Abstract
We used implanted miniature data loggers to obtain the first measurements of body temperature from a free-ranging anthropoid primate. Vervet monkeys (Chlorocebus pygerythrus) living in a highly seasonal, semi-arid environment maintained a lower mean 24-h body temperature in winter (34.6 ± 0.5 °C) than in summer (36.2 ± 0.1 °C), and demonstrated increased heterothermy (as indexed by the 24-h amplitude of their body temperature rhythm) in response to proximal environmental stressors. The mean 24-h amplitude of the body temperature rhythm in summer (2.5 ± 0.1 °C) was lower than that in winter (3.2 ± 0.4 °C), with the highest amplitude for an individual monkey (5.6 °C) recorded in winter. The higher amplitude of the body temperature rhythm in winter was a consequence primarily of lower 24-h minimum body temperatures during the nocturnal phase, when monkeys were inactive. These low minimum body temperatures were associated with low black globe temperature (GLMM, β = 0.046, P < 0.001), short photoperiod (β = 0.010, P < 0.001) and low rainfall over the previous 2 months, which we used as a proxy for food availability (β = 0.001, P < 0.001). Despite the lower average winter minimum body temperatures, there was no change in the lower modal body temperature between winter and summer. Therefore, unlike the regulated physiological adjustments proposed for torpor or hibernation, these minimum winter body temperatures did not appear to reflect a regulated reduction in body temperature. The thermoregulatory plasticity nevertheless may have fitness benefits for vervet monkeys.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In seasonally-variable habitats, mammals are faced with the challenge of maintaining body temperature while being exposed to dynamic thermal environments and contending with fluctuations in food and water availability. At low environmental temperatures, typically in conjunction with food scarcity, some mammals—termed heterotherms—employ physiological mechanisms such as torpor or hibernation (i.e., prolonged torpor) that result in a decrease in energy expenditure (Geiser 2004). Torpor is defined as a state of inactivity and reduced responsiveness (IUPS Thermal Commission 2001), typically associated with a temporary, but substantial, reduction in body temperature below body temperature thresholds of about 35 °C (Willis 2007) to 30 °C (Barclay et al. 2001). However, recent studies have revealed significant seasonal and daily variation in body temperature in species that do not employ torpor or hibernation (for example, Pereira et al. 2002; Ostrowski et al. 2003; Arnold et al. 2004; Grigg et al. 2009; Hetem et al. 2010; Maloney et al. 2011). Thermoregulatory strategies amongst mammals, therefore, might more accurately be described as falling on a continuum, rather than as a dichotomy between homeothermy and heterothermy (Angilletta et al. 2010; Boyles et al. 2011a, 2013).
Indeed, body temperatures above the defined threshold for torpor, but below the homeothermic norm, still may confer significant energy savings, by reducing metabolic rate in much the same way as torpor does, namely by reducing the gradient between the body and environmental temperature (Angilletta et al. 2010). In temperate ungulates, like red deer, Cervus elaphus (Arnold et al. 2004), and alpine ibex, Capra ibex (Signer et al. 2011), lower body temperatures during periods of low environmental temperatures and food availability were associated with metabolic rates being lowered by as much as 60 %. In contrast, desert mammals like the Arabian oryx, Oryx leucoryx (Hetem et al. 2010) and western grey kangaroo, Macropus fuliginosus (Maloney et al. 2011), have lower body temperatures in hot periods, when food quality is poorest. In such environments, heterothermy, in the form of a greater 24-h amplitude of the body temperature rhythm, may arise from both a lower minimum daily body temperature and a higher maximum daily body temperature (Hetem et al. 2010). When access to water is restricted, an animal’s ability to maintain body temperature via evaporative cooling is compromised. By reducing or reversing the gradient between environmental and body temperature, a higher diurnal maximum body temperature (Schmidt-Nielsen et al. 1956; Mitchell et al. 2002; Hetem et al. 2010) reduces environmental heat gain and consequently the body water demands of evaporative cooling. So both lower minimum body temperature and higher maximum body temperature, in the 24-h rhythm, may benefit mammals in a dry, resource-poor environment.
Heterothermy, characterised by a greater amplitude of the daily body temperature rhythm as a result of lower minimum or higher maximum daily body temperatures, or both, is an example of the physiological plasticity which mammals may employ to cope with environmental stress (Fuller et al. 2010). However, studies of body temperature patterns in free-ranging mammals have focussed on small (<5 kg) species, which employ torpor or hibernation (e.g. Geiser 2004; Turbill et al. 2011) and on large ungulates (e.g. Hetem et al. 2010; Signer et al. 2011). The lack of data from smaller free-ranging mammals that do not employ torpor or hibernation currently constrains the development of a broader understanding of the functional mechanisms and the potential ecological implications of thermoregulatory plasticity (Boyles et al. 2011a).
