley (1962). Extractable (with ammonium acetate) K was determined by flame photometry.
In a soil (1:5, w/v) aqueous extract, water-soluble C was determined by wet oxidation with K2Cr2O7 and measurement of the absorbance at 590 nm (Sims and Haby 1971). Water-soluble carbohydrates and total carbohydrates were determined as reported by Brink et al. (1960).
Dehydrogenase activity was determined according to Benefield et al. (1977). Briefly soil (1 g) at 60% of its field capacity was treated with 0.2 ml 0.4% INT (2-p-iodophenyl-3-p-nitrophenyl-5-phenyltetrazolium chloride) for 20 h at 22°C in the dark. The INTF (iodo-nitrotetrazolium formazan) formed was extracted with 10 ml methanol by shaking vigorously for 1 min and filtering through Whatman no. 5 filter paper; INTF was measured spectrophotometrically at 490 nm.
Urease and N-(-benzoyl-l-argininamide (BAA) hydrolysing activities were determined in 0.1 M phosphate buffer at pH 7; 1 M urea (Tabatabai and Bremner 1972) and 0.03 M BAA (Ladd and Butler 1972) were used as substrates, respectively. Two millilitre buffer and 0.5 ml substrate were added to 0.5 g sample, which was incubated at 30°C (for urease) or 39°C (for protease) for 90 min. Both activities were determined as the NH4+ released in the hydrolysis reaction.
Acid phosphatase activity was determined using p-nitrophenyl phosphate disodium (PNPP, 0.115 M) as substrate. Two millilitre 0.5 M sodium acetate buffer at pH 5.5 (Tabatabai and Bremner 1969) and 0.5 ml substrate were added to 0.5 g soil and incubated at 37°C for 90 min. The reaction was stopped by cooling at 2°C for 15 min. Then, 0.5 ml 0.5 M CaCl2 and 2 ml 0.5 M NaOH were added, and the mixture was centrifuged at 2,287g for 5 min. The p-nitrophenol (PNP) formed was determined by spectrophotometry at 398 nm. Controls were made in the same way, although the substrate was added before the CaCl2 and NaOH.
β-Glucosidase was determined using p-nitrophenyl-β-d-glucopyranoside (PNG, 0.05 M) as substrate. This assay is based on the release and detection of PNP. Two millilitre 0.1 M maleate buffer pH 6.5 and 0.5 ml substrate were added to 0.5 g soil and incubated at 37°C for 90 min. The reaction was stopped by adding 0.1 M Tris-hydroxymethyl aminomethane (THAM) pH 12.0 according to Tabatabai (1982). The amount of PNP was determined at 398 nm (Tabatabai and Bremner 1969).
Physical analysis
The percentage of stable aggregates was determined by the method described by Lax et al. (1994). Sieved (0.2–4 mm) soil (4 g) was placed on a small 0.250-mm sieve and wetted by spray. After 15 min the soil was subjected to an artificial rainfall of 150 ml with energy of 270 Jm−2. The remaining soil on the sieve was placed in a previously weighed capsule (T), dried at 105°C and weighed (P1). Then, the soil was soaked in distilled water and, after 2 h, passed through the same 0.250-mm sieve with the assistance of a small stick to break the remaining aggregates. The residue remaining on the sieve, which was made up of plant debris and sand particles, was dried at 105°C and weighed (P2). The percentage of stable aggregates with regard to the total aggregates was calculated by (P1− P2)×100/(4− P2+ T).
Percentage of colonised root and growth parameters
For mycorrhizal assays three subsamples from the upper, middle and lower root system were taken. Sampling was based on root colour and morphology to get a mixed age sample and avoiding woody roots. The percentage of root length colonised by AM fungi was calculated by the gridline intersect method (Giovannetti and Mosse 1980) after staining with trypan blue (Phillips and Hayman 1970). Fresh and dry (105°C, 5 h) weights of shoots and roots, basal stem diameters and heights of the seedlings were measured at the end of the nursery period and every 3 months after planting.
