Abstract
Since it has become appreciated that soil nematode assemblages are abundant, diverse and contribute to soil nutrient turnover, they have been increasingly used as indicators of soil condition. Use of nematodes as functional indicators relies on the allocation of nematodes to feeding groups and reproductive strategies; in both cases groupings are uncertain. Species within feeding groups vary in their food resources and response to environmental variables, as shown by the difficulties in managing plant-pathogenic nematodes. Therefore species-level discrimination is necessary to permit further advances in understanding the role of nematodes in soil processes and thus in ecosystem resilience. Analysis of published nematode lists shows that among the bacterial-feeding nematodes Cephalobidae are often the most abundant group in soils; Rhabditidae may increase following a resource pulse; in stressed, natural environments Plectidae may be important. To be comparable with other biota, nematode biodiversity assessment requires species-level identification. In many jurisdictions such identification will be difficult due to inadequate systematic knowledge of the nematode fauna.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nematodes are abundant (>3 million m−2 at some sites) and their assemblages diverse (>200 species at some sites), with their species composition reflecting substrate texture, climate, biogeography, organic inputs, and both natural and anthropic disturbances (Cobb 1915; Tietjen 1989; Yeates 1984; Neher 2001). Some 30 years ago soil nematodes were commonly regarded as deleterious to agricultural production. Laboratory experiments and field studies have since demonstrated that those nematodes that feed on bacteria and fungi play important roles in influencing the turnover of the soil microbial biomass and thus in the availability of plant nutrients (Bardgett et al. 1999). In particular, bacterial-feeding microfauna enhance nutrient release directly through excretion and indirectly through maintaining bacterial populations in a logarithmic growth phase. It has been estimated that approximately 40% of nutrient mineralisation in certain ecosystems is due to nematodes and other soil fauna as they feed on microbial populations (De Ruiter et al. 1993). The overall positive contribution of the various nematode feeding types to soil and thus ecosystem processes is now accepted (Yeates 1987; Bongers and Ferris 1999; Bardgett et al. 1999). This understanding comes from the combination of laboratory and field studies (i.e. from microcosms and ecosystems; e.g. Yeates 1984; Ingham at al. 1985).
As awareness of the diversity and ecological significance of nematodes has increased they have increasingly been used as indicators in the areas of biodiversity and sustainability, with the general presumption that "more is better". A range of information-rich indices has been used to summarise the attributes of nematode assemblages. In this paper, I first review developments in soil biology that have been associated with the use of indices (including "biodiversity") and then argue that the current use of indices is inadequate to determine the functional role of nematodes in ecosystems.
Indices in the last decade
Initial use of nematodes as soil indicators was related to prediction of economic crop loss, particularly using preplant estimates of particular nematodes such as the cyst nematodes (Heteroderidae). As the positive contributions of nematodes to ecosystem processes were appreciated it was necessary to summarise extensive faunistic data in information-rich indices. Initially, typical ecological indices based on the proportional contribution of each nominal taxon, such as Shannon-Weiner diversity (H′) and dominance (λ), were used for nematode assemblages (Yeates 1984; Wasilewska 1979). They, and "cumulative species curves", were also widely used in interpreting marine nematode assemblages (Boucher 1990; Lambshead et al. 1983; Tietjen 1989).
Increasing interest in biodiversity and the environment, concerns about maintaining the productive capacity of agricultural soils, and interpretation of a growing knowledge of the contribution of nematodes to soil and ecosystem processes have resulted in a wider use of indices. Bongers' (1990) Maturity Index (MI) and Plant Parasite Index (PPI) provided focussed tools for assessing the response of nematode assemblages to disturbance and have been widely applied. Although initially designed to compare conditions in adjacent plots they have been applied among a range of plots, soils and regions. There are, however, fundamental concerns about both Bongers' (1990) allocation of families to coloniser-persister (cp) categories (Yeates 1994; De Goede et al. 1993; Fiscus and Neher 2002; Yeates et al. 2002) and the validity of the high level discrimination between "plant parasitic" and "free-living" forms (Yeates et al. 1993a; Wasilewska 1994; Yeates 1994; Berney and Bird 2001) that underpin the indices. While differences in rK values (reproductive strategies) between plants or animals in different habitats can be found, no general rules exist and the concept has limited explanatory powers (Townsend et al. 2000); before 1990 workers had been circumspect about applying the rK concept to nematodes (Jones 1980; Yeates 1984). Furthermore, the allocation of nematodes to feeding groups (Wieser 1953; Yeates et al. 1993a, 1993b; Bongers and Bongers 1998) required significant extrapolation. The food resources used by the delicate-speared Tylenchidae and Tylodoridae, and the spear-bearing Belondiridae, are poorly documented and highly uncertain.