The lack of data on thermoregulation in free-ranging non-Strepsirrhine primates is particularly notable. Research on thermoregulation in free-ranging primates has been restricted to the small, nocturnal Malagasy lemurs (Schmid and Speakman 2000; Dausmann et al. 2004; Schülke and Ostner 2007) and the similar-sized lesser galago, Galago moholi, of South Africa (Mzilikazi et al. 2006; Nowack et al. 2010). These Strepsirrhine primates display torpor during the cool, dry season, when food availability is limited (Schülke and Ostner 2007; Nowack et al. 2010). With the exception of baboons, Papio hamadryas (Brain and Mitchell 1999) in the Namib Desert, there have been no other body temperature measurements in free-ranging non-Strepsirrhine primates. Measurements in Namib baboons were confined to the daytime, and were intermittent; they showed that free-ranging baboons exhibited amplitudes of body temperature of up to 4 °C when the environment was hot and water was scarce. Lower amplitudes of the body temperature rhythm were typical of baboons exposed to high temperatures in the laboratory, with access to drinking water (Mitchell et al. 2009). Heterothermy resulting from a water deficit therefore may be present in baboons, as it is in some large ungulates (Mitchell et al. 2002; Hetem et al. 2010). Whether lower minimum 24-h body temperatures, during periods of reduced energy availability, are exhibited by free-ranging primates that do not enter torpor is unknown.
The aim of our study was to measure 24-h body temperature continuously across seasons in free-ranging vervet monkeys living in a semi-arid, seasonal environment (Pasternak et al. 2013), to quantify the proximal influence of environmental stressors on thermoregulatory precision or heterothermy (as indexed by the 24-h amplitude of the body temperature rhythm). The strictly diurnal activity patterns (McFarland et al. 2013) and relatively large body size of vervet monkeys distinguish them from those Strepsirrhini that enter torpor. We present seasonal patterns of body temperature and assess whether our study animals maintained strict homeothermy, independently of environmental conditions, or whether thermoregulatory precision was a function of proximal environmental stressors.
Materials and methods
All experimental procedures were approved by the University of the Witwatersrand Animal Ethics Screening Committee (clearance number AESC 2010/41/04).
Study site and animals
The study took place at Samara Private Game Reserve, Eastern Cape Province, South Africa (32°22′S, 24°52′E). Samara Private Game Reserve is situated in the semi-arid Nama Karoo. We collected data from two groups of habituated vervet monkeys that occupied adjacent territories constrained to a narrow corridor of riparian vegetation, dominated by Acacia karroo, transected by a stream that provided the monkeys with a permanent natural source of water during the study. The study troops had no contact with human settlements and food and water availability were not manipulated by humans.
Twenty-five adult vervet monkeys, captured during April and July 2011, underwent surgery for implantation of data loggers. Of those 25 monkeys, 12 (six males and six females) were recaptured in March 2012 and the data loggers were removed surgically. Mean body mass of monkeys for which data are presented was 4.3 ± 1.3 kg (five females: 3.4 ± 1.3 kg, four males: 5.6 ± 1.5 kg).
Surgical procedures
The monkeys were captured by immobilization with a blowpipe (Dan-Inject system, Zoo Blow 1.25, Dan-inject APS, Børhup, Denmark), via a dart containing ketamine (50 mg, Anaket-V, Bayer (Pty) Ltd., Johannesburg, South Africa) and midazolam (2.5 mg, Dormicum, Roche Products (Pty) Ltd., Johannesburg, South Africa). Once recumbent (~5 min), the monkeys were transported to a temporary surgical facility within 5 km of their site of capture. Surgery procedures were similar to those described in McFarland et al. (2013). At the temporary theatre anaesthesia was maintained as necessary with 0–2 % isoflurane (Isofor, Safe Line Pharmaceuticals (Pty) Ltd., Johannesburg, South Africa) administered in 100 % oxygen via an endotracheal tube. Respiratory rate, peripheral haemoglobin oxygen saturation, heart rate, end-tidal carbon dioxide (LifeSense® Vet LS1-10R, Medair AB, Hudiksvall, Sweden), blood pressure (Cardell® veterinary vital signs monitor, Model 9403, Sharn veterinary, Inc., Tampa, USA) and rectal temperature (thermocouple thermometer, BAT-12, Physitemp Instruments Inc., New Jersey, USA) were monitored at 5 min intervals throughout the surgical procedure. Monkeys were kept warm during surgery with heating pads. An ointment (Terra-Cortril, hydrocortisone: Pfizer laboratories (Pty) Ltd., Johannesburg, South Africa) was applied to the eyes to keep them moist. The monkeys received intramuscular long-acting antibiotic (Peni LA Phenix, penicillin: 0.001 mg kg−1, Virbac Animal Health, Johannesburg, South Africa), anti-inflammatory (Rimadyl, carprofen: 3 mg kg−1, Pfizer Laboratories Ltd., Johannesburg, South Africa) and analgesic (Temgesic, buprenorphine: 0.02 mg kg−1, Kyron Laboratories Ltd., Johannesburg, South Africa). During surgery the animals were maintained on a drip (Ringers lactate solution, B. Braun Medical Ltd., Northriding, South Africa) administered at 0.6 ml min−1.
