Abstract
Interactive effects of a combined application of urea and compost on the fates of urea-N and net mineralization of compost-N in three soils with different contents of organic-C and inorganic-N were examined through an aerobic 6-week incubation study. Soils were each subjected to four treatments of urea and compost applied at rates of 0 and 0 mg N kg-1 (control), 115 and 0 mg N kg-1, 0 and 115 mg N kg-1, and 70 and 45 mg N kg-1, respectively. The interactive effects of a combined application of compost and urea on their N transformations varied depending on the contents of indigenous inorganic-N and organic-C in soils. Urea hydrolysis was increased by compost blending only in soils with a relatively low organic-C content. Compost blending increased N immobilization, thus decreasing nitrification of urea-derived N in soils with high organic-C and inorganic-N contents, whereas the reverse was observed in soils with low nutrient contents. Urea blending, by providing inorganic-N, consistently increased net mineralization of compost-N irrespective of soil characteristics, although the increase was much smaller in soil with high indigenous inorganic-N. From the results, it could be concluded that a combined application of chemical fertilizer would improve the compost use efficiency by increasing mineralization of compost-N particularly in soil with a low inorganic-N content. This study also suggests that compost blending would increase immobilization of urea-N in soils with high C and N contents, whereas it would increase nitrification of fertilizer-N in soils with low nutrients contents, thus resulting in increased NO3 - leaching.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing the compost application, which is necessary to satisfy the complete N requirement of a crop, is largely required due to the low nutrient content and plant availability of compost-N as compared to chemical fertilizers (Eghball and Power 1999). However, because excessive applications of compost increase the levels of P and other ions in soil, combining composts with chemical fertilizer is considered as an appealing alternative (Sikora and Enkiri 2001). As suggested by Eghball and Power (1999), applying P-based compost can be the most effective method of using composts when soil P build-up is of concern.
To optimize the use of compost and supplement the amount of additional fertilizer-N needed during the plant growth period, the rate of net N mineralization of compost in the soil must first be determined (Hadas and Portnoy 1994). Net N mineralization of compost has shown to be low, <15% of total N in 32 weeks (Hadas and Portnoy 1994) and <5% in 90 days (Benitez et al. 1998), and is related to the compost characteristics, such as type of raw material, composting method, maturity, and C/N ratio of compost, as well as compost application rate, characteristics of the soil, and other climatic conditions (Hadas et al. 1996; Whalen et al. 2001). However, the effect of fertilizer blending on compost-N mineralization is not yet well understood. Because N fertilization lowers the C/N ratio of the soil, thereby enhancing the mineralization of soil organic matter (Belay et al. 2002), blending with fertilizer-N may change the net mineralization of compost-N.
On the other hand, compost blending may increase the immobilization of fertilizer-N, because compost added to soil can serve as a substrate for soil microorganisms and induce microbial activity and growth (Hadas et al. 1996). The resulting enhanced immobilization of fertilizer-N influences crop availability and loss of fertilizer-N (Choi et al. 2001). Therefore, better knowledge of the effects of compost blending on the fate and recovery of fertilizer-N would enable improvement in the utilization of N from fertilizers and composts.
In this study, therefore, we examined the effects of a combined application of compost and fertilizer on the mineralization of compost and the fate of fertilizer in soils through an aerobic incubation study. To do so, we compared the interactive effects of the combined application using three soils with different characteristics, since the interactive effects of both inputs may vary depending on such characteristics as indigenous organic matter content, texture, and nutrient status of the soil mixed with the inputs.
Materials and methods
Soil, urea, and compost
For the incubation experiment, surface soil (0~20 cm) was sampled from three cultivated soil types: soil A (mesic family of Typic Dystrudepts), soil B (mixed, mesic family of Typic Udifluvents), and soil C (artificially disturbed soils). Soils A and B have been cultivated mainly with fertilizer-N at 200–400 kg ha-1 year-1 for at least 50 years under upland conditions, and soil C with mainly compost at 15,000–25,000 kg ha-1 year-1 for about 10 years under plastic-film greenhouse conditions. Due to its steep slope, soil A was susceptible to water erosion, which causes losses of soil particles and nutrients. Physico-chemical properties of the soils are given in Table 1. Organic-C contents were in the order of soil C (32.4 g kg-1)>soil B (15.0 g kg-1)>soil A (8.1 g kg-1). Inorganic-N concentration also differed significantly among soils, decreasing in the order of soil B>soil C>soil A.