Decomposition processes in soil, although ultimately dependent on the plant resource base, are often allocated to either the bacterial-based energy channel (or pathway) or the slower fungal-based channel (Moore and Hunt 1988). The ratio between the abundance of these two functional groups gives an index of the relative contribution of the channels; it is helpful to express this ratio as a Nematode Channel Ratio [NCR = B/(B+F) where B and F are, respectively, the relative contributions of bacterial-feeding and fungal-feeding nematodes to total nematode abundance] which is constrained to have values between 1 (totally bacterial-mediated) and 0 (totally fungal-mediated). For example, in Nebraska, United States, the decomposition index for an organic system was 0.839 whereas high input, continuous corn had NCR = 0.554 (Neher and Olson 1999). Similarly, in The Netherlands integrated plots had NCR = 0.966 and conventional plots a lower value (NCR = 0.736; data for 1990; Bouwman and Zwart 1994). Both diversity and cp-linked approaches have also been used in such comparisons of nematode assemblages (Wardle et al. 2001; Ferris et al. 2001). Bardgett et al. (2001) have provided evidence that soil microbial communities of heavily grazed sites are dominated by bacterial-based energy channels of decomposition, whereas in systems that are less intensively grazed, or completely unmanaged, fungi have a proportionally greater role.
Reanalysis of nematode assemblage data for cultivated soils by Fiscus and Neher (2002) differentiated between physical and chemical disturbance factors, and resulted in tillage sensitivity (TS) and chemical sensitivity (CS) ratings for taxa. These ratings often conflicted with assigned cp values. When investigating nematode population growth in undisturbed soils at various soil moisture tensions, Yeates et al. (2002) found a greater increase in Cephalobus (nominal cp group 2) than in Rhabditis or Pristionchus (nominal cp group 1). The relatively large populations of Cephalobus they found after 35 days were, however, in agreement with the dominance of Cephalobidae under field conditions world-wide. These two studies have significant implications for the general applicability of Bongers' cp groups, MI and PPI, particularly when added to prior concerns.
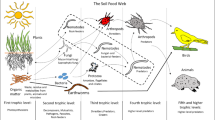
When soil processes are being considered, interpretation of nematode assemblages must not be made in isolation. The nematode populations interact functionally with populations of other soil biota including both nematodes grazing on microbial food resources and predation (or grazing) on nematodes by fungi and invertebrates. Knowledge has advanced from the stage when fluxes through the nematode populations themselves were considered central (Yeates 1973; Sohlenius 1979; Wasilewska 1979), and indices based solely on nematodes may hinder further progress.
The relative use of bacteria and fungi by nematodes reflects differences in decomposition pathways or channels between differing management regimes or ecosystems, as described above. Ratios such as NCR can be powerful tools in analysing both ecosystem processes and nematode assemblages. However, given the uncertainties inherent in, and reservations about, the allocation of nematodes to feeding groups and the lack of general applicability of cp groups, there must be serious doubt about combining these two approaches as proposed by Ferris et al. (2001). The combination may have value in particular, local treatment comparisons (such as initially envisaged for the Maturity Index). However, comparing results between studies and soil types (i.e. situations in which the composition of the nematode assemblage may be quite different), when allocation of taxa to both feeding groups and cp groups are uncertain, is questionable.
Indices, by their nature, condense information. There is, as a corollary, a trend to reduce the level of identification for use in indices. Indeed, the Maturity Index is described as being based on "nematode species composition" but relies on family level discrimination. However, feeding habits and reproduction potential are known to vary within nematode families and detailed identification is necessary for accurate interpretation.
Biodiversity
Soil nematode assemblages have been used as indicators of soil conditions with an underlying assumption that larger, more diverse assemblages reflect "more healthy" soils and are thus "desirable". However, (a) there are continuing debates as to the factors controlling biodiversity (e.g. stress, restrained competition, production, habitat structure; Colinvaux 1993), (b) soil populations are part of complex ecosystems (Boyd and Murray 2001), (c) the relationship between above- and below-ground diversity (and system function) has not been clarified (Wardle 2002), and (d) there is no unambiguous evidence to support the view that diversity or complexity predictably affect the stability of ecosystem properties or processes (Cragg and Bardgett 2001; Wardle 2002).
Wolters (2001) has described how those ecosystem processes that are distributed discontinuously across a broad suite of organisms may persist after species are lost but ecosystem traits discontinuously distributed among a few species will be very sensitive to species loss. It has been demonstrated that increasing trophic diversity of soil nematode assemblages increases nutrient turnover and plant growth (Ingham et al. 1985), that a series of nematode species may sequentially utilise a single resource (Yeates 1987; Ferris et al. 1996). Further, "improvement" of native grasslands may decrease nematode species richness (Yeates and King 1997) and there is significant short-range spatial heterogeneity among soil nematode assemblages (Ettema 1998; Mikola and Sulkova 2001; Ettema and Yeates 2003). It is also apparent that, at the functional group level, there are subtle differences among species that are manifest only under altered conditions or over long time periods (Yeates et al. 1999; Duffy 2002). Thus, following the arguments of Adams and Wall (2000), there is a clear need to investigate the sensitivity of ecosystem functions to changes in species or functional group composition and richness among soil nematodes. There is a range of species within each nematode trophic group and each key group must be represented, and active, if contributions to ecosystem processes are to continue. Each group of, for example, bacterial-feeding nematodes has somewhat different functional attributes and is commonly represented by several/many species in an area. Although yet to be experimentally tested (Hunt and Wall 2002), it seems axiomatic that for resilience the natural diversity within each group should be maintained. Plectidae, Cephalobidae and Rhabditidae are commonly the most abundant of these bacterial-feeding nematodes but other families are significant in some systems.