Statistical analysis
Mycorrhizal inoculation effects on measured variables were tested by a one-way analysis of variance, and comparisons among means were made using the least significant difference (LSD) test, calculated at P <0.05. Correlation analysis between all the soil parameters measured was carried out using Pearson’s rank correlation coefficients. Statistical procedures were carried out with the software package SPSS 11.0 for Windows.
Results
Changes in soil nutrient properties
At the end of the growth period, the treatment with G. claroideum was very effective for increasing the concentration of available P in the rhizosphere soil of O. europaea and R. sphaerocarpa (by about 62% and 42%, respectively, with respect to control plants). Inoculation with the mixture of native AM fungi had no significant effect on the nutrient levels (total N, available P and extractable K) of the rhizosphere soil of the two shrub species.
Changes in plant growth
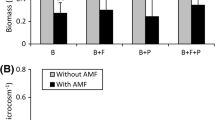
Eighteen months after planting, plant survival was about 90% for all treatments and plant species. At the time of planting, shoot and root dry weights of the native AM fungi-colonised or G. claroideum-colonised O. europaea plants were slightly greater than non-inoculated plants (Fig. 1). At the end of the first year of growth in the field, the O. europaea plants inoculated with G. claroideum or the mixture of native AM fungi had a greater shoot and root biomass than non-inoculated plants (without significant differences between mycorrhizal treatments). Eighteen months after planting, inoculation with the mixture of native AM fungi or with G. claroideum increased the shoot dry weight of O. europaea plants about 11.6-fold and 4.7-fold, respectively, in comparison with the control plants (Fig. 1).
At the time of planting, there were no significant differences in growth between non-inoculated and inoculated R. sphaerocarpa seedlings. The mixture of native AM fungi was more effective in increasing root and shoot dry weight than G. claroideum during the spring growth period (from 3 to 6 months after planting). These differences in seedling growth between mycorrhizal inoculation treatments decreased during the summer growth period, so that the effects of inoculation with G. claroideum or with the mixture of native AM fungi on R. sphaerocarpa growth were generally similar at 9 months. After 18 months, shoot dry weight of R. sphaerocarpa seedlings inoculated with the mixed native AM fungi was 226% and 88% greater than that of the non-inoculated seedlings and the G. claroideum-colonised seedlings, respectively. Similar results were obtained for root dry weight of R. sphaerocarpa plants (Fig. 1).
Changes in aggregate stability, total organic C, C fractions and root colonisation
Six and 18 months after planting, the inoculation with G. claroideum significantly increased the aggregate stability in the rhizosphere soil of O. europaea; however, the treatment with the mixture of native AM fungi was more effective in increasing the aggregate stability of the rhizosphere soil of R. sphaerocarpa seedlings 18 months after planting (Table 2).
At 18 months after planting, the water-soluble C content of the rhizosphere soil of O. europaea was only increased by G. claroideum treatment and that of the rhizosphere soil of R. sphaerocarpa by both mycorrhizal treatments. At the end of the growth period, both fungal treatments significantly increased the water-soluble carbohydrates content of the rhizosphere soils of O. europaea and R. sphaerocarpa seedlings; G. claroideum was more effective in increasing this C fraction than the mixture of native AM fungi. From 6 to 12 months after planting, both mycorrhizal treatments significantly enhanced the total carbohydrate content of the rhizosphere soil of O. europaea seedlings, without significant differences between the two treatments; however, at the end of the growth period, only G. claroideum treatment increased, by about 78%, the total carbohydrate content with respect to the control soil. The mixture of native AM fungi or G. claroideum inoculum enhanced this C fraction in the rhizosphere soil of R. sphaerocarpa seedlings 18 months after planting (Table 2). The content of total organic C of the rhizosphere soil of O. europaea seedlings was only increased by G. claroideum at the end of the growth period.
Six months after planting and at the end of the growth period, both inoculation treatments produced a similar level of root colonisation in O. europaea and R. sphaerocarpa seedlings (around 80% at the end of the growth period). The natural colonisation observed in the non-inoculated plants was similar in both shrub species (around 10% at the end of the growth period; Table 2).