A 100 × 100 mm patch on the ventral abdominal surface of each monkey was shaved and sterilized with Hibitane (chlorhexidine gluconate in alcohol, Astra Zeneca, Johannesburg, South Africa). A local anaesthetic (Lignocaine: 40 mg, Bayer (Pty) Ltd., Johannesburg, South Africa) was administered subcutaneously and a 30 mm midline incision was made through the skin and linea alba, and the muscle and skin layers were sutured closed. The surgical wound was treated with a germicidal wound spray (F10, Health and Hygiene, Johannesburg, South Africa). The mean duration of anaesthesia was 50 ± 15 min. The monkeys recovered in a warm, dark cage, before being released (about 1–2 h post-surgery), out of sight of other monkeys, near the rest of their troop. The monkeys were followed during daylight hours to monitor their recovery post-surgery. All study animals successfully rejoined their groups and resumed normal behaviour on the day following surgery. When the monkeys were recaptured, a similar surgical procedure was followed to remove the loggers. The wounds had healed and there were no signs of infection from the implant surgery.
Body temperature measurement
The temperature-sensitive data loggers (mlog T1C, Sigma Delta Technologies, Perth, Australia) had dimensions of 30 × 25 × 10 mm and a mass of approximately 20–25 g when coated in wax; less than 1 % of monkey body mass. The loggers were coated with an inert wax (Sasolwax 1276; Sasol, Johannesburg, South Africa) and dry-sterilized in formaldehyde vapour before implantation. The loggers were set to measure body temperature at 5 min intervals at a resolution of 0.06 °C (see Fig. 1 for representative body temperature trace). Before implanting, the loggers were calibrated against a precision thermometer (Quat 100, Heraeus, Hanau, Germany) in an insulated water bath. Three of the loggers failed shortly after they were launched and therefore provided no data. Of the nine monkeys for which we present data, eight were implanted in April 2011 and one in July 2011. Further loggers failed after November, but five still were working at explant surgery. So we have nearly a year of body temperature measurements for five monkeys, and a minimum of 7 months for all nine monkeys. For the time during which nine loggers were recording, there were no systematic differences between the data recorded by the loggers that remained functional and by those that failed subsequently.
Environmental measurement
Black globe temperatures (°C) were measured at 30 min intervals by a weather station (Hobo U12, Onset Computer Corporation, Pocasset, USA) on the reserve, in an open clearing exposed to direct sunlight throughout the day. Black globe temperature incorporates the influence of air temperature, solar radiation and wind speed, and is therefore a better measure of the thermal environment than is air temperature alone (Hetem et al. 2007). Rainfall was measured on site by means of a standard 100 mm rain gauge. Total rainfall was recorded after each rainfall event. We used cumulative rainfall as a proxy for food availability. It has been shown for various sub-Saharan sites that primary production is correlated with rainfall (Le Houerou 1984; Barton et al. 1992). For baboons in central Kenya, rainfall was significantly correlated with food availability, with the total rainfall over the two previous months being the strongest predictor of food availability in the current month (Barton et al. 1992). Because baboons and vervet monkeys have broadly similar diets (Skinner and Chimimba 2005), we used total rainfall over the two previous months as a proxy for food availability in the current month. The times of sunrise and sunset for Samara Private Game Reserve were obtained from the United States Naval Observatory (http://aa.usno.navy.mil/data). Photoperiod was calculated as the number of hours of daylight between sunrise and sunset.
Data analysis
From the original 5-min measures of body temperature, the daily (24 h) minimum, maximum, mean, and amplitude of the body temperature rhythm were calculated. To analyse seasonal variations, we used Pearson product-moment correlations between parameters of the body temperature rhythm and days, for the period between the winter (21 June) and summer (22 December) solstices. We calculated each parameter for each monkey, and averaged these values across all monkeys, to obtain a mean value for each parameter for the group of monkeys per day.