In a microcosm study using Collembola, Cragg and Bardgett (2001) found idiosyncratic effects on litter decomposition processes when fewer nominal species of Collembola were added. They found the processes to be driven by the impact of the dominant collembolan present. As abiotic and multi-trophic interactions affect soil processes they cautioned on transfer to field soils. Similarly Liiri et al. (2002) found only weak relationships between soil microarthropod diversity and its recovery following disturbance by drought. These two reports support the earlier summary of Laasko and Setälä (1999) that manipulative experiments have shown high trophic redundancy and functional differences between organisms in food webs, and are reflected in the implied need for diversity within functional groups in Hunt and Wall's (2002) ecosystem modelling. Problems arising from spatially inadequate sampling and from studying microfaunal groups whose systematics are more straightforward have been highlighted by André et al. (2002). Clearly adequate sampling and species identification are critical in analysing the contribution of both microarthropods and nematodes to soil processes.
A range of techniques has been used to estimate the quantitative and qualitative contribution of micro-organisms to soil processes. Soil microbial biomass is useful in studying long-term soil changes and soil enzymes to assess specific activities (Nannipieri et al. 1990, 2002; Smith and Paul 1990). Degens and Harris' (1997) variation of the BIOLOG system to measure in situ catabolic potential of microbial communities overcomes many problems inherent in applying the BIOLOG approach to soils (Nannipieri et al. 2002). Values of Degens and Harris' index are bounded by the number of substrates compared, and ranges of 16–22 (for 36 substrates; Degens 1998; Degens et al. 2000) and 11–23 (for 25 substrates; Schipper et al. 2001) have been found. In soil nematodes, six putative feeding or functional groups are typically recognised, and calculation of an index based on the relative contribution of these to the assemblage has a smaller range (e.g. 2.7–3.2; Freckman and Ettema 1993; Neher and Olson 1999). In the light of the uncertainty of allocation to these groups and the small range there must be serious reservations about the application of such an index to soil nematodes. This situation emphasises the need to discriminate the whole range of taxa present in functional groups.
Nematode grazing on soil microbes affects their standing crop (i.e. soil microbial biomass; Cragg and Bardgett 2001; Sonnemann et al. 1999). Nematodes are grazed by other soil biota (Yeates and Wardle 1996). Food specificity is widespread among soil-inhabiting nematodes (Yeates 1998; Venette and Ferris 1998). Within the constraints of soil type, soil moisture and temperature, the nature of the food resources (e.g. plant cultivar, C:N ratio of litter, bacterial species) influences the species and dominance within each nematode trophic group. There are, however, problems in adequately discriminating functional species. For soil nematodes, this can be exemplified by the delay in recognising the complex of species and races of Globodera rostochiensis and G. pallida (Stone 1973, 1977), and the long-term coexistence of two Panagrolaimus species identified by Sohlenius (1988).
Contribution of bacterial-feeding nematodes
The soil microbial biomass is "the eye of the needle through which all nutrients must pass" (Jenkinson 1977) and, as described above, laboratory and field studies have shown the importance of microbial grazing, and especially bacterial-grazing, by nematodes in increasing plant nutrient cycling (Bardgett et al. 1999). This section therefore re-examines some data on the contribution of bacterial-feeding nematodes to nematode assemblages. For practical reasons instantaneous population estimates are used; no account is taken of seasonal or predation-induced population changes.
Classical Maturity Index sites
The data of Ettema and Bongers (1993) show that over 72% of the nematode assemblage under their trial on bare ground in The Netherlands were classified as bacterial-feeding, and that of the bacterial-feeding nematodes the Cephalobidae made the greatest proportional contribution to the total nematode fauna under "bare ground control" (0.354–0.320). In the two disturbed treatments, Cephalobidae were the dominant group (0.283, 0.502), except at 5 and 7 weeks after disturbance (Table 1). No fungal-feeding nematodes were reported to be present. Nematode diversity, as measured by Shannon-Weiner's H′, increased with time in both the disturbed treatments.
If Freckman and Ettema's (1993) data from Michigan are averaged by annual and perennial crops, annual crops, with pulses of organic matter input, have Rhabditidae as the most abundant group of bacterial feeders (0.201), while under perennial crops Cephalobidae predominated (0.163; Table 1). While bacterial-feeding nematodes were relatively more abundant in soil under annual crops than under perennial crops (0.458 vs 0.399), the decomposition and diversity indices were similar under the two regimes.