Changes in soil biochemical properties
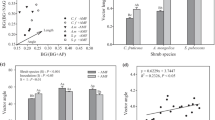
Six months after planting, urease, β-glucosidase, dehydrogenase and BAA-hydrolysing activities of the rhizosphere soil of O. europaea were not significantly affected by either mycorrhizal inoculation treatments. One year after planting, inoculation with G. claroideum or the mixture of native AM fungi stimulated all enzyme activities of the rhizosphere soil. The highest increase in enzyme activities due to both inoculation treatments were observed after 18 months, with the highest increase observed for acid phosphatase activity. At the end of the growth period, acid phosphatase or dehydrogenase activities showed no significant differences between the two mycorrhizal inoculation treatments (Fig. 2).
Changes in the biochemical properties of the rhizosphere of Olea europaea subsp. sylvestris, in response to inoculation with the allochthonous AM fungus G. claroideum or with a mixture of native AM fungi, during an 18-month growth period. For each enzyme activity and sampling, values sharing the same letter are not significantly different (P <0.05), according to the LSD test
Six months after planting, inoculation with G. claroideum increased significantly the urease activity of the rhizosphere soil of R. sphaerocarpa. Urease activity was stimulated by inoculation with G. claroideum or the mixture of native species, 1 year after planting and at the end of the growth period, without significant differences between the fungal treatments. One year after planting, the mixture of native AM fungi was more effective in increasing acid phosphatase and dehydrogenase activities of the rhizosphere soil of R. sphaerocarpa than the G. claroideum treatment. Eighteen months after planting, the two mycorrhizal treatments increased similarly all enzyme activities of the rhizosphere soil of R. sphaerocarpa seedlings, except that inoculation with G. claroideum was more effective in increasing the acid phosphatase activity (about 276% greater with respect to the control soil; Fig. 3).
Changes in the biochemical properties of the rhizosphere of Retama sphaerocarpa, in response to inoculation with the allochthonous AM fungus Glomus claroideum or with a mixture of native AM fungi, during an 18-month growth period. For each enzyme activity and sampling, values sharing the same letter are not significantly different (P <0.05), according to the LSD test
Discussion
This study confirms the key role of mycorrhizae in sustaining the plant cover in soils that are deficient in nutrients (particularly in N and P), as well as showing the necessity of including mycorrhizal inoculation to guarantee plant performance in revegetation programmes for degraded areas where the mycorrhizal inoculum potential is low. This fact is supported by the low effectivity of natural colonisation for increasing the growth of non-inoculated plants. Moreover, the growth of both shrub species (O. europaea and R. sphaerocarpa) was correlated significantly (P <0.01) with the level of colonisation by AM fungi in their roots. The mycorrhizal inoculation treatments showed different levels of effectiveness in improving the performance of the two shrub species. The mixture of native AM fungi was more effective in increasing shoot dry weight of O. europaea and R. sphaerocarpa plants than was G. claroideum, despite the fact that the percentage of root colonisation was similar with the two inoculation treatments. Our results also indicated that the inoculation treatment with the greater number of AM fungal taxa, and, therefore, with the higher AM fungal biodiversity, resulted in higher productivity and biomass yield.
