Abstract
A field study was conducted to investigate the long-term effect of surface application of sewage sludge composts vs chemical N fertilizer on total N, total C, soluble organic C, pH, EC, microbial biomass C and N, protease activity, deaminase activity, urease activity, gross and net rates of N mineralization and nitrification, CO2 evolution, and N2O production. Soil samples were taken from five depths (0–15, 15–20, 20–30, 30–40, and 40–50 cm) of a long-term experiment at the University of Tokyo, Japan. Three fields have been receiving sewage sludge composted with rice husk (RH), sawdust (SD), or mixed chemical fertilizer NPK (CF), applied at the rate of 240 kg N ha−1 each in split applications in summer and autumn since 1978. Significantly higher amounts of total N and C and soluble organic C were found in the compost than in the CF treatments up to the 40-cm soil depth, indicating improved soil quality in the former. In the CF treatment, soil pH values were significantly lower and electrical conductivity values were significantly higher than those of compost-treated soils of up to 50 cm depth. Soil microbial biomass C and N, CO2 evolution, protease, deaminase, and urease activities were significantly higher in the compost than in the CF treatments due to greater availability of organic substrates that stimulated microbial activity. Gross N mineralization rates determined by 15N dilution technique were eight and five times higher in the SD and RH treatments than in the CF treatment, respectively, probably due to high levels of microbial and enzyme activities. Net N mineralization rates were also significantly higher in the compost treatments and were negative in the CF treatment indicating immobilization. Net nitrification rates were higher in compost treatments and negative in the CF treatment. Nitrous oxide productions from compost treatments were higher than the CF treatment due to the greater availability of mineral N as a result of higher mineralization and nitrification rates and soluble organic C in the former. Most of the measured parameters were highest in the surface soil (0–15 cm) and were significantly higher in the SD treatment than in the RH treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among organic wastes, sewage sludge needs special attention for its safe disposal because of the unpleasant odor, potential risk of the spread of infectious diseases, and the presence of heavy metals such as Zn, Cu, Pb, Cd, and Ni which pose a risk to human health through the accumulation of potentially toxic elements through the food chain (Wilkinson et al. 2001). Therefore, in many developed countries, including Japan, a significant amount of sewage sludge is first treated to remove the unpleasant smell and pathogenic microorganisms, and then composted with other materials such as rice husk (RH) or sawdust (SD). Such composts are now widely used in Japan as a major source of plant nutrients, and particularly N. Approximately 230,000 ton sewage sludge compost have been annually applied to agricultural lands as a source of N (Ministry of Construction Japan 1998).
In Japan, farmers use both commercially available composts and N fertilizers to meet the plant demand for nutrients, especially N. Compost can enrich soil with other macronutrients such as P, K, S, Ca, Mg, and micronutrients (Zaman et al. 2002a). The application of compost to agricultural or forest land may also have beneficial effects on soil physiochemical and biological properties and processes due to the presence of available C and nutrients in it. For example in one of our incubation studies, we observed that the long-term application of SD compost and RH compost significantly improved soil biological properties and processes in the surface soil (Zaman et al. 2002a). Other studies (Kunito et al. 2001; Albiach et al. 2000; Zaman et al. 1999b) also found increased microbial and enzyme activity in surface soils after a single or repeated application of organic wastes.
The nutrients (especially N) in compost are found in a variety of organic forms (Zaman et al. 2002a), which are released at different rates by a diverse group of microorganisms and enzymes (Zaman et al. 1999a). When composts are applied to soil, only a small fraction of the total organic N is mineralized during a growing season and the rest is either released in the following season or transformed into more resistant forms such as humus N, which accumulates in soil. As a result of repeated long-term applications of compost, the organic substrates in compost (organic N and C), and heavy metals (such as Zn, Cu, Cd, and Pb) may build up and move downward into the lower soil depths. The accumulation of such organic substrates and their subsequent mineralization may also have an impact on soil quality and future compost or fertilizer applications. However, limited information is available on long-term application of compost onto agricultural land because most previous studies on compost application were either short-term or focused on surface soil (Witter et al. 2000; Saviozzi et al. 1999). For example, Zaman et al. (2002b) reported increased soil microbial biomass and extracellular enzyme activity up to a soil depth of 20 cm after a single application of organic wastes to pasture soils. However, the stimulated microbial and enzyme activity returned to the level of the control soil after 6 months. The accumulation of heavy metals at different soil depths is beyond the scope of this paper and has been reported by Goto et al. (2002).
