Abstract
Many plants produce plant secondary metabolites (PSM) that inhibit digestive enzymes of herbivores, thus limiting nutrient availability. In response, some specialist herbivores have evolved digestive enzymes that are resistant to inhibition. Monoterpenes, a class of PSMs, have not been investigated with respect to the interference of specific digestive enzymes, nor have such interactions been studied in avian herbivores. We investigated this interaction in the Greater Sage-Grouse (Phasianidae: Centrocercus urophasianus), which specializes on monoterpene-rich sagebrush species (Artemisia spp.). We first measured the monoterpene concentrations in gut contents of free-ranging sage-grouse. Next, we compared the ability of seven individual monoterpenes present in sagebrush to inhibit a protein-digesting enzyme, aminopeptidase-N. We also measured the inhibitory effects of PSM extracts from two sagebrush species. Inhibition of aminopeptidase-N in sage-grouse was compared to inhibition in chickens (Gallus gallus). We predicted that sage-grouse enzymes would retain higher activity when incubated with isolated monoterpenes or sagebrush extracts than chicken enzymes. We detected unchanged monoterpenes in the gut contents of free-ranging sage-grouse. We found that three isolated oxygenated monoterpenes (borneol, camphor, and 1,8-cineole) inhibited digestive enzymes of both bird species. Camphor and 1,8-cineole inhibited enzymes from chickens more than from sage-grouse. Extracts from both species of sagebrush had similar inhibition of chicken enzymes, but did not inhibit sage-grouse enzymes. These results suggest that specific monoterpenes may limit the protein digestibility of plant material by avian herbivores. Further, this work presents additional evidence that adaptations of digestive enzymes to plant defensive compounds may be a trait of specialist herbivores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Specialist herbivores regularly consume diets containing high concentrations of plant secondary metabolites (PSMs) (Shipley et al. 2009). These chemicals act to deter feeding by altering the homeostasis of the herbivore through various mechanisms (Dearing et al. 2005a). One common mode of action is to inhibit digestive enzymes of herbivores (Kohl and Dearing 2011; Rhoades 1977; Oh and Hoff 1986; Chauhan et al. 2007; Welsch et al. 1989; Feeny 1969). These interactions may limit nutrient availability to the animal and potentially impact fitness (DeGabriel et al. 2009). In response, herbivores have evolved mechanisms that enhance resistance to enzyme inhibition, such as elevating gut pH or producing surfactants that limit PSM—enzyme interactions (Berenbaum 1980; Martin and Martin 1984). Other herbivores may produce unique forms of digestive enzymes that are resistant to inhibition by PSMs. For example, many insect herbivores produce digestive enzymes that are resistant to proteinaceous enzyme inhibitors (Jongsma and Bolter 1997). Similarly, populations of Bryant’s woodrat (Cricetidae: Neotoma bryanti) produce digestive enzymes that are resistant to inhibition by the phenolic-rich resin produced by creosote bush (Zygophyllaceae: Larrea tridentata) (Kohl and Dearing 2011).
Monoterpenes are a class of chemical compounds that have not been investigated as digestive inhibitors (Stirby et al. 1987) despite their potency at inhibiting other enzymes, such as acetylcholinesterase (Miyazawa et al. 1997; Perry et al. 2000). This research gap likely stems from the fact that these compounds, due to their physicochemical properties, are readily and quickly absorbed through the intestinal wall and unlikely to interact with digestive enzymes. For example, many mammalian herbivores absorb >95 % of ingested monoterpenes (Boyle et al. 1999; Foley et al. 1987; Sorensen et al. 2004; Shipley et al. 2012). However, several specialist herbivores, such as the Stephen’s woodrat (Cricetidae: Neotoma stephensi) and the Greater Sage-Grouse (Phasianidae: Centrocercus urophasianus) exhibit reduced absorption of monoterpenes and excrete considerable amounts of unchanged monoterpenes in their feces (Sorensen et al. 2004; Thacker et al. 2012). Thus, there is the potential for unabsorbed monoterpenes to interact with digestive enzymes in these animals. If unabsorbed PSMs do inhibit digestive enzymes, specialist herbivores are predicted to produce digestive enzymes that are more resistant to inhibition by monoterpenes, similar to what has been observed with other classes of PSMs in other specialist herbivores (Jongsma and Bolter 1997; Kohl and Dearing 2011).
We focused on the Greater Sage-Grouse (hereafter, sage-grouse), a specialist avian herbivore. Sage-grouse are seasonal dietary specialists on sagebrush (Asteraceae: Artemisia spp.; Wallestad et al. 1975). Across the year, sagebrush leaves comprise 62 % of the diet of sage-grouse and are the only component of their diet in winter (Wallestad et al. 1975). Sagebrush leaves are heavily defended by high concentrations of diverse mixtures of several classes of PSMs, especially monoterpenes (Kelsey et al. 1982; Welch and McArthur 1981), phenolics (Wilt et al. 1992; Wilt and Miller 1992) and sesquiterpene lactones (Kelsey et al. 1976; Shafizadeh et al. 1974). Monoterpenes influence diet selection by sage-grouse (Remington and Braun 1985; Frye et al. 2013) and other vertebrate herbivores (Bray et al. 1991; Ulappa et al. 2014). Specifically, sage-grouse select black sagebrush (A. nova) over Wyoming big sagebrush (A. tridentata wyomingensis) due, in part, to its lower concentration of total monoterpenes (Frye et al. 2013). Further, sage-grouse select individual plants and patches of black sagebrush with lower concentrations of individual monoterpenes while also selecting for the highest protein content (Frye et al. 2013). Sage-grouse also excrete unchanged monoterpenes in their feces (Thacker et al. 2012) and thus may experience interactions between monoterpenes and digestive enzymes.