Commercially available composted manure, a mixture of sawdust and manure (pig and poultry) prepared by aerobic fermentation, was used as a compost amendment. Physico-chemical characteristics of the compost are described in Table 1. Compost was ground to pass a 2-mm sieve and used for the incubation experiment. For the fertilizer amendment, 15N-labelled urea (5.0 15N atom %) was used.
Incubation experiment
Soil samples were air-dried, passed through a 2-mm sieve, and pre-incubated for 7 days at 25±0.5°C. During pre-incubation, the water content of each soil was kept at 3% lower than its respective water content equivalent to a soil matric potential of −33 kPa (Table 1). Sixty-five grams (dry basis) of each of the pre-incubated soil samples was placed separately in 250-ml beakers. After 7 days of pre-incubation, urea solution (U) and compost solid (C) were added to each beaker at rates of 0 mg urea-N kg-1 and 0 mg compost-N kg-1 (control), 115 mg urea-N kg-1 and 0 mg compost-N kg-1 (SU treatment), 0 mg urea-N kg-1 and 115 mg compost-N kg-1 (SC treatment), and 70 mg urea-N kg-1 and 45 mg compost-N kg-1 (SUC treatment), respectively. Finally, the water content of each soil was set at −33 kPa of soil matric potential by adding distilled water. The samples were then mixed homogeneously with a spatula. Subsequently, the beakers were covered with aluminium foil perforated with several needle holes to ensure gas exchange, and incubated at 25±0.5°C for 0.5, 7, 14, 28, and 42 days. The beakers were weighed every day, and distilled water was added as necessary to maintain −33 kPa of soil matric potential. All treatments were triplicated.
Chemical analyses
At each sampling time, the pH (1:1) of each soil sample (5 g, dry basis) was measured with a pH meter (DMP 200, DMS, Korea). To determine the urea and inorganic-N (NH4 + and NO3 -) contents, 40 g (dry basis) of each soil sample was extracted with 200 ml of 2 M KCl-PMA solution. The urea concentration in the KCl-PMA extracts was measured using the diacetyl monoxime method (Bremner 1982). One-third of the KCl-PMA extracts was steam-distilled using MgO and Devarda alloy to determine the inorganic-N concentration (Keeney and Nelson 1982). The remaining extract was steam-distilled, adjusted to pH 2~3 with 0.1 N H2SO4 or NaOH, and concentrated under infrared lamps for the analysis of 15N atom % according to the procedures of Choi et al. (2001). The resulting fine powder [(NH4)2SO4] of the dried sample was analysed for 15N atom % by a combustion method using a stable isotope ratio mass spectrometer (Isoprime-EA, Micromass, UK) coupled with an elemental analyser (Feast and Dennis 1996). Accuracy and reproducibility of the analysis, checked with a reference material (RM 8548: IAEA-N2) from the International Atomic Energy Agency, were better than 0.4 and 0.2‰, respectively.
For the analysis of immobilized urea-15N, inorganic N was completely removed from the soil sample through KCl-PMA extraction following the procedures of Choi et al. (2001). The inorganic-N free sample was dried at 80°C and ground to a very fine powder. Total N content and 15N atom % were determined by the combustion method mentioned above.
Calculations and statistical analysis
The amount of N derived from 15N-urea (NDFU) was calculated for NH4 +, NO3 -, and the 2 M KCl-PMA non-extractable organic-N pool using the following equation:
where, T is the total amount of N in each N pool in the urea-treated samples, A S is the 15N atom % excess in the urea-treated samples, and A F is the 15N atom % excess in the applied urea. Recovery of urea-15N in each pool was calculated as % of the amount of applied urea-N.
At each time interval, net N mineralization of the compost was calculated based on the accumulation of inorganic-N in soil. For the SC treatment, the net amount of N mineralized from the compost was calculated as difference in the concentrations of inorganic N between the SC treatment and the treatment without compost (control). For the SUC treatment, the net mineralization of compost was calculated by subtracting the NDFU of SUC plus the total inorganic-N concentration of the control treatment from the total inorganic-N concentration of the SUC treatment (Hadas and Portnoy 1994). The net mineralization of compost is presented as a percentage of total compost-N applied.
Data were statistically analysed using Generalized Linear Models procedures (SAS Institute 1989). Least significant differences (LSD) were used to compare significant differences at the 95% confidence level among treatments.