This study was carried out to determine the long-term effect of compost application, in comparison to N fertilizer application, on soil chemical properties, soil microbial and enzyme activity, gross and net N mineralization rates, net nitrification rate and N2O production in both surface and sub-surface soils. Data from this study will improve our understanding of the effects of long-term application of sewage sludge composts vs N fertilizer on soil biochemical and biological processes and the sustainability of soil fertility.
Materials and methods
The field experiment
The site, located in Tama farm, Graduate School of Agricultural and Life Sciences, the University of Tokyo, Nishi-Tokyo, Japan, has a temperate and humid climate. The long-term average annual temperature is 15.9°C and average annual rainfall is about 1,500 mm. The rainy season falls in the summer months of June and July and summer precipitation usually exceeds its evapotranspiration potential. The soil, developed from volcanic ash, is a Typic Melanudand (US Soil Taxonomy 1998) and has a silt loam texture at the surface.
Three fields (60-m length ×4.5-m width) have been set up at Tama farm. Three N treatments, sewage sludge compost with SD, sewage sludge compost with RH and mixed chemical fertilizer NPK (CF), have been applied to surface soils of these fields at the rate of 240 kg N ha−1 each in two split applications since 1978. One dose is applied before sowing of corn (Zea maize L.) in July and the other before the sowing of barley (Hordeum vulgare L.) in October/November. Both SD and RH composts are a mixture of sewage sludge with SD and RH, respectively, and are manufactured at a compost plant in Japan. The chemical properties of the two composts are listed in Table 1. Other farm practices included removal of crop residues after each harvest and application of lime to adjust soil pH to 6 after every 3–5 years (Goto et al. 1997). Composites of four soil samples from each of the five soil depths (i.e., 0–15, 15–20, 20–30, 30–40, and 40–50 cm) were collected from each field before the summer application in July 2001. The composite soil sample from each depth was sieved (<2 mm) to remove plant roots and litter materials and stored below 4°C until analyses for different chemical (Table 2) and microbial properties and processes were carried out. The moisture content of the field-moist soil samples was 62% of the water filled pore space (WFPS).
Gross and net N mineralization rates
Two sets of soil samples (25 g soil on an oven-dry basis) from the three top soil layers of each field were incubated (in four replicates) for gross N mineralization rates using the 15N dilution technique as described by Barraclough (1995). Each soil sample was placed into a 120-ml plastic container and was uniformly mixed with 1 ml 15N 99 at.%-enriched NH4Cl solution, giving 2 μg 15N g−1 soil. The rationale of adding 1 ml solution at the rate of 2 μg 15N g−1 soil was to avoid the stimulation of microbial activity by adding large amounts of water and NH4 +. Overall, 72 field-moist soil samples were labeled with 15N and incubated at 20°C, similar to the field temperature at the time of soil collection (July). After 24 h, 36 15N-labeled soil samples were taken out and immediately extracted with 50 ml 2 M KCl after shaking for 1 h and analyzed for NH4 + concentration using the nitroprusside method described by Anderson and Ingram (1989). This provided the N data after a 1-day incubation. The remaining 36 15N-labeled soil samples were taken out after 2 days incubation and similarly extracted with 50 ml 2 M KCl. The 15N enrichment of the inorganic N in the KCl extracts was determined with the diffusion method described by Stark and Hart (1996) and an isotope ratio mass spectrometer (Europa Scientific, United Kingdom). The gross rate of N mineralization was calculated from the rate at which 15N abundance in the labeled NH4 + pool and the changes in the size of the NH4 + pool declined over time (Barraclough 1995).
where m is gross N mineralization (μg N g−1 soil day−1), A o * is 15N excess abundance in the NH4 + pool after 1 day, A t * is 15N excess abundance in the NH4 + pool after 2 days, Co is the size of the NH4 + pool (μg N g−1 dry soil) after 1 day, θ is the rate at which the NH4 + pool changes in size and t is the incubation time (1 day).