Pastures
Under a series of 13 grazed New Zealand pastures bacterial-feeding nematodes made up 0.198–0.499 of the nematode assemblage (Yeates 1984). Cephalobidae predominated among bacterial-feeding nematodes, except that in one year Plectidae were dominant at Kaitoke and, following irrigation (i.e. effectively a pulse of resource availability), in Otiake soil Rhabditidae predominated (Table 2). For three New Zealand soils there is information on the nematode assemblage for systems with and without earthworms (Table 2; Yeates 1981). In all samples Cephalobidae were the predominant bacterial-feeders, but in every case in the absence of earthworms they make a much greater contribution (1.7- to 11.6-fold). Earthworms both alter decomposition rates and increase porosity; either factor could influence the populations of Cephalobidae.
In three Welsh soils pastures under organic management, Cephalobidae made a greater contribution than under conventional management (Table 2; Yeates et al. 1997). The only site at which Rhabditidae outnumbered Cephalobidae was conventionally managed. Furthermore, under both management regimes the proportion of Cephalobidae increased with the clay content of the soil (loam > silt > sand). In the loam and sand the proportions of bacterial-feeding nematodes were lower under organic management, but in all three soils NCR was lower at the organic sites (i.e. nematodes associated with fungal-based decomposition were relatively more numerous). Nematode diversity was consistently higher under organic pastures than under conventional pastures.
At four out of five Polish grassland sites on peat, Rhabditidae were relatively more abundant than Cephalobidae (Table 2; Wasilewska 1999). Values for NCR and H′ were similar across the five sites.
Cropped sites
At all five sites in a cropping sequence in South Australia, Cephalobidae were the predominant bacterial-feeding nematodes (Table 3; Yeates and Bird 1994). Their greatest proportion (0.394) was under semi-natural shrubland that had the highest proportion of bacterial-feeding nematodes (0.636) and which presumably had the lowest pulse of inputs. Nematode diversity was lowest in the annual crops.
Pasture and cropped sites on a silty clay loam in New Zealand had 40–68% bacterial-feeding nematodes and a predominance of Rhabditidae, except after 34 years cropping when the structurally degraded soil had a predominance of Plectidae (Cephalobidae outnumbered Rhabditidae 1.2x; Table 3; Saggar et al. 2001). In the silt loam, Cephalobidae predominated in all treatments. In neither of these soils was there a marked shift in NCR or H′ with cropping.
In two Swedish trials, Cephalobidae predominated, even in buried barley straw where NCR was only 0.44–0.50 (Table 3; Sohlenius 1989; Sohlenius and Boström 1984). Nematode diversity was greater in soil than in litter. On two sampling dates, the bacterial-feeding nematode assemblage in both unploughed grass leys and ploughed grass in Sweden was dominated by Cephalobidae (Table 3).
In North Carolina, Rhabditidae were relatively more abundant than Cephalobidae in both conventionally and organically cropped soils on 1993 but the opposite was the case in 1994 (Table 3; Neher 1999). A series of sites in Nebraska showed Rhabditidae, NCR and H′ to be greater under organic management (Table 3; Neher and Olson 1999).
Soil growing millet after a 21-year fenced fallow had the lowest proportion of bacterial-feeding nematodes of seven sites in Senegal; this site also had the lowest NCR value (Table 3; Villenave et al. 2001). At all sites Cephalobidae were the dominant group of bacterial-feeding nematodes.
Forest, scrub and natural sites
Twenty-two years after planting Pinus radiata in a pumice soil in New Zealand both the proportion of bacterial-feeding nematodes and nematode diversity in the surface 0–10 cm soil decreased with increasing density of trees (Table 4; Yeates et al. 2000). Cephalobidae were the dominant bacterial-feeding nematode group. While the upper 20 cm of soil under P. radiata included over 70% bacterial-feeding nematodes, their contribution to 80-cm depth was erratic. However, Cephalobidae were the predominant group at every depth (Table 4).
In a uniform New Zealand soil under three land uses the predominant group of bacterial-feeding nematodes varied (Yeates 1996). Under improved pasture Cephalobidae were predominant, while Rhabditidae dominated in native forest. The regenerating shrubland had the lowest NCR, the highest diversity, and Plectidae were the most abundant bacterial-feeding nematode group (Table 4). Over 12 months Plectidae were the dominant bacterial-feeders in a Danish Fagus forest (Yeates 1973); this was a site at which Tardigrada replaced Mononchidae as the main predator on nematodes (Yeates and Wardle 1996).
Summer nematode assemblages under a halophyte in an Israeli desert were dominated by Cephalobidae but in bare ground between plants Rhabditidae dominated (Table 4; Liang et al. 2002).
In subarctic shrub heathland and two Signy Island moss sites, Plectidae were the dominant group of bacterial-feeding nematodes (Table 4; Spaull 1973). In continental Antarctica, two sites were dominated by Cephalobidae and Panagrolaimidae, respectively (Freckman and Virginia 1998; Sinclair and Sjursen 2001).