Results and discussion
Urea hydrolysis
The pattern of urea hydrolysis was significantly ( P <0.05) different among soils irrespective of compost application (Table 2). After 0.5 day of incubation, the % of added urea-N remaining as urea in the SU and SUC treatments was 62.8% and 56.4% for soil A, 41.9% and 29.0% for soil B, and 10.9% and 9.2% for soil C, respectively, thus showing that hydrolysis was fastest in soil C, which had the highest soil organic-C content, and slowest in soil A, which had the lowest soil organic-C content (Table 1). Studies examining the effect of soil organic matter content on urease activity have observed that urea hydrolysis increases with increasing soil organic-C, because the soil urease activity depends on the microbial biomass, which is proportional to soil organic matter (McGarity and Meyers 1967; García et al. 1993). After 7 days of incubation, however, no significant differences were observed due to the low concentration of urea.
Compost blending significantly ( P <0.05) decreased the percent of urea recovered in soils A (LSD=3.2) and B (LSD=5.6) at 0.5 day of incubation. Many investigators including Zantua and Bremner (1976) and Pascual et al. (2002) observed that the total urease activity of organic-amended soils was significantly higher than that of the control due to a high level of substrates capable of activating enzyme synthesis by ureolytic microorganisms (Fenn and Hossner 1985). The increased urea hydrolysis rate caused by compost application could also be attributed to urease in the composted manure (Varel 1997). In contrast to soils A and B, compost blending did not result in any significant increase in the rate of urea hydrolysis in soil C, probably due to sufficient indigenous organic matter in the soil (Table 1).
Although hydroxyl ion evolution during urea hydrolysis can cause a considerable pH increase and thus lead to N loss through NH3 volatilization (Martens 2000), the pH of soils in this study was consistently low (<6.0) throughout the incubation (data not shown). The maximum pH was observed at 0.5 day of incubation; the pH values for the SU and SUC treatments were 5.2 and 5.8 in soil A, 5.2 and 5.2 in soil B, and 5.8 and 5.6 in soil C, respectively. Therefore, in this study, the contribution of N loss through NH3 volatilization to total N loss could be assumed to be negligible. The low pH could be due to various reasons such as an acid soil reaction of the soil used in this experiment, the pH buffering capacity of the soil (particularly for soils B and C with high clay or organic matter content), and a low urea hydrolysis rate of soil (particularly for soil A), as reported by Fenn and Hossner (1985). In addition, nitrification, which produces hydrogen ions, would also contribute to the low pH of the soils (Paul and Clark 1989).
Changes in NH4 + and NO3 - concentrations
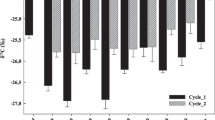
Applications of urea and compost increased the concentrations of NH4 + and NO3 - (Fig. 1). Patterns of inorganic-N concentration changes were different among soil types depending on the concentration of indigenous soil inorganic-N. For urea-treated soils (SU and SUC treatments), the urea hydrolysis rate also influenced the patterns of NH4 + and NO3 - formation. In soils B and C, considerable decreases in NH4 + concentrations were observed 0.5–7 days after treatment (Fig. 1b, c), whereas that of soil A increased during the same period due to slow urea hydrolysis (Fig. 1a). The production pattern of NO3 - followed that of NH4 +. For soils B and C, the NO3 - concentration reached a steady state between 7 and 14 days of incubation (Fig. 1e, f); however, the concentration of NO3 - in soil A increased gradually during 42 days of incubation, indicating slow nitrification (Fig. 1d).
Although total application rates were equal, the concentration of inorganic N (NH4 ++NO3 -) was much higher in the SU treatment than in the SUC treatment (Fig. 1g–i) due to the slow release of inorganic N from compost-N (Hadas and Portnoy 1994). However, the differences in inorganic-N concentrations between SU and SUC treatments in soil A were significantly lower than those in soils B and C. At the end of the incubation, the differences were 13.1 for soil A, 31.2 for soil B, and 22.5 mg N kg-1 for soil C. These results show that net N mineralization from the organic-N pool of soil or compost would be highest in soil A, and lowest in soil B.