Similar to the gross mineralization study, two sets of soil samples (25 g soil on an oven-dry basis) from the three top soil layers of each field were incubated (in four replicates) for net N mineralization and nitrification rates. One set was immediately shaken with 50 ml 2 M KCl for 1 h and analyzed for pre-incubation NH4 + and NO3 − concentrations. The NO3 − concentration was determined by the hydrazine reduction method (Hayashi et al. 1997). The remaining set was incubated at 20°C for 7 days. The field moisture content (62% WFPS) was maintained during the 7-day incubation by adding de-ionized water. After 7 days of incubation, soil samples were extracted with 50 ml 2 M KCl to determine post-incubation NH4 + and NO3 − concentrations. Net N mineralization rates were calculated by subtracting pre-incubation NH4 + and NO3 − concentrations from post-incubation NH4 + and NO3 − concentrations and dividing this difference by 7, the number of incubation days. Net nitrification rates were determined by subtracting the pre-incubation NO3 − concentration from post-incubation NO3 − concentration and then dividing by the number of days of incubation.
Microbial, enzyme, chemical and gas analyses
Soil microbial biomass C and N were determined using the fumigation-extraction method described by Vance et al. (1987) and Brookes et al. (1985). Soil protease activity was determined using the method of Ladd and Butler (1972) modified by Burton and McGill (1992). In this method, soil samples with or without casein solution were incubated at 50°C for 1 h and the concentration of tyrosine released after 1 h incubation was measured. Soil deaminase activity was measured by incubating soil samples with arginine as a substrate at 37°C for 3 h and extracting the NH4 +–N with 2 M KCl as described by Alef and Kleiner (1986). Soil urease activity was assayed according to Kandeler and Gerber (1988). Soil pH (in water) was measured with a pH meter using field-moist soil at a soil:water ratio of 1:2 (v/v). To measure electrical conductivity (EC), a suspension of soil and deionised water at a ratio of 1:5 was shaken on a reciprocal shaker for 30 min and then analyzed with a conductivity meter. Soluble organic C was determined by shaking 20 g soil (oven-dry basis) with 100 ml deionised H2O for 30 min. The mixture was centrifuged at 2,000g for 10 min and filtered through Whatman 42 filter paper. The filtrate was analyzed for organic C by a TOC-analyzer (Shimadzu TOC 5000). To measure CO2 and N2O production, field moist soil samples (20 g oven-dry basis) were placed into 50-ml open-top plastic containers. The plastic containers were then placed into 720-ml jars and incubated at 20°C for 1 month. The lid of each jar was fitted with a rubber septum for collection of gas samples. Two to four milliliters of deionised water was added to maintain humidity in each jar. A 60-ml syringe was used to collect a gas sample from each jar and the gas samples were injected into pre-evacuated 30-ml vials and analyzed for CO2 and N2O concentrations using a gas chromatograph (Shimadzu GC-14B). Gas samples were collected on a weekly basis for a total of 4 weeks. After each collection of gas samples, the lid from each jar was opened for 10 min to reset the gas concentration at the ambient level. The 720-ml jars were used to provide sufficient headspace for the accumulated CO2, which otherwise could cause anaerobic conditions.
Statistical analyses
The three N sources (RH, SD and CF) and five soil depths were replicated four times in a 3×5×4 factorial design. An analysis of variance (ANOVA) was carried out to determine the treatment effects on the measured parameters. Least significant difference values (LSD) were calculated at P <0.05, when the treatment effect was found to be significant. All analyses were performed using SYSTAT (1994).