Sites polluted with heavy metals
In all New Zealand pasture plots receiving artificially contaminated sewage sludge there was an increase in bacterial feeding nematodes as a proportion of total and microbial-feeding nematodes (Table 5; Yeates, unpublished). While untreated sludge resulted in dominance of Rhabditidae, addition of a heavy metal to the sludge was associated with dominance by Cephalobidae, as in the pasture control. In all forest plots the bacterial-feeding nematode fauna of the LFH layer was dominated by Plectidae, irrespective of heavy metal addition with sludge (Table 5).
Experimental addition of copper to a Dutch soil had little effect on the relative contribution of bacterial-feeders to the nematode assemblage (Korthals et al. 1996). Cephalobidae were the most abundant group under all treatments (Table 5).
In an area of accidental spillage of copper-chromium-arsenic in New Zealand, NCR was higher under more contamination while H′ steadily declined with increasing contamination (Table 5; Yeates et al. 1994). However, in all areas Cephalobidae were the dominant group of bacterial-feeding nematodes.
These examples suggest that:
-
1.
in many topsoils, Cephalobidae represent the most numerous group of bacterial-feeding nematodes;
-
2.
resource pulses (e.g. water, organic matter) or differing land management (e.g. organic farming) led to a relative increase in Rhabditidae in some cases and years;
-
3.
conversely, a resource limitation (e.g. heavy metal addition) led to an increase in the proportion of Cephalobidae in some cases;
-
4.
for a given soil texture, a relative decrease in porosity (through structural degradation or absence of earthworms) can result in an increase in the proportion of Cephalobidae;
-
5.
in a geographic region, clay content and Cephalobidae may be correlated (porosity rather than actual clay content presumably being the causal factor); and
-
6.
in coniferous forest, structurally degraded soils, Antarctic soils and successional scrub, Plectidae may be the predominant bacterial-feeding nematodes.
The overall nematode assemblage
In the past 30 years consideration of the contribution of free-living nematodes to soil processes has been associated with significant advances in understanding. Functional/feeding groups have played a key role in that advance, and nematodes are now accepted as playing critical roles in controlling the turnover of the soil microbial biomass and thus in the availability of plant nutrients. The use of indices has played a key part but, by definition, indices limit further advances. There must be concern about the validity of divisions and weightings applied to nematodes [mathematical debate about the most suitable bases for, or form of, indices is discussed by Magurran (1988) and Colinvaux (1993)].
In the marine environment it has been found that use of family-level identification of benthic invertebrates, including nematodes, gives comparable measures of the impact of pollution, as does species identification (Warwick 1988). However, this work did not consider any functional attributes of the systems studied.
Plant nematologists have been concerned with particular nematode groups as plant pathogens, and as each group has been studied in relation to particular soil and crop conditions there has of necessity been increasing sophistication in the systematics of both nematode and plant. Development of biological control of insects has also demonstrated the critical importance of selection of the most appropriate nematode population (sometimes compounded with an appropriate micro-organism; Liu et al. 2000). In both these areas of study, multi-year trials are common in production systems and overall system performance (within the prevalent production or environmental paradigm) is important.
Use of nematodes as indicators of soil, site or ecosystem condition also requires precision. Indices convey summaries of information, and extensive use of the Maturity Index has generally confirmed the types of trends anticipated between local perturbations. Using the Maturity Index for comparison among soil types or regions raises another set of questions.
The contribution of many plant-pathogenic nematodes to agroecosystem processes is demonstrably species (or even "race" or "pathotype") specific. There is no reason for the relation of "free-living" nematodes to ecosystem processes to be any different. Indeed, careful work by Ferris et al. (1996, 2001) has shown successional changes in bacterial-feeding nematodes and the influence of moisture on the changes; nitrogen availability to plants was related to the succession. The effect of many environmental perturbations has been assessed using nematodes as indicators; there are often marked changes in populations, usually assessed at the family or genus level. The base data in some papers show changes at species level, but in most regions there is inadequate systematic knowledge for analysis at this level. In considering the diversity of nematodes in a tropical forest, Bloemers et al. (1997) stressed the substantial inputs required to document adequately the >400 species involved, and the problems in using indices or all-taxon inventories with such information.
When there are marked long-term shifts in specific nematode populations it is critical that the species be identified and the shifts assessed in ecosystem terms. A pulse of input may result in a pulse of Rhabditidae; if there is also an underlying population of Cephalobidae it will probably resume dominance; if the Cephalobidae become locally extinct nematode diversity in the patch will require recolonisation. As diversity and succession within functional groups are of importance, it is essential that any additional tools, including molecular, be species rather than family based.
Conclusion
Soil nematode diversity is high but typically only six functional groups are recognised. Biodiversity is regarded as essential to ensure resilience in basic, underpinning ecological processes. Among the bacterial-feeding nematodes Cephalobidae are often the most abundant group in soils; Rhabditidae may increase following a resource pulse; in stressed, natural environments Plectidae may be important. Potential resilience can only be assessed by addressing diversity within individual functional groups rather than in the nematode assemblage as a whole. In many jurisdictions such assessment will be difficult due to inadequate systematic knowledge of the nematode fauna; in all studies appropriate resources will be necessary to permit adequate identification.