Arbuscular mycorrhizal fungi can interact with other rhizosphere microorganisms (Jeffries et al. 2003) and can affect rhizodeposition and thus the quantity and quality of organic C delivered to the soil via fungal hyphae (Marschner et al. 1997). Indeed, the concentrations of water-soluble C, total and water-soluble carbohydrates were higher in the rhizosphere soil of both shrub species inoculated with AM fungi. It is worth noting that the percentage of colonised root was significantly correlated to these labile C fractions in the rhizosphere soil of O. europaea and R. sphaerocarpa. The higher release of carbohydrates into the rhizosphere of mycorrhizal plants probably affected the composition, activity and size of the rhizosphere soil microflora (Wamberg et al. 2003). In both shrub species, the increase of microbial activity did not depend on the assayed mycorrhizal inoculation treatment. Mycorrhiza-inoculated R. sphaerocarpa was the most effective at increasing dehydrogenase activity (by about 34% with respect to the control). Dehydrogenase activity responded to the treatments in a similar manner to the water-soluble C fractions, i.e. increasing with the mycorrhizal inoculation treatments. A positive correlation between the soluble C fractions and microbial activity exists in soil (Ghani et al. 2003). Water-soluble C, as a component of the labile C pool, may also be sensitive to perturbation and stress in soil-plant ecosystems (Doran and Parkins 1994) and, therefore, it could be used as a sensitive indicator of soil quality. Increased microbiological activity was also revealed by the variations in urease, acid phosphatase, β-glucosidase and BAA-hydrolysing activities. The measurement of these hydrolase activities can provide an early indication of changes in soil fertility, since they are related to the mineralisation of such important nutrient elements as N, P and C (Ceccanti et al. 1994). Enzyme activities also are sufficiently sensitive to indicate perturbations caused by microbial inoculation (Naseby and Lynch 1997). The increases observed in urease, β-glucosidase and BAA-hydrolysing activities may be related mainly to increase of the rhizosphere microbial population as a consequence of the inoculation treatments. To our knowledge, there is no evidence regarding the secretion of urease, β-glucosidase and proteases enzymes by AM fungi. In contrast, increased acid phosphate activity in the rhizosphere of mycorrhizal plants may be due to a direct fungal secretion or an induced secretion by the plant roots, as pointed out by Joner et al. (2000). Phosphatases are enzymes with a relatively broad specificity, capable of hydrolysing various organic phosphate esters, and are involved in the P cycle. The highest increase in phosphatase activity was recorded in the rhizosphere soil of mycorrhizal R. sphaerocarpa, colonised by G. claroideum. The fact that the highest concentrations of available P occurred in the rhizosphere of both shrub species inoculated with G. claroideum may be due to the hydrolysis of organic P compounds catalysed by extracellular fungal phosphatase activities. However, the quantitative contribution of extracellular enzymes to the P nutrition of AM plants is considered to be insignificant (Joner et al. 2000).
Soil structure and other soil properties affect soil quality and fertility, which favour the establishment and viability of a stable plant cover. Indeed, R. sphaerocarpa yield parameters and soil aggregate stability were significantly (P <0.001) correlated. The present study confirms the influence of mycorrhizal inoculation treatments on soil aggregate stability. The mixture of native AM fungi was more effective in increasing the aggregate stability of the rhizosphere soil of R. sphaerocarpa, while that of O. europaea was increased only by G. claroideum. The mechanisms involved in aggregate stabilisation are based on the enmeshment of soil particles by hyphae and roots, and the exudation of polysaccharides (Bearden and Petersen 2000). The water-soluble C fraction is also regarded as one of the key labile components of organic matter responsible for soil aggregation (Puget et al. 1999). The levels of both soil total carbohydrates and water-soluble C were significantly correlated to the percentage stable aggregate in rhizosphere soil of O. europaea (P <0.001). The increased levels of stable aggregates resulting from mycorrhizal inoculation treatments can also be attributed to the proliferation of fungal hyphae in the rhizosphere soil (Roldán et al. 1994; Jeffries and Barea 2000). According to Roldán et al. (1994), the binding effect of roots and hyphae is long-lived, while that of polysaccharides is transient because they are decomposed rapidly by microbes. On the other hand, the fact that the highest microbial activity was in the rhizosphere soil of both mycorrhizal shrub species might be due to high levels of stable aggregates, which protect the organic fraction (on which extracellular enzymes and soil microorganisms are immobilised) from microbial degradation (Nannipieri 1994).
Facilitative interactions between plants have been identified as one of the main processes affecting the composition of vegetation cover in arid and semiarid environments (Maestre et al. 2003). It has been reported that R. sphaerocarpa plants facilitate the introduction of annual and perennial species through self-promoting changes in microclimate and soil fertility, thus acting as “nurse plants” (Moro et al. 1997). We have recently shown that within the relict natural vegetation currently growing in patches in the target ecosystem, the rhizosphere soil of R. sphaerocarpa showed a higher microbial activity than that of the rhizosphere soil of O. europaea (Caravaca et al. 2003c). The improvement in soil quality due to the increase in microbial activity and aggregate stability in the rhizosphere of O. europaea and R. sphaerocarpa plants inoculated with AM fungi can contribute to the nurse role of these species, thus benefiting plant-plant interactions. In summary, AM fungi can enhance the growth of native shrub species both directly, through improved nutrient assimilation or water supply, and indirectly, by favouring the development of rhizosphere microorganisms and their activities, which lead to an improvement of aggregate stability and an acceleration of nutrient cycles.