We investigated the potential for interactions between monoterpenes and the digestive enzyme aminopeptidase-N (APN). This enzyme cleaves terminal amino acids from digested proteins, facilitating absorption by the animal (Sjöström et al. 2002). Since most herbivores are protein-limited (Karasov and Martinez del Rio 2007), maintaining activity of this enzyme is critical for herbivores to obtain sufficient nitrogen. We first measured PSM concentrations in the contents of several gut segments of free-ranging sage-grouse. Next, we tested whether isolated monoterpenes inhibit digestive enzymes of the sage-grouse and a species naïve to sagebrush and associated PSMs, the domestic chicken (Phasianidae: Gallus gallus). In accordance with other herbivores that exhibit counter-adaptations to resist negative impacts of ingested PSMs, we predicted that sage-grouse enzymes would exhibit lower rates of inhibition by isolated monoterpenes than the domestic chicken. We also predicted that whole extracts of sagebrush species would inhibit digestive enzymes of animals due to the presence of PSMs such as monoterpenes, phenolics, and sesquiterpene lactones. Additionally, because free-ranging sage-grouse select black sagebrush over Wyoming sagebrush (Frye et al. 2013), we predicted that extracts of PSMs from black sagebrush would exhibit lower inhibition of digestive enzymes compared to those extracted from Wyoming big sagebrush. Similar to the prediction for isolated monoterpenes, we also predicted that sage-grouse enzymes would exhibit lower rates of inhibition by sagebrush extracts compared to chickens.
Materials and methods
Chemicals
All chemicals were purchased from Sigma–Aldrich (St. Louis, MO, USA) or Fisher Scientific (Pittsburg, PA, USA) and were of ACS grade, except monoterpene standards, which were of GC grade.
Animal collection
Intestinal tracts of sage-grouse (n = 11) were obtained to confirm the presence of unchanged monoterpenes in the intestinal tract. Licensed hunters generously collected birds across Idaho (hunting locations were not disclosed) between December 2011 and February 2012. Whole carcasses were kept on ice while hunters were in the field and then transported to a −20 °C freezer. Intestinal tracts were dissected from each bird. The contents of the small intestine, ceca, and colon were isolated, weighed, and stored at −20 °C prior to analysis. Analysis of gizzard contents confirmed that all birds were consuming exclusively sagebrush leaves, though the species of sagebrush was not determined. Because hunters collected these carcasses in the field, collection and cryopreservation of tissue from these same birds for enzyme assays was not possible.
Intestinal tissues for enzyme assays were obtained from three sage-grouse (one male, two females) collected in south-central Idaho (E 721093, N 4789063) during Nov–Dec 2012. Sage-grouse were collected under approved permits (Idaho permit 110914 and Boise State University IACUC protocol AC11-022). Carcasses were dissected immediately in the field and the proximal section of the small intestine was immediately stored in liquid nitrogen and stored at −80 °C.
Our sample sizes for sage-grouse were limited to three individuals. Sage-grouse have undergone significant population decline (Connelly and Braun 1997), are currently listed as ‘near-threatened’ by the International Union for the Conservation of Nature (Birdlife 2012), and are a candidate species under the Endangered Species Act. Agencies are becoming increasingly reluctant to issue collecting permits. Thus, the collection of additional samples would be subject to substantial criticism from various special interest groups and is not feasible.
Tissues from four chickens were provided by the University of Wisconsin–Madison. Chickens were fed Purina Start and Grow SunFresh Poultry feed and housed under University of Wisconsin–Madison IACUC protocol A00733. The proximal section of the small intestine was immediately frozen and stored at −80 °C. All tissues were mailed to University of Utah on dry ice for enzyme assays.
Extraction of sagebrush PSMs
Black sagebrush and Wyoming big sagebrush were collected during the winter of 2010–2011 from a site in south-central Idaho, USA (42°11′N, 114°46′W) used by wintering sage-grouse. A mosaic of black sagebrush (A. nova) and Wyoming big sagebrush (A. tridentata wyomingensis) dominated the vegetation in this area and included an understory of native grasses, exotic grasses, and forbs. Elevation ranged from approximately 1,550 to 1,750 m and average annual precipitation was 26.3 cm with temperatures during collection ranging from 4.68 to 5.98 °C. Sage-grouse were not collected at this site but it was the same site used to determine selective foraging on species, patches, and plants of sagebrush by sage-grouse (Frye et al. 2013). We extracted PSMs from five individuals of each sagebrush species following a previously established protocol (Durling et al. 2007). We added ethanol to small samples of frozen plants using a 6:1 solvent:plant ratio (mass:mass). Ethanol was used as a solvent for consistency with assays using isolated monoterpenes and its high boiling point compared to other common solvents, given that incubations with tissues were conducted at avian body temperature (40 °C; see below). Samples were then ground with a Polytron PT3100 Mixer (Kinematica, Lucerne, Switzerland) at 12,000 rpm for 30 s. We then incubated the samples at 40 °C for 6 h. The solution was then filtered through Whatman filter paper (grade 1) and stored at −20 °C until enzyme assays were conducted.