Results and discussion
Total N, total C, soluble organic C, pH, and EC
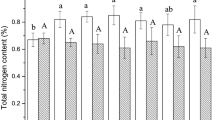
The application of the composts significantly (P <0.05) increased total N, total C, and soluble organic C contents and altered soil pH and EC in surface (0–15 cm) and sub-surface (below 15 cm) soil depths as compared to the CF treatment (Table 2). The SD compost-treated soils had significantly higher amounts of total N than the RH and CF treatments in all five depths. The higher total N of the SD compost (21 g N kg−1; see Table 1) could have resulted in such increases in total soil N content. The higher total N in the upper 0–15 cm was due to the surface application of the composts and mixing by rotary cultivator. The increase in total N in sub-surface soils could be due to the leaching of organic substrates from the surface as a result of high rainfall or irrigation waters (Zaman et al. 2002b). The applications of SD compost also significantly increased total C in all five depths compared to the CF treatment. Like total N, the SD compost treatment resulted in greater increases in soil total C in the upper 0–20 cm than the RH compost-treated soil. This could be due to higher total C (401 g kg−1) in the SD compost than in RH compost (253 g kg−1). Such increases were always higher in the upper 20 cm and became less in the deeper layers. The application of composts also significantly increased soluble organic C in the 0–40 cm soil depths. In the upper 0–30 cm, significantly higher amounts of soluble organic C were found in the SD- than in the RH-treated soil. Our results are consistent with others studies (Stamatiadis et al. 1999; Zaman et al. 2002a). The pH of the two compost-treated soils at 0–30 cm was around 6.3–6.5 while soil pH of the CF-treated soil was slightly lower (Table 2). The decreasing soil pH in the CF treatment, despite lime application (every 3–5 years to all fields to maintain soil pH around 6 during the past 22 years), could be attributed to the acidification effect of ammonium fertilizer (Mulvaney et al. 1997). As reported by Goto and Chino (1996), heavy metals in our experimental fields did not move to sub-surface soil. This could be attributed to the soil pH of above 6 (Table 2) through application of lime as chelation of heavy metals to organic substances is unlikely to occur at low soil pH.
The EC values were significantly higher in the CF-treated soils than in the two compost-treated soils probably due to the addition of large amounts of soluble salts with the chemical fertilizer. Higher EC values were also observed in the deeper soil layers of the CF-treated soils probably because of downward movement of soluble salts from the surface to the sub-surface soils.
Microbial biomass C and N
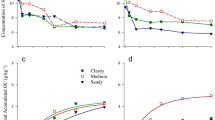
The two compost-treated soils had 2 times higher microbial biomass C than that of the CF-treated soils (Fig. 1a), resulting from continuous application of organic substrates, which the heterotrophs use for maintenance and growth. Microbial biomass C of the SD-treated soil was significantly higher than that of RH compost-treated soil at the 0–15 cm soil depth, and below that depth the two composts had similar microbial biomass C. The changes in microbial biomass C of the two compost-treated soils at different soil depths coincided with the changes in soluble organic C of the corresponding treatments. Increased microbial biomass C in surface soils after sewage sludge compost application have also been reported by Goyal et al. (2000) and Zaman et al. (2002a), but limited information is available about microbial biomass C at the lower soil depths after long-term application of organic composts.
a Soil microbial biomass C and b microbial biomass N as affected by long-term applications of sewage sludge composts or chemical fertilizer N in different soil depths (open square rice husk compost, filled square sawdust compost, hatched square chemical fertilizer). Vertical bars indicate LSD values at P <0.05
The amount of microbial biomass N was also significantly (P <0.05) greater in the compost than in the CF-treated soil up to a depth of 40 cm (Fig. 1b). The SD-treated soil had a higher amount of microbial biomass N than the RH compost-treated soil. The higher level of microbial biomass N in the compost-treated soils could be due to organic substrates added with composts, which stimulate microbial activity and growth. Despite its relatively small size, soil microbial biomass plays an important role in sustainability by conserving plant-available nutrients such as N (Smith and Paul 1990), which can be lost through NO3 − leaching and N2O and N2 production. It is apparent from our study that the long-term application of sewage sludge composts maintained a larger microbial biomass compared to soil treated with chemical fertilizer, which will therefore result in a longer availability of plant nutrients.