References
Adams GA, Wall DH (2000) Biodiversity above and below ground the surface of soils and sediments: linkages and implications for global change. Bioscience 50:1043–1048
André HM, Ducarme X, Lebrun P (2002) Soil biodiversity: myth, reality or conning? Oikos 96:3–24
Bardgett RD, Cook R, Yeates GW, Denton CS (1999) The influence of nematodes on below-ground processes in grassland ecosystems. Plant Soil 212:23–33
Bardgett RD, Jones AC, Jones DL, Kemmitt SJ, Cook R, Hobbs PJ (2001) Soil microbial community patterns related to the history and intensity of grazing in sub-montane ecosystems. Soil Biol Biochem 33:1653–1664
Berney MF, Bird GW (2001) Analysis of nematode community structure (abstract). J Nematol 33:251
Bloemers GF, Hodda M, Lambshead PJD, Lawton JH, Wanless FR (1997) The effects of forest disturbance on diversity of tropical soil nematodes. Oecologia 111:575–582
Bongers T (1990) The maturity index: an ecological measure of environmental disturbance based in nematode species composition. Oecologia 83:14–19
Bongers T, Bongers M (1998) Functional diversity of nematodes. Appl Soil Ecol 10:239–251
Bongers T, Ferris H (1999) Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol Evol 14:224–228
Boucher G (1990) Patterns of nematode species diversity in temperate and tropical subtidal sediments. Mar Ecol 11:133–146
Bouwman LA, Zwart KB (1994) The ecology of bacterivorous protozoans and nematodes in arable soil. Agric Ecosyst Environ 51:145–160
Boyd IL, Murray AWA (2001) Monitoring of a marine ecosystem using responses of upper trophic level predators. J Anim Ecol 70:747–760
Cobb NA (1915) Nematodes and their relationships. In: Yearbook of the United States Department of Agriculture, 1914. Government Printing Office, Washington, D.C., pp 457–490
Colinvaux P (1993) Ecology 2. Wiley, New York
Cragg RG, Bardgett RD (2001) How changes in soil faunal diversity and composition within a trophic group influence decomposition processes. Soil Biol Biochem 33:2073–2081
Degens BP (1998) Microbial functional diversity can be influenced by the addition of simple organic substrates to soil. Soil Biol Biochem 30:1981–1988
Degens BP, Harris JA (1997) Development of a physiological approach to measuring the catabolic diversity of soil microbial communities. Soil Biol Biochem 29:1309–1320
Degens BP, Schipper LA, Sparling GP, Vojvodic-Vukovic M (2000) Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol Biochem 32:189–196
De Goede RGM, Bongers T, Ettema CH (1993) Graphical presentation and interpretation of nematode community structure: c-p triangles. Meded Rijksfac Landbouww Gent 58(2b):743–750
De Ruiter PC, Moore JC, Zwart KB, Bouwman LA, Hassink J, Bloem J, De Vos JA, Marinissen JCY, Didden WAM, Lebbink G, Brussaard L (1993) Simulation of nitrogen mineralization in the below-ground food webs of two winter wheat fields. J Appl Ecol 30:95–106
Duffy JE (2002) Biodiversity and ecosystem function: the consumer connection. Oikos 99:201–219
Ettema CH (1998) Soil nematode diversity: species coexistence and ecosystem function. J Nematol 30:159–169
Ettema CH, Bongers T (1993) Characterization of nematode community colonization and succession in disturbed soil using the Maturity Index. Biol Fertil Soils 16:79–85
Ettema CH, Yeates GW (2003) Nested spatial biodiversity patterns in a New Zealand forest and pasture soil. Soil Biol Biochem (in press)
Ferris H, Venette RC, Lau SS (1996) Dynamics of nematode communities in tomatoes grown in conventional and organic farming systems, and their impact on soil fertility. Appl Soil Ecol 3:161–175
Ferris H, Bongers T, De Goede RGM (2001) A framework for soil food web diagnostics: extension of the nematode faunal analysis concept. Appl Soil Ecol 18:13–29
Fiscus DA, Neher DA (2002) Distinguishing sensitivity of free-living soil nematode genera to physical and chemical disturbances. Ecol Appl 12:565–575
Freckman DW, Ettema CH (1993) Assessing nematode communities in agroecosystems of varying human intervention. Agric Ecosyst Environ 45:239–261
Freckman DW, Virginia RA (1998) Soil biodiversity and community structure in the McMurdo Dry Valleys, Antarctica. In: Priscu JC (ed) Ecosystem dynamics in a polar desert: the McMurdo Dry Valleys, Antarctica. American Geophysical Union, Washington, D.C., pp 323–335
Hunt HW, Wall DH (2002) Modelling the effects of loss of soil biodiversity on ecosystem function. Global Change Biol 8:33–50
Ingham RE, Trofymow JA, Ingham ER, Coleman DC (1985) Interactions of bacteria, fungi and their nematode grazers: effects on nutrient cycling and plant growth. Ecol Monogr 55:119–140