Based on mean 24-h black globe temperatures, we analysed data for “winter”, the three coldest months (June, July and August), and “summer”, the three hottest months (December, January and February). We used paired t tests to compare mean 24-h minimum, 24-h maximum, 24-h mean, and 24-h amplitude of the body temperature rhythm between winter and summer for the six monkeys that had a minimum of 30 sample days in both winter and summer.
The mode of body temperature may approximate the preferred or optimal body temperature (Boyles et al. 2011b). The monkeys had bimodal distributions of body temperature over 24-h. We compared the upper and lower modal body temperatures between winter and summer, by extracting individual modes from a frequency distribution of body temperatures for each monkey for winter and summer, for the six monkeys with a minimum of 30 sample days in each season. The distributions were centred on 0.1 °C intervals across the range 34–42 °C. Analyses were performed with GraphPad Prism v4.0. The Kolmogorov–Smirnov test confirmed normality. If variables were correlated significantly, we conducted linear regression analysis.
We used a generalised linear mixed-effects model (GLMM) to investigate the influence that environmental factors had on the body temperature rhythms of our animals. For reasons that will become apparent (see “Results”), we entered 24-h minimum body temperature as our dependent variable. We entered rainfall over the two previous months, a proxy for food availability, photoperiod and 24-h minimum black globe temperature as our three independent variables. We included sex, mass, and group as control variables to control for their potential effects on body temperature. Finally, we entered individual identity as a random factor to control for the non-independence of data points (Pinheiro and Bates 2000). GLMM analysis was performed using STATA v10.1 software (StataCorp 2007).
Results
Environmental conditions
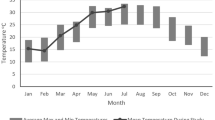
In the period we called winter, the 24-h minimum black globe temperatures ranged between −4.0 and 19.0 °C, being below freezing on 23 % of days, whereas black globe temperatures in our summer period never dropped below zero, with 24-h minimum black globe temperatures ranging between 1.6 and 20.6 °C. In summer, 24-h maximum black globe temperatures ranged between 20.6 and 59.9 °C, and exceeded 40 °C on 54 % of days, compared to only 1 day in winter, during which 24-h maximum black globe temperatures ranged between 7.0 and 41.5 °C. The mean 24-h black globe temperature was lowest in July and highest in January (Fig. 2a), so environmental temperature extremes were delayed by 1 month after photoperiod extremes (Fig. 2b). In winter, the monkeys were exposed to relatively short day lengths (10.4 ± 0.4 h, mean ± SD) compared to in summer (13.8 ± 0.5 h). Compared to the mean annual rainfall between 2000 and 2010 (307 ± 68 mm) our study site experienced unusually high rainfall (450 mm) during the current study period. The total rainfall over the two previous months decreased progressively after logger implantation to a minimum in October, and then reversed, but not to the March maximum (Fig. 2c).
Environmental conditions during the study period. a Mean (±SD between days within the month) 24-h black globe temperature averaged per month over the study period. b Mean (±SD) daily photoperiod averaged per month. c Sum of rainfall over the two previous months, a proxy for food availability, over the duration of the study period
Variation in body temperature
The mean 24-h minimum body temperature was significantly lower in winter (34.6 ± 0.5 °C) than in summer (36.2 ± 0.1 °C; t = 7.45, P < 0.0001, N = 6). The mean 24-h minimum body temperature was lowest in July and highest in January (Fig. 3a, left panel). Thus, the mean 24-h minimum body temperature was positively correlated with the number of days after the winter solstice (Fig. 3a, right panel, r 2 = 0.54, P < 0.0001, N = 185). There was no significant difference in the mean 24-h maximum body temperature between winter (40.3 ± 0.6 °C) and summer (40.6 ± 0.4 °C; t = 0.87, P = 0.42, N = 6). Although the mean 24-h maximum body temperature increased significantly between the winter and summer solstice (Fig. 3b, right panel, r 2 = 0.08, P < 0.0001, N = 185), it was by only 0.1 °C per hundred days, and the regression explained only 8 % of the variation in the 24-h maximum body temperature. The mean 24-h maximum monthly body temperature varied by less than 0.2 °C over the duration of the study period (Fig. 3b, left panel).