CO2 evolution
The CO2 evolution followed a similar trend to that of microbial biomass C and was significantly (P <0.05) higher in the upper 0–30 cm of the compost- than in the CF-treated soils during the 4-week incubation period (Fig. 2). There was a flush of CO2 during the first week of incubation when the amounts of CO2 evolved from the compost-treated soils were 2 times higher than those of the CF-treated soils in the 0–30 cm soil layer. This could be related to the stimulation of microbial activity by soil disturbance such as destruction of soil structure (aggregation), enhanced aeration, exposure of organic substrates which were previously inaccessible to microorganisms, and probably lyses of some microbial cells and optimum incubation temperature. Below 30-cm soil depth, there was no difference among the three treatments probably due to limited availability of organic C. After 2 weeks of incubation, the CO2 evolution in the compost-treated soils decreased but remained significantly higher than those of the CF-treated soils in the 0–15 and 20–30 cm soil layers; in the 15–20 cm soil layer the difference between SD and CF treatments was not significant. After weeks 3 and 4, CO2 evolution further decreased in compost treatments, but it remained significantly higher in the 0–30 cm soil layer than in the same layer of the CF-treated soil. During the 4-week incubation period, the two compost-treated soils had similar CO2 evolution in all soil depths. The higher CO2 evolution in the compost-treated soils compared to the CF-treated soil indicates enhanced microbial activity that is associated with higher microbial biomass C and N (Fig. 1a, b) and greater availability of soluble organic C (Table 2). Carbon dioxide evolution is a useful index to assess the influence of soil conditions and management practices on microbial biomass (Brookes 1995; Nannipieri et al. 1990) because metabolically active heterotrophs release CO2 when they utilize soluble organic C (metabolizable) as a substrate for their maintenance and growth.
Protease, deaminase, and urease activity
The long-term application of sewage sludge composts had a significant effect (P <0.05) on protease, deaminase and urease activities in surface and sub-surface soils (Fig. 3a–c). Protease activity was significantly higher in the compost-treated soils than in the CF-treated soil (Fig. 3a). Protease activity decreased with increasing soil depths. The enhanced protease activity in compost-treated soils could be attributed to the higher organic C (Table 1) and increased microbial biomass (Fig. 1a, b). The SD-treated soil exhibited higher protease activity than the RH-treated soil in all soil depths but these differences were not statistically significant. Despite the accumulation of heavy metals such as Cu and Zn in the compost-treated soils (Goto et al. 2002), the enzyme activity was not affected.
Like protease, the RH and SD compost-treated soils had significantly higher deaminase activities in the 0–30 cm soil depth than the CF-treated soil (Fig. 3b). In the 0–20 cm layer, the deaminase activities of the RH and SD compost-treated soils were 2 times higher than those of the CF-treated soil. The deaminase activities of the two compost treatments in the top 30 cm were similar. Like protease, the increased deaminase activities are likely to be associated with increased microbial biomass due to abundant availability of organic substrates.
Urease activities of the compost-treated surface and sub-surface soils were significantly higher than those of the CF-treated soils (Fig. 3c). The higher urease activity could be due to the enhanced microbial activity (Figs. 1, 2) as a result of increased readily available organic C (Table 2) in the compost-treated soils. The urease activities of the top 20 cm of the RH and SD compost-treated soils were 2 times higher than that of the CF-treated soil. Urease activity of the 0–20 cm layer was significantly higher in the SD-treated soil than in the RH-treated soil, but below that depth the differences disappeared. The low level of urease activity in the CF-treated soil further indicates that mineral N without sufficient amounts of available organic substrates may not have any impact on soil urease activity.