Confirmation of PSMs in gut contents and extracts
We measured the concentrations of seven individual monoterpenes in the contents of gut segments from free-ranging sage-grouse. We also verified the presence of the same individual monoterpenes in the ethanol extracts of sagebrush used in inhibition assays. Monoterpenes were analyzed by placing 100 mg wet weight of content from each intestinal segment or 50 μL of sagebrush extract into an airtight headspace vial. Concentrations of individual monoterpenes and total monoterpenes were determined using headspace gas chromatography with an Agilent 7694 headspace sampler coupled with an Agilent 6890 N gas chromatograph (GC). 1 mL of headspace gas was injected into a J&W DB-5 capillary column (30 m × 250 µm × 0.25 µm). Operating conditions for the headspace sampler were as follows: oven temperature at 100 °C, loop temperature at 110 °C, transfer line temperature at 120 °C, a vial equilibrium time of 20 min, a pressurization time of 0.20 min, a loop fill time of 0.50 min, a loop equilibrium time of 0.20 min, and an injection time of 0.50 min. Operating conditions for the GC were as follows: splitless injector at 250 °C, flame ionization detector at 300 °C, oven temperature at 40 °C for 2 min, then increasing 3 °C/min to 60 °C, then increasing 5 °C/min to 120 °C, then increasing 20 °C/min to 300 °C, and held at 300 °C for 7 min. The make-up gas was nitrogen and the carrier gas was helium. The inlet pressure was 80 kPa with a flow rate of 1.0 mL/min. Retention times and peak areas of individual monoterpenes were calculated using Agilent OpenLab version A.01.05.
We verified the relative retention times of individual monoterpenes with co-chromatography using a mixture of standards (10 mg/mL concentration) of camphene (CAS #79-92-5), camphor (76-22-2), borneol (464-45-9), p-cymene (99-87-6), 1,8-cineole (470-67-7), α-pinene (7785-26-4), and β-pinene (18172-67-3). We estimated the relative concentrations of individual monoterpenes in the intestinal content material by comparing AUC of peaks in gut samples to AUC of a known volume of 10 mg/mL monoterpene standards. This method does not allow us to account for the composition of enantiomers of individual monoterpenes, but does allow us to confirm at least one enantiomer is present in our samples. Several unidentified monoterpenes were also detected. We calculated concentration of monoterpenes in mM using the assumption that wet gut contents had a density equivalent to water. We estimated total monoterpene concentration in mM by adding peaks from all identified and unidentified monoterpenes and using averages of AUCs and molar masses of the known monoterpenes that were detected in samples.
Enzyme Inhibition Assays
We investigated aminopeptidase-N from the proximal small intestine of sage-grouse (n = 3) and chickens (n = 4). Tissues were thawed and rinsed in ice-cold physiological saline solution to remove gut contents and thus remove microbial enzymes. Tissues were homogenized in a buffer of 350 mM mannitol in 1 mM N-2-hydroxyethylpiperazine-N′-2-ethanosulfonic acid (HEPES)-KOH, pH 7.0. Intestinal homogenates were then incubated with PSMs to allow for inhibition. For isolated compounds, we incubated 12 μL of intestinal homogenate with 4 μL of isolated monoterpenes diluted in ethanol to the following concentrations (mM): 50, 100, 200, 400, and 800. This mixture resulted in the following final concentrations of monoterpenes: 12.5, 25, 50, 100, and 200 mM. For whole sagebrush PSMs, we incubated 12 μL of intestinal homogenate with 4 μL of the ethanol extract. For control samples we incubated 12 μL of intestinal homogenate with 4 μL of ethanol. All samples were incubated at 40 °C for 10 min. We then measured APN activity using l-alanine-p-nitroanilide as a substrate, which is cleaved into p-nitroaniline as a reaction product that can be quantified. To start the reaction, we added 2.5 μL of the incubated homogenate mixture (with or without PSMs) to 250 μL of assay mix (2.0 mM l-alanine-p-nitroanilide in one part of 0.2 M NaH2PO4/Na2HPO4, pH 7.0, and one part of deionized H2O). The reaction solution was incubated for 20 min at 40 °C and terminated with 750 μL of ice-cold 2 N acetic acid. Assays from each sample were run with technical duplicates and activity was averaged for each sample. Additionally, we always corrected against a blank tube where tissue was added after the acetic acid to prevent the reaction from occurring. Duplicate 200 μL aliquots of all final reactions were transferred to a 96-well plate and absorbance was measured at 384 nm. Activity (rate of product formation/g tissue/min) was determined using a standard curve for the product p-nitroaniline. We calculated relative enzyme activity by dividing the activity of PSM-exposed tissues by the activities of the control samples, which were incubated only with ethanol.
It is worth noting that the addition of ethanol did not inhibit enzyme activities. We measured enzyme activities of all sage-grouse and chicken samples by incubating 12 μL of intestinal homogenate with 4 μL of ethanol as described above. Another set of assays was conducted simultaneously where tissues were incubated with 4 μL of homogenizing buffer. There was no difference in activity between assays with ethanol and those with homogenizing buffer (Wilcoxon signed-rank test: P = 0.41).
Statistics
Concentrations of monoterpenes were compared across gut segments (small intestine, ceca and colon) using a Tukey’s HSD test. For inhibition with isolated monoterpenes, we generated inhibition curves for three individual sage-grouse and four individual chickens. We compared inhibition between sage-grouse and chickens using a repeated measures ANOVA for each of the seven individual monoterpenes with avian species as a variable and monoterpene concentration as the repeated variable. We applied the Huynd-Feldt Correction to the ‘Concentration’ and ‘Concentration × Bird species’ terms to address the violation of sphericity in our data (Huynh and Feldt 1976).