Effect of compost on the fate of urea-15N
Pattern of urea-N distribution and the recovery of urea-derived N were slightly different among soils (Fig. 2). In soil A, unlike soils B and C, urea-derived NH4 + was detected until 28 days after treatment due to slow hydrolysis of urea (Table 2). At a given sampling time, the percentage of urea-N recovered as NO3 - was much higher in SUC than in SU treatment, whereas the reverse was observed for NH4 +. At the end of incubation, when most inorganic N was found in the NO3 - pool, the % of urea-N in the NO3 - pool was significantly ( P <0.05, LSD=3.7) higher in the SUC (80.2%) than in the SU (74.5%) treatment, indicating an increased nitrification of urea-derived N due to compost blending. Choi et al. (2001) reported that a combined application of compost enhanced the microbial immobilization of fertilizer-derived NH4 +; however, an organic amendment may increase nitrification rather than immobilization since the effect of organic inputs on nitrification potentials is soil-specific (Honeycutt et al. 1991; Chao and Chao 1997). Therefore, this result suggests that compost application might increase nitrifiers as well as heterotrophic populations in soil A having low indigenous organic-C and inorganic-N contents (Chao and Chao 1997).
Recovery of added urea-15N in the urea (■), NH4 + (□), NO3 - (▨), organic N (▦), and unaccounted-for portion (▩) in a urea-amended soil ( SU treatment) and b compost plus urea-amended soil ( SUC treatment). Values are % of added urea-N and the means of triplicates. Vertical bars indicate SDs of the means
Unlike in soil A, compost application increased immobilization of urea-derived N in soils B and C, which had a relatively high content of indigenous organic-C and inorganic-N. In soil B, the % of urea-N immobilized was increased significantly by compost blending ( P <0.05, LSD=1.2) from 4.1% for SU to 7.5% for SUC at the end of the incubation, while that accumulated in the NO3 - pool decreased significantly (LSD=2.2) from 87.0% for SU to 82.5% for the SUC treatment. However, in spite of the much higher soil organic-C content of soil B, the % of immobilized urea-N in soil B was about 3 times lower than that in soil A, which could be attributed to the characteristics of soil B. Firstly, because indigenous soil inorganic-N, which occupies the same N pool as added urea-N for microbial immobilization, was more abundant in soil B than in soil A (Table 1), microbial immobilization of added urea-N in soil B might have been lowered by the co-existence of soil inorganic-N as compared to soil A. Secondly, because nitrifiers compete with heterotrophs for soil NH4 + (Verhagen and Laanbroek 1991), fast nitrification in soil B (Fig. 1b, e) would have prevented microbial immobilization of NH4 + (Recous and Mary 1990). After 7 days of incubation, >80% of the added urea-N was found as NO3 - in soil B, whereas >60% was found as NH4 + in soil A (Fig. 2). This result corresponds to the observations of Shi and Norton (2000), who reported that the nitrification rate and nitrifier population size increased in NH4 +-abundant soil.
In soil C with a high organic matter content, immobilization of added urea-N occurred immediately after urea addition (Fig. 2), irrespective of the compost application, which indicates stimulation of microbial activity in the organic-C-rich soil due to the added fertilizer-N, as suggested by Blagodatsky et al. (1998). After 0.5 day of incubation, the % of immobilized urea-N was 24.9% for SU and 26.0% for SUC (Fig. 2). At the end of incubation, however, it decreased to 18.1% for SU, but increased to 32.3% for SUC, indicating an enhanced ( P <0.05, LSD=2.0) microbial immobilization of urea-N following compost application (Hadas et al. 1996). Unlike in soil B, fast microbial immobilization decreased nitrification of urea-derived N in soil C, and this pattern was more obvious in the soil receiving both urea and compost, thus resulting in a significant ( P <0.05, LSD=2.5) decrease in the % of urea-N recovered in the NO3 - pool at the end of incubation from 70.2% for SU to 59.0% for the SUC treatment.
At the end of the incubation, the unaccounted-for portions of added urea-N in the SU treatment were 7.8% for soil A, 8.9% for soil B, and 11.7% for soil C. However, with simultaneous compost blending, the unaccounted-for portions significantly ( P <0.05) decreased to 3.5% in soil A (LSD=2.1) and 8.5% in soil C (LSD=1.9), whereas no significant difference was observed in soil B. The unaccounted-for portions could be attributed to denitrification in the heterogeneous anaerobic sites of these soils (Choi et al. 2001).