In conclusion, mycorrhizal inoculation with the mixture of native AM fungi was the more effective treatment for stimulating the growth of the shrub species. The establishment of mycorrhizal shrub species favoured the reactivation of soil microbial activity, which was linked to an increase in aggregate stability. Finally, the improved soil physical and microbiological quality could facilitate the establishment and development of new plants in the surrounding area, which would aid the revegetation of semiarid ecosystems.
References
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Azcón-Aguilar C, Palenzuela J, Roldán A, Bautista S, Vallejo R, Barea JM (2003) Analysis of the mycorrhizal potential in the rhizosphere of representative plant species from desertification-threatened Mediterranean shrublands. Appl Soil Ecol 22:29–37
Barea JM, Azcón R, Azcón-Aguilar C (1992) Vesicular-arbuscular mycorrhizal fungi in nitrogen-fixing systems. Method Microbiol 24:391–416
Bearden BN, Petersen L (2000) Influence of arbuscular mycorrhizal fungi on soil structure and aggregate stability of vertisols. Plant Soil 218:173–183
Benefield CB, Howard PJA, Howard DM (1977) The estimation of dehydrogenase activity in soil. Soil Biol Biochem 9:67–70
Brink RH, Dubar P, Lynch DL (1960) Measurement of carbohydrates in soil hydrolysates with anthrone. Soil Sci 89:157–166
Caravaca F, Barea JM, Figueroa D, Roldán A (2002) Assessing the effectiveness of mycorrhizal inoculation and soil compost addition for reafforestation with Olea europaea subsp. sylvestris through changes in soil biological and physical parameters. Appl Soil Ecol 20:107–118
Caravaca F, Alguacil MM, Figueroa D, Barea JM, Roldán A (2003a) Re-establishment of Retama sphaerocarpa as a target species for reclamation of soil physical and biological properties in a semi-arid Mediterranean area. For Ecol Manage 182:49–58
Caravaca F, Barea JM, Palenzuela J, Figueroa D, Alguacil MM, Roldán A (2003b) Establishment of shrub species in a degraded semiarid site after inoculation with native or allochthonous arbuscular mycorrhizal fungi. Appl Soil Ecol 22:103–111
Caravaca F, Figueroa D, Barea JM, Azcón-Aguilar C, Palenzuela J, Roldán A (2003c) The role of relict vegetation in maintaining physical, chemical and biological properties in an abandoned Stipa-grass agroecosystem. Arid Land Res Manage 17:103–111
Carrillo-García A, Luz JL, Bashan Y, Bethlenfalvay GJ (1999) Nurse plants, mycorrhizae, and plant establishment in a disturbed area of the Sonoran desert. Restor Ecol 7:321–335
Ceccanti B, Pezzarossa B, Gallardo-Lancho FJ, Masciandaro G (1994) Bio-tests as markers of soil utilization and fertility. Geomicrobiol J 11:309–316
Doran JW, Parkins TB (1994) Defining and assessing soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA (eds) Defining soil quality for a sustainable environment. Soil Science Society of America, Madison, Wis., pp 3–21
FAO (1998) Soil map of the world: revised legend. Food Agriculture Organization of the United Nations, p 119
Ghani A, Dexter M, Perrott KW (2003) Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol Biochem 35:1231–1243
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol 84:489–499
Herrera MA, Salamanca CP, Barea JM (1993) Inoculation of woody legumes with selected arbuscular mycorrhizal fungi and rhizobia to recover desertified Mediterranean ecosystems. Appl Environ Microbiol 59:129–133
Jeffries P, Barea JM (2000) Arbuscular mycorrhiza—a key component of sustainable plant-soil ecosystems. In: Hock B (ed) The Mycota. IX. Fungal associations. Springer, Berlin Heidelberg New York, pp 95–113
Jeffries P, Gianinazzi S, Perotto S, Turnau K, Barea JM (2003) The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol Fertil Soils 37:1–16