For extracts of whole sagebrush PSMs, we measured enzyme inhibition of tissues from one sage-grouse individual and one chicken individual using extracts from five individual plants for each of the two sagebrush species. We compared inhibition using a one-sample t test against a mean of 1 for each sagebrush and bird species combination. The two sagebrush species were compared using t tests for each avian species. Using one chicken and one sage-grouse allowed us to minimize enzyme variability among individual birds and instead compare the variation in inhibition by PSMs across individual plants for each sagebrush species. The objective of these assays was to compare the inhibitory properties of sagebrush species; therefore, we used five individual plants from each sagebrush species.
Results
We detected individual monoterpenes in various segments of the intestinal tract of free-ranging sage-grouse (Table 1). The highest concentrations were detected in the colon, while the ceca exhibited very low concentrations (Table 1). Cymene was not detected in any sample. Camphor was present in the highest concentration in all gut segments (Table 1). We detected the presence of the same isolated monoterpenes in the ethanol extracts of sagebrush.
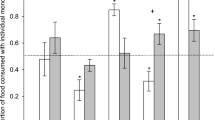
Isolated monoterpenes varied in their capacity to inhibit APN enzymes. Borneol, camphor, and 1,8-cineole all exhibited significant inhibition as a function of monoterpene concentration (Table 2, Fig. 1). At the highest concentrations of monoterpenes these compounds reduced APN activity to 32–78 % of original activity, depending on the compound and avian species. All other monoterpenes (α-pinene, β-pinene, camphene, and cymene) did not inhibit APN enzymes (Table 2; Fig. 1).
Effects of isolated monoterpenes on sage-grouse (n = 3) and chicken (n = 4) APN enzymes. Points represent the mean ± SEM of relative enzyme activity. Compounds followed by an “I” significantly inhibited APN activity. Compounds labeled with an asterisk exhibited differential inhibition between sage-grouse and chickens
Sage-grouse exhibited differential inhibition kinetics compared to chickens for some monoterpenes. Camphor and 1,8-cineole were more potent against chicken enzymes than sage-grouse enzymes (Table 2; Fig. 1). At the highest concentration of camphor, chicken enzymes were inhibited by 50 % of capacity, while sage-grouse enzymes were only inhibited by 22 %. Similarly, at the highest concentration of 1,8-cineole, chicken enzymes were inhibited by 63 % of capacity, while sage-grouse enzymes were only inhibited by 22 %.
Interestingly, incubation with β-pinene seemed to increase relative APN activity in the chicken samples, but not the sage-grouse samples (Fig. 1). While this difference was not statistically significant (Table 2), it was noteworthy and unique compared to all other monoterpenes.
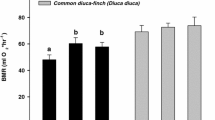
Extracts from both sagebrush species significantly inhibited the chicken APN enzyme (one-sample t test against a mean of 1: P = 0.037 and P = 0.038 for black and Wyoming sagebrush, respectively; Fig. 2). However, these extracts did not inhibit the sage-grouse APN enzyme (P = 0.14 and P = 0.35 for black and Wyoming sagebrush, respectively; Fig. 2). Within a bird species, the level of enzyme inhibition was similar regardless of sagebrush species (t test comparing inhibition of sagebrush species: P > 0.65 for both chicken and sage-grouse; Fig. 2).
Effects of sagebrush extracts on sage-grouse and chicken APN enzymes. N = 5 individual plants for each bar. Bars represent the mean ± SEM of relative enzyme activity. Inhibition assays were conducted using samples from one chicken and one sage-grouse, which allowed us to minimize enzyme variability among individual birds and instead compare the variation in inhibition by PSMs across individual plants for each sagebrush species
Discussion
The co-evolutionary arms race between plants and herbivores has been instrumental in shaping terrestrial ecology (Dearing et al. 2005a; Freeland and Janzen 1974; McArthur et al. 1991). One site of this arms race is the intestinal tract, where many PSMs limit digestibility by herbivores, and in response, herbivores have evolved digestive enzymes that are tolerant to inhibitory compounds (Jongsma and Bolter 1997; Kohl and Dearing 2011). Most examples of this trend have been identified in herbivores feeding on plants containing tannins or proteinaceous protease inhibitors (Jongsma and Bolter 1997; McArthur et al. 1991). Here, we add monoterpenes as a class of chemical compounds that may limit digestibility of proteins through inhibition of digestive enzymes. Most research to date has been conducted on insects (Jongsma and Bolter 1997) and to a lesser extent, mammals (Kohl and Dearing 2011). Our research suggests that an avian herbivore exhibits a similar adaptation, i.e., digestive enzymes that are tolerant of inhibitory compounds in the plants they consume.