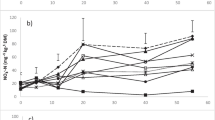
Effect of urea on net mineralization of compost
Net mineralization and immobilization of compost-N was affected by urea application as well as by the characteristics of the soils used (Fig. 3). Compared to the indigenous inorganic-N (11.4% of total-N) in compost (Table 1), the portion of inorganic-N in the added compost-N increased sharply at 0.5 day, but decreased at 7 days of incubation as a result of net N immobilization. However, thereafter the portion of inorganic-N rose again until 42 days of incubation, indicating net N mineralization. In particular, the magnitude of initial mineralization and subsequent immobilization of compost N was further enhanced in the presence of urea (Fig. 3b).
The initial mineralization of organic-N from compost was probably due to microbial decomposition of the easily decomposable part of the compost, which is generally heterogeneous with more than two components decomposing at different rates (Hadas et al. 1996). Kuzyakov et al. (2000) also suggested that the release of inorganic-N without a lag phase is common after the addition of an readily available organic substance, because the microbial community dose not have to adapt to the added substance. The pattern of net mineralization between 0.5 and 7 days after treatment was very similar to the result of Hadas and Portnoy (1994), who attributed the decrease in net mineralization of compost-N to N assimilation by the growing microbial biomass stimulated by organic-C and inorganic-N in the compost. Release of inorganic-N from compost following net immobilization after 7 days of incubation also corresponds to the observation of Hadas and Portnoy (1994).
Net mineralization of compost-N at the end of the incubation estimated by subtracting the initial inorganic-N was 14.5% for soil A, 7.6% for soil B, and 13.4% for soil C under the SC treatment. The significantly lower net mineralization of compost-N in soil B ( P <0.05) might be due to the fast nitrification of NH4 +, which is the N source for heterotrophs decomposing organic matter (Bendi and Richter 2002). As seen in Table 1, higher contents of clay and silt in soil B could be another reason for lower N mineralization, since clay and silt fractions protect the amended organics from microbial decomposition (Sørenson et al. 1996). With simultaneous urea blending, net N mineralization was further enhanced to 18.5% (LSD=1.4), 8.9% (LSD=1.2), and 21.0% (LSD=2.0), respectively, compared to that without urea blending. This result could be attributed to an increase in heterotrophic activity caused by the combined application of organic and inorganic fertilizers as suggested by Gyoal et al. (1999). Chantigny et al. (1999) also suggested that decomposition of organic substances was accelerated by inorganic-N addition in soils with low N availability. Therefore, the considerably high concentration of indigenous inorganic-N in soil B (Table 1) could have resulted in a relatively low effect of urea blending on the mineralization of compost-N.
Considering the recovery of added urea-15N and temporal changes in NH4 + and NO3 -concentrations, the results of this study show that contents of indigenous organic-C and inorganic-N in soils affected the interactive effect of a combined application of urea and compost on N transformations (Table 3). Compost blending increased urea hydrolysis in soils A and B, but did not in soil C which had a high content of indigenous organic-C. Particularly, in soil A with low indigenous C and N contents, compost blending enhanced nitrification rather than immobilization of urea-derived N while the reverse was the case for soils B and C. In contrast, the effect of urea blending on the mineralization of compost-N was significant irrespective of soil characteristics, but this increasing effect would be negligible in soil B under field conditions since the significant difference was very low for soil B. These results suggest that a combined application of chemical fertilizer would increase the compost use efficiency by increasing compost-N mineralization particularly in soils with relatively low indigenous inorganic-N contents, but also suggest that compost blending would increase the nitrification of fertilizer-N in soils with low C and N contents, and thus enhance NO3 - leaching which may cause a reduction in water quality.