Measurements of the heavy metal content of the soils are beyond the scope of our study and they were monitored and presented by Goto et al. (2002), who report that most of the added heavy metals (Zn, Cu, and Pb) were found in the 0–20 cm soil depth. Heavy metals like Zn and Cu are known to decrease enzyme activity (Witter et al. 2000). As discussed by Nannipieri (1994), it is difficult to assess the inhibitory effects of heavy metals on soil enzyme activities because these effects are masked by the enhancement effects of the added C, which stimulates microbial growth and thus enzyme activities. However our results suggest that sewage sludge can be used as an alternative source of fertilizer to agricultural land if managed properly.
Gross and net N mineralization rates and net nitrification rates
The long-term application of sewage sludge composts had a positive effect on gross and net rates of N mineralization, and net nitrification rates (Fig. 4a–c). In the 0–30 cm soil layer, gross N mineralization rates were significantly higher in the SD- than in the RH-treated soils (Fig. 4a). In the surface soil (0–15 cm), the SD-treated soil exhibited the highest gross N mineralization rate (8.6 μg N g−1 soil day−1) followed by the RH-treated soil (5.2 μg N g−1 soil day−1) and by the CF-treated soil (1.2 μg N g−1 soil day−1). Gross N mineralization rates in the 15–20 and 20–30 cm soil layers were 2 and 8 times lower than those of the surface soil layer (0–15 cm). The higher gross N mineralization rates in compost-treated soils were due to the higher availability of organic N, as well as to higher microbial activity including protease and deaminase activities, which catalyze two reactions of the mineralization process.
a Gross-, and b net-N mineralization rates, and c net nitrification rate as affected by long-term applications of sewage sludge composts or chemical fertilizer N in different soil depths (open square RH compost, filled square SD compost, hatched square CF). Vertical bars indicate LSD values at P <0.05
Net N mineralization rates were also significantly affected by the long-term applications of sewage sludge composts and chemical fertilizers (Fig. 4b). The SD compost-treated soils exhibited significantly higher net N mineralization rates than the RH compost-treated soils in both the surface and sub-surface soil. Chemical fertilizer-treated soils showed negative net N mineralization rates indicative of N immobilization. Comparing both net and gross N mineralization rates, it was clear that net N mineralization rates were 9 times lower than gross N mineralization rates in compost-treated soils. Stottlemyer and Toczydlowski (1999) also observed higher gross N mineralization rates than net N mineralization rates. Zaman and Chang (2004) observed negative net N mineralization rates and positive gross N mineralization rates for similar treatments during an incubation study. Holmes and Zak (1994) stated that the turnover rates of microbial biomass exert a greater control on the availability of N from organic sources than the size of microbial biomass and the turnover rates can be quantified by measuring gross N mineralization rates.
Net nitrification rates were significantly higher in the SD- and RH-treated soils than in the CF-treated soil in the 0–30 cm layer (Fig. 4c). The high nitrification rates were probably due to faster net N mineralization as a result of stimulated microbial activity in compost-treated soils as discussed earlier. The CF-treated soils exhibited negative net nitrification rates in the three soil layer depths due to NO3 − immobilization and denitrification since NO3 − leaching and plant uptake were absent from the incubation study. However, NO3 − immobilization only occurs if NH4 + concentrations are very low in the soil (Rice and Tiedje 1989). Further investigation of this point is needed.
N2O production
The N2O productions were significantly higher in the 0–15 and 15–20 cm layers of the compost-treated soils than in the respective layers of the CF-treated soils during the 4-week incubation period (Fig. 5). The higher N2O emission could be attributed to high NO3 − concentrations as a result of faster net N mineralization and nitrification and higher concentration of soluble organic C in compost- than in CF-treated soils. Both N2O and N2 production via denitrification are favored by NO3 − and soluble organic C availability (Bremner 1997). Nitrous oxide emission also showed some temporal variations and increased during the first 3–4 weeks, probably due to the release of organic substrates which were not accessible previously. The higher N2O emissions from compost-treated soils highlights the need for quantifying N2O emissions from agricultural and forest land in Japan particularly where composts are used instead of chemical fertilizer. Future research should focus on quantifying N2O emissions under field conditions since environmental factors are likely to influence such emissions.