Some specific monoterpenes inhibited the APN enzyme of sage-grouse and chickens while others did not. The idiosyncratic inhibition by some monoterpenes is consistent with other studies. While monoterpenes are potent at inhibiting acetylcholinesterase (Perry et al. 2000; Miyazawa et al. 1997), research on a related enzyme, butyrylcholinesterase, found that only one of 21 isolated terpenes was inhibitory (Savelev et al. 2004). Although the mechanism of inhibition of APN by monoterpenes is not known, monoterpenes competitively inhibit other enzymes (acetylcholinesterase) by binding to the hydrophobic active site of the enzyme (Perry et al. 2000; Miyazawa et al. 1997). Other PSMs, such as tannins, can lower enzyme activity by binding to the substrate and limiting interactions with the digestive enzyme, rather than inhibiting the digestive enzyme itself (Mole and Waterman 1986). It is unlikely that this mechanism is occurring in our study, given that we found species differences in the susceptibility to inhibition by monoterpenes. The structure–activity relationships of enzyme inhibition by monoterpenes is poorly understood, but seems unrelated to lipophilicity or molecular size of the monoterpene in other enzyme studies (Perry et al. 2000), though these trends may vary among different enzymes (Savelev et al. 2004). In our study, the monoterpenes that inhibited APN (borneol, camphor, and 1,8-cineole) were larger compounds (≥152 Da), more water-soluble (>700 mg/L), and all contained oxygen atoms, while the compounds that did not inhibit APN (camphene, cymene, α-pinene, β-pinene) were smaller (≤136 Da), less water-soluble (<25 mg/L), and did not contain oxygen (Howard 1997; Krzysztof 2006; Cheng et al. 2013). These results suggest that molecular size, solubility, or chemical structure may play a role in inhibition of APN by monoterpenes. Similarly, oxygenated monoterpenes are highly potent inhibitors of ruminal microbial activity, while monoterpene hydrocarbons are not inhibitory (Oh et al. 1967, 1968). Overall, understanding the mechanism by which monoterpenes, differing chirality of monoterpenes, other classes of PSMs, or mixtures of PSMs inhibit APN or other digestive enzymes deserves further attention.
Consistent with our hypothesis, sage-grouse APN retained higher relative activity when exposed to monoterpenes compared to chicken enzymes. For example, 1,8-cineole only inhibited sage-grouse enzyme activity by 22 % while it inhibited chicken enzymes by 63 %. Similarly, extracts of sagebrush inhibited activity of chicken APN, but not sage-grouse APN. These findings align with the work of others demonstrating that specialist insects and mammals have evolved digestive enzymes that are more tolerant to inhibitory compounds than generalists (Jongsma and Bolter 1997; Kohl and Dearing 2011). Our results add an avian herbivore to this pattern. Specifically, resistance to APN inhibition may allow sage-grouse and other specialists to feed on PSM-rich plants without experiencing decreases in digestibility of protein. Our study is limited to a two-species comparison; thus investigations of other avian herbivores may further elucidate the nature of enzyme adaptation to terpene-rich diets. Another promising study system may be the smallest avian herbivores, plantcutter birds (Cotingidae: Phytotoma spp.), which specialize on mesquite (Fabaceae: Prosopis spp.) and boxthorn (Solanaceae: Lycium spp.) (Bucher et al. 2003; Ríos et al. 2014; Rosina and Romo 2012). These plants contain some monoterpenes, as well as alkaloids and phenolics (Pisani and Distel 1998; Yao et al. 2011). Interestingly, plantcutters have nearly twice the APN activity of a similarly sized omnivorous passerine (Meynard et al. 1999). It would be interesting to compare digestive enzyme inhibition by various classes of PSMs between plantcutters and omnivorous passerines.
Mechanisms underlying the production of enzymes that are resistant to inhibition have been well studied in other plant-animal interactions. For example, herbivorous insects produce digestive enzymes with unique sequences that render them tolerant to inhibition by proteinaceous inhibitors (Jongsma et al. 1995). Resistance to tannins can be brought about by post-translational modification of proteins, such as glycosylation (Sarni-Manchado et al. 2008). It is unknown whether these mechanisms would also make digestive enzymes more resistant to inhibition by monoterpenes.
The monoterpene β-pinene exhibited the unique effect of seeming to increase enzyme activity in chicken samples, but not sage-grouse samples. β-pinene increases the relative activity of NADH dehydrogenase in yeast, likely by increasing membrane fluidity (Uribe et al. 1985). APN is located in the cell membranes of intestinal cells (Sjöström et al. 2002), and thus increasing membrane fluidity may also increase its relative activity. Incubation with β-pinene did not alter the relative APN activity of sage-grouse samples, suggesting that their cell membranes may be resistant to these effects. However, these ideas are largely speculative.
Our analyses of the contents of the intestinal tracts of sage-grouse confirm that a portion of ingested monoterpenes are not absorbed or altered by sage-grouse and may interact with digestive enzymes. The ceca exhibited the lowest monoterpene concentrations of the three regions, which may be accomplished through microbial degradation of monoterpenes (Adams et al. 2013), whereas the large intestine exhibited the highest concentrations. It would be interesting to compare enzyme activity across gut regions, as many birds express digestive enzymes in their ceca and colons (McWhorter et al. 2009; Siddons 1972). We predict that enzyme activity might be highest where monoterpene concentrations are lowest, thus avoiding inhibition by these compounds. This notion is supported by the fact that absorptive capacity is high in the ceca of sage-grouse, compared to higher absorption in the small intestinal observed in most other birds (Obst and Diamond 1989).
Concentrations of specific monoterpenes in the small intestine (0–1.2 mM) were quite low compared to the ranges used in our inhibition assays with isolated monoterpenes (25–200 mM). However, our technique measured concentrations in total luminal contents. Concentrations may be highly heterogeneous throughout the gut and between the lumen and gut surface (Takahashi 2011). This heterogeneity can depend on flow rates and flow behavior of the gut, as well as viscosity of the gut contents (Takahashi 2011). Thus, some digestive enzymes may experience higher concentrations of monoterpenes in vivo. Additionally, concentrations exhibited inter-individual variability. For example, camphor measurements in the small intestine and colon were as high as 5 mM. Such concentrations would likely reduce enzyme efficiency in chickens, but not in sage-grouse. Even slight reductions in digestive efficiency may have fitness consequences for animals, especially given that dietary protein is often limited in herbivores. Regulating intestinal concentrations of monoterpenes below thresholds that cause inhibition of digestive enzyme may explain selection for sagebrush with lower concentrations of monoterpenes by free-ranging sage-grouse (Frye et al. 2013).