Jenkinson DS (1977) The soil biomass. N Z Soil News 25:213–218
Jones FGW (1980) Some aspects of the epidemiology of plant parasitic nematodes. In: Polti J, Kranz J (eds) Comparative epidemiology: a tool for better disease management. Pudoc, Wageningen, pp 71–92
Korthals GW, Alexiev AD, Lexmond TM, Kammenga JE, Bongers T (1996) Long-term effects of copper and pH on the nematode community in an agroecosystem. Environ Toxicol Chem 15:979–985
Laasko J, Setälä H (1999) Sensitivity of primary production to changes in the architecture of belowground food webs. Oikos 87:57–64
Lambshead PJD, Platt HM, Shaw KM (1983) The detection of differences among assemblages of marine benthic species based on an assessment of dominance and diversity. J Nat Hist 17:859–874
Liang W, Mouratov S, Pinhasi-Adiv Y, Avigad P, Steinberger Y (2002) Seasonal variation in the nematode communities associated with two halophytes in a desert ecosystem. Pedobiologia 46:63–74
Liiri M, Setälä H, Haimi J, Pennanen T, Fritze H (2002) Relationship between soil microarthropod species diversity and plant growth does not change when the system is disturbed. Oikos 96:137–149
Liu J, Poinar GO, Berry RE (2000) Control of insect pests with entomopathogenic nematodes: the impact of molecular biology and phylogenetic reconstruction. Ann Rev Entomol 45:287–306
Magurran AE (1988) Ecological diversity and its measurement. Croon Helm, London
Mikola J, Sulkova P (2001) Responses of microbial-feeding nematodes to organic matter distribution and predation in experimental soil habitat. Soil Biol Biochem 33:811–817
Moore JC, Hunt HW (1988) Resource compartmentation and the stability of real ecosystems. Nature 333:261–263
Nannipieri P, Ceccanti B, Grego S (1990) Ecological significance of the biological activity in soil. In: Bollag J-M, Stotzky G (eds) Soil biochemistry, vol 6. Dekker, New York, pp 293–355
Nannipieri P, Kandeler E, Ruggiero P (2002) Enzyme activities and microbiological and biochemical processes in soil. In: Burns RG, Dick RP (eds) Enzymes in the environment. Dekker, New York, pp 133
Neher DA (1999) Nematode communities in organically and conventionally managed agricultural soils. J Nematol 31:142–154
Neher DA (2001) Role of nematodes in soil health and their use as indicators. J Nematol 33:161–168
Neher DA, Olson RK (1999) Nematode communities in soils of four farm cropping management systems. Pedobiologia 43:430–438
Ruess L, Michelsen A, Schmidt IK, Jonasson S, Dighton J (1998) Soil nematode fauna of a subarctic heath: potential nematicidal action of plant leaf extracts. Appl Soil Ecol 7:111–124
Saggar S, Yeates GW, Shepherd TG (2001) Cultivation effects on soil biological properties, microfauna and organic matter dynamics in Eutric Gleysol and Gleyic Luvisol soils in New Zealand. Soil Till Res 58:55–68
Schipper LA, Degens BP, Sparling GP, Duncan LC (2001) Changes in microbial heterotrophic diversity along five plant successional sequences. Soil Biol Biochem 33:2093–2103
Sinclair BJ, Sjursen H (2001) Terrestrial invertebrates abundance across a habitat transect in Keble Valley, Ross Island, Antarctica. Pedobiologia 45:134–145
Smith JL, Paul EA (1990) The significance of soil microbial biomass estimations. In: Bollag J-M, Stotzky G (eds) Soil biochemistry, vol 6. Dekker, New York, pp 357–396
Sohlenius B (1979) A carbon budget for nematodes, rotifers and tardigrades in a Swedish coniferous forest soil. Holarct Ecol 2:30–40
Sohlenius B (1988) Interactions between two species of Panagrolaimus in agar cultures. Nematologica 34:208–217
Sohlenius B (1989) Ploughing of a perennial grass ley effect on the nematode fauna. Pedobiologia 33:199–210
Sohlenius B, Boström S (1984) Colonization, population development and metabolic activity of nematodes in buried barley straw. Pedobiologia 27:67–78
Sonnemann I, Dogan H, Klein A, Pieper B, Ekschmitt K, Wolters V (1999) Response of soil microflora to changes in nematode abundance evidence for large scale effects in grassland soil. J Plant Nutr Soil Sci 162:385–391
Spaull VW (1973) The Signy Island terrestrial reference sites. IV. The nematode fauna. Br Antarct Surv Bull 37:94–96
Stone AR (1973) Heterodera pallida n. sp. (Nematoda: Heteroderidae), a second species of potato cyst nematode. Nematologica 18:591–606
Stone AR (1977) Recent developments and some problems in the taxonomy of cyst-nematodes, with a classification of the Heteroderoidea. Nematologica 23:273–288
Tietjen JH (1989) Ecology of deep-sea nematodes from the Puerto Rico Trench area and Hatteras Abyssal Plain. Deep Sea Res 36:1579–1594
Townsend CR, Harper JL, Begon M (2000) Essentials of ecology. Blackwell Science, Malden, Mass.