References
Albiach R, Canet R, Pomares F, Ingelmo F (2000) Microbial biomass content and enzymatic activities after the application of organic amendments to a horticultural soil. Biores Tech 75:43–48
Alef K, Kleiner D (1986) Arginine ammonification, a simple method to estimate microbial activity potential in soils. Soil Biol Biochem 18:233–235
Anderson JM, Ingram ISI (1989) Colorimetric determination of ammonium. In: Anderson JM, Ingram ISI (eds) Tropical soil biology and fertility. ISSS, CAB International, Wallingford, pp 42–43
Barraclough D (1995) 15N isotope dilution techniques to study soil nitrogen transformations and plant uptake. Fertil Res 42:185–192
Bremner JM (1997) Sources of nitrous oxide in soils. Nutr Cycl Agroecosyst 49:7–16
Brookes PC (1995) The use of microbial parameters in monitoring soil pollution by heavy metals. Biol Fertil Soils 19:269–279
Brookes PC, Landemann A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 7:837–842
Burton DL, McGill WB (1992) Spatial and temporal fluctuation in biomass, nitrogen mineralizing reactions and mineral nitrogen in a soil cropped to barley. Can J Soil Sci 72:31–42
Goto S, Chino M (1996) Behavior of heavy metals in plants and soils amended with activated sludge of various Zn levels (in Japanese with English summary). Jpn J Soil Sci Plant Nutr 67:299–303
Goto S, Chino M, Yamagishi J, Kumazawa K (1997) Heavy metal accumulation in soil associated with long-term application of sewage sludge compost (in Japanese with English summary). Jpn J Soil Sci Plant Nutr 68:156–162
Goto S, Hayashi H, Yamagishi J, Yoneyama T, Chino M (2002) Accumulation and lateral movement of heavy metals in soil with long-term application of sewage sludge compost (in Japanese with English summary). Jpn J Soil Sci Plant Nutr 73:391–396
Goyal S, Sakamoto K, Inubushi K (2000) Microbial biomass and activities along an Andosol profile in relation to soil organic matter level. Microb Environ 15:143–150
Hayashi A, Sakamoto K, Yoshida T (1997) A rapid method for determination of nitrate in soil by hydrazine reduction procedure (in Japanese with English summary). Jpn J Soil Sci Plant Nutr 68:322–326
Holmes WE, Zak DR (1994) Soil microbial biomass dynamics and net nitrogen mineralization in Northern hardwood ecosystems. Soil Sci Soc Am J 58:238–243
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–72
Kunito T, Saeki K, Goto S, Hayashi H, Oyaizu H, Matsumoto S (2001) Copper and zinc fractions affecting microorganisms in long-term sludge amended soils. Biores Tech 79:135–146
Ladd JM, Butler JH (1972) Short-term assays of soil proteolytic enzyme activity using proteins and dipeptide derivates as substrates. Soil Biol Biochem 4:19–30
Ministry of Construction Japan (1998) Annual statistics of water treatment and related activities. pp 48–49
Mulvaney RL, Khan SA, Mulvaney CS (1997) Nitrogen fertilizers promote denitrification. Biol Fertil Soils 24:211–220
Nannipieri P (1994) The potential use of soil enzymes as indicators of productivity, sustainability and pollution. In: Pankhurst CE, Doube BM, Gupta VVSR, Grace PR (eds) Soil biota. Management in sustainable farming systems. CSIRO, East Melbourne, Australia, pp 238–244
Nannipieri P, Grego S, Ceccanti B (1990) Ecological significance of the biological activity in soil. In: Bollag JM, Stotzky G (eds) Soil biochemistry, vol 6. Dekker, New York, pp 293–355
Rice CW, Tiedje JM (1989) Regulation of nitrate assimilation by ammonium in soils and in isolated soil microorganisms. Soil Biol Biochem 21:597–602