The ecological relevance of our studies is better revealed by the use of whole sagebrush extracts. The low monoterpene concentrations of these extracts significantly inhibited chicken enzymes by 14 %, but did not inhibit sage-grouse enzymes. We hypothesize that differences in enzyme structure yielded differences between avian species. Further, we hypothesize that maintenance of enzyme activities aids sage-grouse in specializing on sagebrush.
We did not find any evidence that differences in enzyme inhibition might underlie the preference by sage-grouse for black sagebrush over Wyoming sagebrush (Frye et al. 2013), given that the two extracts yielded similar effects. Lack of differential inhibition by sagebrush species may reflect our focus on the inhibitory capacities of monoterpenes. Sagebrush species are also known to produce many phenolics (Wilt et al. 1992) and sesquiterpene lactones (Kelsey et al. 1976) that vary among species, which may also inhibit digestive enzymes (Min et al. 2003; Tadera et al. 2006). The extraction solvent we used (ethanol) extracts these other PSM classes. Although differences in total phenolics between Wyoming and black sagebrush have not been detected (Frye et al. 2013), differential concentrations or presence of other classes of PSMs, such as individual phenolics or sesquiterpene lactones, in our whole-plant extract may confound interpretation of the APN inhibition assays with regards to specific monoterpenes.
While we present evidence that monoterpenes have the capacity to inhibit digestibility, further work is needed to demonstrate these effects in vivo. Many animals absorb >95 % of ingested terpenes (Boyle et al. 1999; Foley et al. 1987; Sorensen et al. 2004; Shipley et al. 2012) and concentrations in the gut may be below effective doses and thus these interactions may not always occur in vivo. Additionally, animals may compensate through other mechanisms such as the production of surfactants to prevent PSM-protein interactions (Martin and Martin 1984) or slowing gut transit time to maximize the extraction of nutrients (Karasov and Douglas 2013). Several studies have demonstrated that ingestion of terpene-rich plants such as sagebrush or juniper lowers nitrogen and dry matter digestibility in herbivores (Dearing et al. 2005b; Ngugi et al. 1995). However, these plants often have other PSMs such as tannins and phenolics that may inhibit protein digestion (Dearing et al. 2005b; Wilt et al. 1992). Feeding trials that augment artificial diets with isolated monoterpenes are difficult due to the volatility of these compounds. While feeding trials of this nature have been conducted (Dearing et al. 2000; Wiggins et al. 2003), nutrient digestibility has not been investigated. Further experiments will reveal the capacity of monoterpenes and other PSMs to inhibit digestion and the adaptations that specialist herbivores have to overcome these challenges.
References
Adams AS, Aylward FO, Adams SM, Erbilgin N, Aukema BH, Currie CR, Suen G, Raffa KF (2013) Mountain pine beetles colonizing historical and naive trees are associated with a bacterial community highly enriched in genes contributing to terpene metabolism. Appl Environ Microbiol 79:3468–3475
Berenbaum M (1980) Adaptive significance of midgut pH in larval Lepidoptera. Am Nat 115:138–146
Birdlife International (2012) Centrocercus urophasianus. Accessed 15 Nov 2014
Boyle R, McLean S, Foley WJ, Davies NW (1999) Comparative metabolism of dietary terpene, p-cymene, in generalist and specialist folivorous marsupials. J Chem Ecol 25:2109–2126
Bray RO, Wambolt CL, Kelsey RG (1991) Influence of sagebrush terpenoids on mule deer preference. J Chem Ecol 17:2053–2062
Bucher EH, Tamburini D, Abril A, Torres P (2003) Folivory in the white-tipped plantcutter Phytotoma rutila: seasonal variations in diet composition and quality. J Avian Biol 34:211–216
Chauhan A, Gupta S, Mahmood A (2007) Effect of tannic acid on brush border disaccharidases in mammalian intestine. Ind J Exp Biol 45:353–358
Cheng C, Liu XW, Du FF, Li MJ, Xu F, Wang FQ, Liu Y, Li C, Sun Y (2013) Sensitive assay for measurement of volatile borneol, isoborneol, and the metabolite camphor in rat pharmacokinetic study of Borneolum (Bingpian) and Borneolum syntheticum (synthetic Bingpian). Acta Pharm Sinic 34:1337–1348
Connelly JW, Braun CE (1997) Long-term changes in Sage Grouse Centrocercus urophasianus populations in western North America. Wildl Biol 3:229–234
Dearing MD, Mangione AM, Karasov WH (2000) Diet breadth of mammalian herbivores: nutrient versus detoxification constraints. Oecologia 123:397–405
Dearing MD, Foley WJ, McLean S (2005a) The influence of plant secondary metabolites on the nutritional ecology of herbivorous terrestrial vertebrates. Ann Rev Ecol Evol Syst 36:169–185
Dearing MD, McLister JD, Sorensen JS (2005b) Woodrat (Neotoma) herbivores maintain nitrogen balance on a low-nitrogen, high-phenolic forage, Juniperus monosperma. J Comp Physiol B 175:349–355