References
Blagodatsky SA, Yevdokimov IV, Larionova AA, Richter J (1998) Microbial growth in soil and nitrogen turnover: model calibration with laboratory data. Soil Biol Biochem 30:1757–1764
Belay A, Claassens AS, Wehner FC (2002) Effect of direct nitrogen and potassium and residual phosphorus fertilizers on soil chemical properties, microbial components and maize yield under long-term crop rotation. Biol Fertil Soils 35:420–427
Bendi DK, Richter J (2002) A critical review of some approaches to modelling nitrogen mineralization. Biol Fertil Soils 35:168–183
Benitez C, Bellido E, Gonzalez JL, Medina M (1998) Influence of pedological and climatic factors on nitrogen mineralization in soils treated with pig slurry compost. Bioresour Technol 63:147–151
Bremner JM (1982) Ntrogen-Urea. In: Parge AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd edn. (Agronomy monograph no. 9) ASA, SSSA, Madison, Wis., pp 643–698
Chantigny MH, Angers DA, Prévost D, Simard RR, Chalifour F (1999) Dynamics of soluble organic C and C mineralization in cultivated soils with varying N fertilization. Soil Biol Biochem 31:543–550
Chao WL, Chao CC (1997) Nitrogen transformation in tropical soils: influence of fertilization and crop species. Agric Ecosyst Environ 64:11–17
Choi WJ, Jin SA, Lee SM, Ro HM, Yoo SH (2001) Corn uptake and microbial immobilization of 15N-labeled urea-N in soil as affected by composted pig manure. Plant Soil 235:1–9
Eghball B, Power JF (1999) Phosphorus- and nitrogen-based manure and compost applications: corn production and soil phosphorus. Soil Sci Soc Am J 63:895–901
Feast NA, Dennis PF (1996) A comparison of methods for nitrogen isotope analysis of groundwater. Chem Geol 129:167–171
Fenn LB, Hossner LR (1985) Ammonia volatilization from ammonium or ammonium-forming nitrogen fertilizers. Adv Soil Sci 1:123–169
García CT, Hernández T, Costa F, Ceccanti B, Ganni A (1993) Hydrolases in the organic matter fractions of sewage sludge. Changes with composting. Bioresour Technol 45:44–52
Gyoal S, Chander K, Mundra MC, Kapoor KK (1999) Influence of inorganic fertilizers and organic amendments on soil organic matter and soil microbial properties under tropical conditions. Biol Fertil Soils 29:196–200
Hadas A, Portnoy R (1994) Nitrogen and carbon mineralization rates of composted manures incubated in soil. J Environ Qual 23:1184–1189
Hadas A, Kautsky L, Portnoy R (1996) Mineralization of composted manure and microbial dynamics in soil as affected by long-term nitrogen management. Soil Biol Biochem 28:733–738
Honeycutt CW, Potaro LJ, Halteman WA (1991) Predicting nitrate formation from soil, fertilizer, crop residue, and sludge with thermal units. J Environ Qual 20:850–856
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Parge AL, Miller RH, Keeney DR(eds) Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd edn. (Agronomy Monograph no. 9) ASA, SSSA, Madison, Wis. pp. 643–698
Kuzyakov Y, Friedel JK, Stahr (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Martens DA (2000) Nitrogen cycling under different soil management system. Adv Agron 70:143–192
McGarity JW, Meyers MG (1967) A survey of urease activity in soils of northern New South Wales. Plant Soil 27:217–238
Pascual JA, Moreno JL, Hernández T, García C (2002) Persistence of immobilized and total urease and phosphatase activities in a soil amended with organic wastes. Bioresour Technol 82:73–78
Paul EA, Clark FE (1989) Soil microbiology and biochemistry. Academic Press, San Diego, Calif.
Recous S, Mary B (1990) Microbial immobilization of ammonium and nitrate in cultivated soils. Soil Biol Biochem 22:913–922
SAS Institute (1989) SAS/STAT user’s guide version 6, 4th edn. SAS, Cary, N.J.
Shi W, Norton JM (2000) Microbial control of nitrate concentrations in an agricultural soil treated with dairy waste compost or ammonium fertilizer. Soil Biol Biochem 32:1453–1457
Sikora LJ, Enkiri NK (2001) Uptake of 15N fertilizer in compost-amended soils. Plant Soil 235:65–73
Sørenson P, Ladd JN, Amato N (1996) Microbial assimilation of 14C of ground and unground plant materials decomposing in a loamy sand and a clay soil. Soil Biol Biochem 28:1425–1434
Varel VH (1997) Use of urease inhibitors to control nitrogen loss from livestock waste. Bioresour Technol 62:11–17
Verhagen FJM, Laanbroek HJ (1991) Competition for ammonium between nitrifying and heterotrophic bacteria in dual energy-limited chemostats. Appl Environ Microbiol 57:3255–3263
Whalen JK, Chang C, Olson BM (2001) Nitrogen and phosphorus mineralization potentials of soils receiving repeated annual cattle manure applications. Biol Fertil Soils 34:334–341
Zantua MI, Bremner JM (1976) Production and persistence of urease activity in soils. Soil Biol Biochem 8:369–374
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, KH., Choi, WJ., Han, GH. et al. Urea-nitrogen transformation and compost-nitrogen mineralization in three different soils as affected by the interaction between both nitrogen inputs. Biol Fertil Soils 39, 193–199 (2004). https://doi.org/10.1007/s00374-003-0704-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-003-0704-4