Joner EJ, van Aarle IM, Vosatka M (2000) Phosphatase activity of extra-radical arbuscular mycorrhizal hyphae: a review. Plant Soil 226:199–210
Ladd JN, Butler JH (1972) Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivative as substrates. Soil Biol Biochem 4:19–30
Lax A, Díaz E, Castillo V, Albaladejo J (1994) Reclamation of physical and chemical properties of a salinized soil by organic amendment. Arid Soil Res Rehabilit 8:9–17
Maestre FT, Cortina J, Bautista S, Bellot J (2003) Does Pinus halepensis facilitate the establishment of shrubs in Mediterranean semi-arid afforestation? For Ecol Manage 176:147–160
Marschner P, Crowley DE, Higashi M (1997) Root exudation and physiological status of a root-colonizing fluorescent pseudomonad in mycorrhizal and non-mycorrhizal pepper (Capsicum annuum L). Plant Soil 189:11–20
Moro MJ, Pugnaire FI, Haase P, Puigdefábregas J (1997) Effect of the canopy of Retama sphaerocarpa on its understorey in a semiarid environment. Funct Ecol 11:425–431
Murphy J, Riley JP (1962) A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nannipieri P (1994) The potential use of soil enzymes as indicators of productivity, sustainability and pollution. In: Pankhurst CE, Doube BM, Gupta VVSR, Grace PR (eds) Soil biota: management in sustainable farming systems. CSIRO, Australia, pp 238–244
Naseby DC, Lynch JM (1997) Rhizosphere soil enzymes as indicators of perturbation caused by a genetically modified strain of Pseudomonas fluorescens on wheat seed. Soil Biol Biochem 29:1353–1362
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–161
Puget P, Angers DA, Chenu C (1999) Nature of carbohydrates associated with water-stable aggregates of two cultivated soils. Soil Biol Biochem 31:55–63
Requena N, Pérez-Solis E, Azcón-Aguilar C, Jeffries P, Barea JM (2001) Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Appl Environ Microbiol 67:495–498
Roldán A, Díaz G, Albaladejo J (1992) Effect of VAM-fungal inoculation on growth and phosphorus uptake of two Hedysarum species in a Xeric Torriorthent soil from southeast Spain. Arid Soil Res Rehabilit 6:33–39
Roldán A, García-Orenes F, Lax A (1994) An incubation experiment to determine factors involving aggregation changes in an arid soil receiving urban refuse. Soil Biol Biochem 26:1699–1707
Sims JR, Haby VA (1971) Simplified colorimetric determination of soil organic matter. Soil Sci 112:137–141
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic, San Diego
Tabatabai MA (1982) Soil enzymes. In: Page AL, Miller EM, Keeney DR (eds) Methods of soil analysis. American Society of Agronomy and Soil Science Society of America, Madison, Wis., pp 501–538
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenol phosphate in assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tabatabai MA, Bremner JM (1972) Assay of urease activity in soil. Soil Biol Biochem 30:1333–1341
Wamberg C, Christensen S, Jakobsen I, Müller AK, Sørensen SJ (2003) The mycorrhizal fungus (Glomus intraradices) affects microbial activity in the rhizosphere of pea plants (Pisum sativum). Soil Biol Biochem 35:1349–1357
Yeomans JC, Bremner JM (1988) A rapid and precise method for routine determination of organic carbon in soil. Commun Soil Sci Plant Anal 19:1467–1476
Acknowledgements
This research was supported by the EC + CICYT co-financed FEDER programme (1FD97-0507 FOREST). We acknowledge the technical support of Paisajes del Sur and TRAGSA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alguacil, M.M., Caravaca, F. & Roldán, A. Changes in rhizosphere microbial activity mediated by native or allochthonous AM fungi in the reafforestation of a Mediterranean degraded environment. Biol Fertil Soils 41, 59–68 (2005). https://doi.org/10.1007/s00374-004-0788-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-004-0788-5