DeGabriel JL, Moore BD, Foley WJ, Johnson CN (2009) The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal. Ecology 90:711–719
Durling NE, Catchpole OJ, Grey JB, Webby RF, Mitchell KA, Foo LY, Perry NB (2007) Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem 101:1417–1424
Feeny PP (1969) Inhibitory effect of oakleaf tannin production on the hydrolysis of proteins by trypsin. Phytochem 8:2119–2126
Foley WJ, Lassak EV, Brophy J (1987) Digestion and absorption of Eucalyptus essential oils in greater glider (Petauroides volans) and brushtail possums (Trichosurus vulpecula). J Chem Ecol 13:2115–2130
Freeland WJ, Janzen DH (1974) Strategies in herbivory by mammals: the role of plant secondary compounds. Am Nat 108:269–289
Frye GG, Connelly JW, Musil DD, Forbey JS (2013) Phytochemistry predicts habitat selection by an avian herbivore at multiple spatial scales. Ecology 94:308–314
Howard PH (1997) Handbook of environmental fate and exposure data for organic chemicals. CRC Press, Boca Raton
Huynh H, Feldt LS (1976) Estimation of the Box correction for degrees of freedom from sample data in randomised block and split-plot designs. J Educ Stat 1:69–82
Jongsma MA, Bolter C (1997) The adaptation of insects to plant protease inhibitors. J Insect Physiol 43:885–895
Jongsma MA, Bakker PL, Peters J, Bosch D, Stiekema WJ (1995) Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition. Proc Natl Acad Sci 92:8041–8045
Karasov WH, Douglas AE (2013) Comparative digestive physiology. Compr Physiol 3:741–783
Karasov WH, Martinez del Rio C (2007) Physiological Ecology: How Animals Process Energy, Nutrients, and Toxins. Princeton University Press, Princeton
Kelsey RG, Morris MS, Shafizadeh F (1976) The use of sesquiterpene lactones as taxonomic markers in the shrubby species of Artemisia (section tridentatae) in Montana. J Range Manage 29:502–505
Kelsey RG, Stephens JR, Shafizadeh F (1982) The chemical constituents of sagebrush foliage and their isolation. J Range Manage 35:617–622
Kohl KD, Dearing MD (2011) Induced and constitutive responses of digestive enzymes to plant toxins in an herbivorous mammal. J Exp Biol 214:4133–4140
Krzysztof C (2006) Aqueous solubility of liquid monoterpenes at 293 K and relationship with calculated log P value. Yakugaku Zasshi 126:307–309
Martin MM, Martin JS (1984) Surfactants: their role in preventing the precipitation of proteins by tannins in insect guts. Oecologia 61:342–345
McArthur C, Hagerman AE, Robbins CT (1991) Physiological strategies of mammalian herbivores against plant defenses. In: Palo RT, Robbins CT (eds) Plant defenses against mammalian herbivory. CRC Press, Boca Raton, USA, pp 103–114
McWhorter TJ, Caviedes-Vidal E, Karasov WH (2009) The integration of digestion and osmoregulation in the avian gut. Biol Rev 84:533–565
Meynard C, López-Calleja MV, Bozinovic F, Sabat P (1999) Digestive enzymes of a small avian herbivore, the Rufous-tailed Plantcutter. Condor 101:904–907
Min BR, Barry TN, Attwood GT, McNabb WC (2003) The effect of condensed tannins on the nutrition and health of ruminants fed fresh temperate forages: a review. Anim Feed Sci Technol 106:3–19
Miyazawa M, Watanabe H, Kameoka H (1997) Inhibition of acetylcholinesterase activity by monoterpenoids with a p-menthane skeleton. J Agric Food Chem 45:677–679
Mole S, Waterman PG (1986) Tannic acid and proteolytic enzymes: enzyme inhibition or substrate deprivation? Phytochem 26:99–102
Ngugi RK, Hinds FC, Powell J (1995) Mountain big sagebrush browse decreases dry matter intake, digestibility, and nutritive quality of sheep diets. J Range Manage 48:487–492
Obst BS, Diamond JM (1989) Interspecific variation in sugar and amino acid transport by the avian cecum. J Exp Zool 252:117–126
Oh H, Hoff JE (1986) Effect of condensed grape tannins on the in vitro activity of digestive proteases and activation of their zymogens. J Food Sci 51:577–580
Oh HK, Sakai T, Jones MB, Longhurst WM (1967) Effect of various essential oils isolated from Douglas fir needles upon sheep and deer rumen microbial activity. Appl Microbiol 15:777–784
Oh HK, Jones MB, Longhurst WM (1968) Comparison of rumen microbial inhibition resulting from various essential oils isolated from relatively unpalatable plant species. Appl Microbiol 16:39–44
Perry NSL, Houghton PJ, Theobald A, Jenner P, Perry EK (2000) In-vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol 52:895–902
Pisani JM, Distel RA (1998) Inter- and intraspecific variations in production of spines and phenols in Prosopis caldenia and Prosopis flexuosa. J Chem Ecol 24:23–36
Remington TE, Braun CE (1985) Sage grouse food selection in winter, North Park, Colorado. J Wildl Manage 49:1055–1061
Rhoades DF (1977) The antiherbivore chemistry of Larrea. In: Mabry TJ, Hunziker JH, DiFeo DR (eds) Creosote bush: biology and chemistry of Larrea in New World deserts. Hutchinson and Ross, Stroudsberg, pp 135–175