Saviozzi A, Biasci A, Riffaldi R, Levi-Minzi R (1999) Long-term effects of farmyard manure and sewage sludge on some soil biochemical characteristics. Biol Fertil Soils 1:100–106
Smith JL, Paul EA (1990) The significance of soil microbial biomass estimations. In: Bollag JM, Stotzky G (eds) Soil biochemistry, vol 6. Dekker, New York, pp 357–386
Stamatiadis S, Doran JW, Kettler T (1999) Field and laboratory evaluation of soil quality changes resulting from injection of liquid sewage sludge. Appl Soil Ecol 12:263–272
Stark JM, Hart SC (1996) Diffusion technique for preparing salt solutions, kjeldahl digests, and persulfate digests for nitrogen-15 analysis. Soil Sci Soc Am J 60:1846–1855
Stottlemyer R, Toczydlowski D (1999) Nitrogen mineralization in a mature boreal forest, Isle Royale, Michigan. J Environ Qual 28:709–720
SYSTAT (1994) SYSTAT 5.04 for Windows. SYSTAT, Evanston, Ill.
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wilkinson JM, Hill J, Livesey CT (2001) Accumulation of potentially toxic elements in the body tissues of sheep grazed on grassland given repeated applications of sewage sludge. Anim Sci 72:179–190
Witter E, Gong-Ping, Baath E, Marstorp H, Gong P (2000) A study of the structure and metal tolerance of the soil microbial community six years after cessation of sewage sludge applications. Environ Tox Chem 19:1983–1991
Zaman M, Chang SX (2004) Substrate type, temperature, and moisture content affect gross and net N mineralization and nitrification rates in agroforestry systems. Biol Fertil Soils 39:269–279
Zaman M, Di HJ, Cameron KC, Frampton CM (1999a) Gross nitrogen mineralization and nitrification rates and their relationships to enzyme activities and the soil microbial biomass in soils treated with dairy shed effluent and ammonium fertilizer at different water potentials. Biol Fertil Soils 29:178–186
Zaman M, Di HJ, Cameron KC (1999b) A field study of gross rates of N mineralization and nitrification rates and their relationships to microbial biomass and enzyme activities in soils treated with dairy effluent and ammonium fertilizer. Soil Use Manag 15:188–194
Zaman M, Di HJ, Sakamoto K, Goto S, Hayashi H, Inubushi K (2002a) Effect of sewage sludge compost and chemical fertilize application on microbial biomass and N mineralization rates. Soil Sci Plant Nutr 48:195–201
Zaman M, Cameron KC, Di HJ, Inubushi K (2002b) Changes in mineral N, microbial biomass and enzyme activities in different soil depths after surface applications of dairy shed effluent and chemical fertilizer. Nutr Cycl Agroecosyst 63:275–290
Acknowledgements
We thank the Japan Society for Promotion of Science (JSPS) for funding this project, Michael Miyattah-Kporgbe and Yu-ichi Suzuki, Chiba University, Japan, for their assistance, Dr Tsuneo Matsumaru and Mr Ei-Ichi Otsuka, Chiba Prefecture Agricultural Research Center, for C, N and 15N analyses, and the Graduate School of Agricultural and Life Sciences, the University of Tokyo, Tama Farm for using their long-term experimental plots. Last but not least, we extend our sincere thanks to Dr H.J. Di (Soil and Physical Sciences Group, Lincoln University, New Zealand), Munib Akhtar (Honda UK) and Shazia Zaman (Canterbury University, New Zealand) for their critical comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaman, M., Matsushima, M., Chang, S.X. et al. Nitrogen mineralization, N2O production and soil microbiological properties as affected by long-term applications of sewage sludge composts. Biol Fertil Soils 40, 101–109 (2004). https://doi.org/10.1007/s00374-004-0746-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-004-0746-2