Venette RC, Ferris H (1998) Influence of bacterial type and density on population growth of bacterial-feeding nematodes. Soil Biol Biochem 30:949–960
Villenave C, Bongers T, Ekschmidt K, Djigal D, Chotte JL (2001) Changes in nematode communities following cultivation of soils after fallow periods of different length. Appl Soil Ecol 17:43–52
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components. Princeton University Press, Princeton, N.J.
Wardle DA, Barker GM, Yeates GW, Bonner KI, Ghani A (2001) Introduced browsing mammals in natural New Zealand forests: aboveground and belowground consequences. Ecol Monogr 71:587–614
Warwick RM (1988) The level of taxonomic discrimination required to detect pollution effects on marine benthic communities. Mar Pollut Bull 19:259–268
Wasilewska L (1979) The structure and function of soil nematode communities in natural ecosystems and agrocoenoses. Pol Ecol Stud 5:97–145
Wasilewska L (1994) The effect of age of meadows on succession and diversity in soil nematode communities. Pedobiologia 38:1–11
Wasilewska L (1999) Soil nematode response to root production in grasslands on fen peat soils. Pol J Ecol 47:231–246
Wieser W (1953) Die Beziehung zwischen Mundhöhlengestalt, Ernährungsweise und Vorkommen bei freilebenden marinen Nematoden. Ark Zool 4:439–484
Wolters V (2001) Biodiversity of soil animals and its function. Eur J Soil Biol 37:221–227
Yeates GW (1973) Nematoda of a Danish beech forest. II. Production estimates. Oikos 24:179–185
Yeates GW (1981) Soil nematode populations depressed in the presence of earthworms. Pedobiologia 22:191–195
Yeates GW (1984) Variation in soil nematode diversity under pasture with soil and year. Soil Biol Biochem 16:95–102
Yeates GW (1987) How plants affect nematodes. Adv Ecol Res 17:61–113
Yeates GW (1994) Modification and qualification of the nematode maturity index. Pedobiologia 38:97–101
Yeates GW (1996) Diversity of nematode faunae under three vegetation types on a pallic soil in Otago, New Zealand. N Z J Zool 23:401–407
Yeates GW (1998) Feeding in free-living soil nematodes: a functional approach. In: Perry RN, Wright DJ (eds) Physiology and biochemistry of free-living and plant-parasitic nematodes. CAB International, Wallingford, pp 245–269
Yeates GW, Bird AF (1994) Some observations on the influence of agricultural practices on the nematode faunae of some South Australian soils. Fund Appl Nemat 17:133–145
Yeates GW, King KL (1997) Soil nematodes as indicators of the effect of management of grasslands in the New England Tablelands (NSW): comparison of native and improved grasslands. Pedobiologia 41:526–536
Yeates GW, Wardle DA (1996) Nematodes as predators and prey: relationships to biological control and soil processes. Pedobiologia 40:43–50
Yeates GW, Bongers T, De Goede RGM, Freckman DW, Georgieva SS (1993a) Feeding habits in soil nematode families and genera—an outline for soil ecologists. J Nematol 25:315–331
Yeates GW, Wardle DA, Watson RN (1993b) Relationships between nematodes, soil microbial biomass and weed management strategies in maize and asparagus cropping systems. Soil Biol Biochem 25:869–876
Yeates GW, Orchard VA, Speir TW, Hunt JL, Hermans MCC (1994) Impact of pasture contamination by copper, chromium, arsenic timber preservative on soil biological activity. Biol Fertil Soils 18:200–208
Yeates GW, Bardgett RD, Cook R, Hobbs PJ, Bowling PJ, Potter JF (1997) Faunal and microbial diversity in three Welsh grassland soils under conventional and organic management regimes. J Appl Ecol 34:453–470
Yeates GW, Wardle DA, Watson RN (1999) Responses of soil nematode populations, community structure and temporal variability to agricultural intensification over a seven-year period. Soil Biol Biochem 31:1721–1733
Yeates GW, Hawke MF, Rijske WC (2000) Changes in soil fauna and soil conditions under Pinus radiata agroforestry regimes during a 25-year tree rotation. Biol Fertil Soils 30:391–406
Yeates GW, Dando J, Shepherd TG (2002) Pressure plate studies to determine how soil moisture affects access of bacterial-feeding nematodes to food in soil. Eur J Soil Sci 53:355–365
Acknowledgements
This work was funded by the Foundation for Research, Science and Technology (N.Z.) under contracts CO9X0016 and C09X0004. Richard Bardgett and the referees made constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeates, G.W. Nematodes as soil indicators: functional and biodiversity aspects. Biol Fertil Soils 37, 199–210 (2003). https://doi.org/10.1007/s00374-003-0586-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-003-0586-5