Ríos J, Zarco A, Mosca-Torres ME, Sabat P (2014) Dieta de Phytotoma rutila (Passeriformes: Cotingidae) en el desierto del Monte central, Argentina. Gayana (Concepción) 78:21–24
Rosina M, Romo M (2012) Reproducción y alimentación de Phytotoma raimondii, cortarrama peruana en El Gramadal, Ancash. Revista Peruana de Biología 19:167–173
Sarni-Manchado P, Canals-Bosch J-M, Mazerolles G, Cheynier V (2008) Influence of the glycosylation of human salivary proline-rich proteins on their interactions with condensed tannins. J Agric Food Chem 56:9563–9569
Savelev SU, Okello EJ, Perry EK (2004) Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother Res 18:315–324
Shafizadeh F, Bhadane NR, Kelsey RG (1974) Sesquiterpene lactones of sage brush: constituents of Artemisia tripartita. Phytochem 13:669–670
Shipley LA, Forbey JS, Moore BD (2009) Revisiting the dietary niche: when is a mammalian herbivore a specialist? Integr Comp Biol 49:274–290
Shipley LA, Davis EM, Felicetti LA, McLean S, Forbey JS (2012) Mechanisms for eliminating monoterpenes in sagebrush by specialist and generalist rabbits. J Chem Ecol 38:1178–1189
Siddons RC (1972) Effect of diet on disaccharidase activity in the chick. Brit J Nutr 27:343–352
Sjöström H, Norén O, Olsen J (2002) Structure and function of aminopeptidase N. In: Langner J, Ansorge S (eds) Cellular peptidases in immune functions and diseases 2. Springer, USA, pp 25–34
Sorensen JS, Turnbull CA, Dearing MD (2004) A specialist herbivore (Neotoma stephensi) absorbs fewer plant toxins than does a generalist herbivore (Neotoma albigula). Physiol Biochem Zool 77:139–148
Stirby KD, Wambolt CL, Kelsey RG, Havstad KM (1987) Crude terpenoid influence on in vitro digestibility of sagebrush. J Range Manage 40:244–248
Tadera K, Minami Y, Takamatsu K, Matsuoka T (2006) Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol 52:149–153
Takahashi T (2011) Flow behavior of digesta and the absorption of nutrients in the gastrointestine. J Nutr Sci Vitaminol 57:265–273
Thacker ET, Gardner DR, Messmer TA, Guttery MR, Dahlgren DK (2012) Using gas chromatography to determine winter diets of greater sage-grouse in Utah. J Wildl Manage 76:588–592
Ulappa AC, Kelsey RG, Frye GG, Rachlow LA, Shipley LA, Bond L, Pu X, Forbey JS (2014) Plant protein and secondary metabolites influence diet selection in a mammalian specialist herbivore. J Mamm 95:834–842
Uribe S, Ramirez J, Peña A (1985) Effects of β-pinene on yeast membrane functions. J Bacteriol 161:1195–1200
Wallestad R, Peterson JG, Eng RL (1975) Foods of adult sage-grouse in central Montana. J Wildl Manage 39:628–630
Welch BL, McArthur ED (1981) Variation of monoterpenoid content among subspecies and accessions of Artemisia tridentata grown in a uniform garden. J Range Manage 34:380–384
Welsch CA, Lachance PA, Wasserman BP (1989) Effects of native and oxidized phenolic compounds on sucrase activity in rat brush border membrane. J Nutr 119:1737–1740
Wiggins NL, McArthur C, McLean S, Boyle R (2003) Effects of two plant secondary metabolites, cineole and gallic acid, on nightly feeding patterns of the common brushtail possum. J Chem Ecol 29:1447–1464
Wilt FM, Miller GC (1992) Seasonal variation of coumarin and flavonoid concentrations in persistent leaves of Wyoming big sagebrush (Artemisia tridentata ssp. wyomingensis: Asteraceae). Biochem Syst Ecol 20:53–67
Wilt FM, Geddes JD, Tamma RV, Miller GC, Everett RL (1992) Interspecific variation of phenolic concentrations in persistent leaves among six taxa from subgenus Tridentatae of Artemisia (Asteraceae). Biochem Syst Ecol 20:41–52
Yao X, Peng Y, Xu LJ, Li L, Wu QL, Xiao PG (2011) Phytochemistry and biological studies of Lycium medicinal plants. Chem Biodivers 8:976–1010
Acknowledgments
We would like to thank Dr. William Karasov, Dr. Mark Cook, and Taylor Jarmes for assistance with obtaining tissues from chickens. We also thank S. Vasilchenko, N. Wiggins, falconers T. Maechtle, D. Skinner, and H. Quade as well as Gus, the German short-haired pointer, Jack and Kenna the English setters, Grace and Bob the gyrfalcons, and Gabriel the gyrfalcon/peregrine falcon hybrid for assistance with collecting tissues in the field. This research was funded by the Idaho Department of Fish and Game, Idaho Governor’s Office for Species Conservation to J. S. F, a University of Utah Undergraduate Research Opportunities Grant to E. P, the National Science Foundation (DEB-1146194 and IOS-1258217 to J. S. F, DEB-1210094 to M. D. D and K. D. K, and DBI-1400456 to K. D. K), and Idaho INBRE Program-NIH Grant #P20 GM103408 to J.S.F., This is a contribution from Idaho Federal Aid in Wildlife Restoration Project W-160-R.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Kohl, K.D., Pitman, E., Robb, B.C. et al. Monoterpenes as inhibitors of digestive enzymes and counter-adaptations in a specialist avian herbivore. J Comp Physiol B 185, 425–434 (2015). https://doi.org/10.1007/s00360-015-0890-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-015-0890-z