Body temperature parameters averaged per month (left panels) and per day between the winter and summer solstice (right panels). Left panels mean (±SD), a 24-h minimum, b 24-h maximum, c 24-h mean, and d 24-h amplitude of the daily body temperature rhythm averaged per months for the duration of the study period. SD is the variability between individual monkeys. N is the sample size (monkeys) per month. Right panels mean 24-h minimum (a y = 0.005 (x) + 35.95), 24-h maximum (b y = 0.001 (x) + 39.33), 24-h mean (c y = 0.004 (x) + 37.57), and 24-h amplitude (d y = −0.004 (x) + 3.38) of the body temperature rhythm across days between the winter and summer solstices
Since the mean 24-h minimum body temperature increased significantly between winter and summer, with no significant change in the mean 24-h maximum body temperature, the 24-h mean body temperature was significantly lower in winter (37.7 ± 0.2 °C) than it was in summer (38.2 ± 0.1 °C: t = 5.37, P < 0.01, N = 6), and increased significantly across days between the winter and summer solstices (Fig. 3c, right panel, r 2 = 0.61, P < 0.0001, N = 185). Like minimum 24-h body temperature, mean 24-h body temperature was lowest in July and highest in January (Fig. 3c, left panel). The low 24-h minimum body temperature in winter, with no significant change in the 24-h maximum body temperature, resulted in a significantly greater 24-h amplitude of the body temperature rhythm in winter (3.2 ± 0.4 °C) than in summer (2.5 ± 0.1 °C; t = 5.47, P < 0.01, N = 6), and the mean 24-h amplitude of the body temperature rhythm decreased linearly between the winter and summer solstices (Fig. 3d, right panel, r 2 = 0.42, P < 0.0001, N = 185). In summer, the highest 24-h amplitude of the body temperature rhythm recorded for an individual monkey was 4 °C. In winter, the 24-h amplitude of the body temperature rhythm for individual monkeys exceeded 4 °C on 12 ± 11 % of days, with the highest 24-h amplitude of the body temperature rhythm recorded for any individual monkey being 5.6 °C. As expected, the 24-h amplitude of the body temperature rhythm was lowest in January and highest in July (Fig. 3d, left panel).
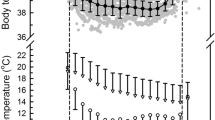
In both winter and summer, the phase of the nycthemeral rhythm of body temperature was closely associated with the light:dark cycle (Fig. 4), with body temperatures being highest during the 7 diurnal period. A sharp increase in body temperature occurred before sunrise in summer, but after sunrise in winter. After that increase, in both winter and summer, body temperature increased slowly during the day and peaked shortly before sunset, before decreasing rapidly to the nocturnal mean. A notable difference between the winter and summer nycthemeral rhythms of body temperature for monkeys was the extent of individual variation in the body temperature before dawn and during the day in winter compared to summer (Fig. 4).
As was apparent especially in the winter data, the 24-h rhythm of body temperature did not appear to be sinusoidal. Rather, it appeared square-wave, as was confirmed by the frequency distribution of body temperature (Fig. 5). The lower modal body temperature was 37.1 °C in winter (Fig. 5a) and 37.3 °C in summer (Fig. 5b). These values were not significantly different (t = 2.10, P = 0.09, N = 6) in monkeys with at least 30 days of data in each season. The upper modal body temperature was 39.0 °C in winter (Fig. 5a) and 38.6 °C in summer (Fig. 5b), a difference that was significant (t = 5.94, P < 0.01, N = 6).
Mean (+SD) frequency distribution of observed body temperatures, divided into 0.1 °C categories, over the 24-h period for nine monkeys in winter (a N = 92 days) and six monkeys in summer (b N = 91 days). Vertical dotted lines indicate lower and upper modal body temperatures. SD is the variability between individual monkeys
GLMM analysis, across the full study period, revealed that the 24-h minimum body temperature was positively correlated with 24-h minimum black globe temperature, photoperiod, and rainfall over the previous 2 months (Table 1). Minimum body temperature also was positively correlated with body mass.
Discussion
Our study provides the first continuous measurements of body temperature for any free-ranging cercopithecine primate. Our vervet monkeys displayed seasonal variations in body temperature, with the 24-h amplitude of the body temperature rhythm decreasing significantly (by ~0.8 °C) from mid-winter to mid-summer. The mean 24-h amplitude of the body temperature rhythm in winter was more than twice that predicted for a mammalian species of 5 kg body mass (i.e. ~1.5 °C; Mortola and Lanthier 2004), and higher than that previously reported for capuchin monkeys, Cebus albifrons, owl monkeys, Aotus trivirgatus, rhesus macaques, Macaca mulatta, and squirrel monkeys, Saimiri sciureus, in laboratory conditions (i.e. 1–2 °C; Refinetti and Menaker 1992). The highest recorded 24-h amplitude of the body temperature rhythm in any of our vervet monkeys was 5.6 °C. The higher 24-h amplitude of the body temperature rhythm in winter compared to summer was a consequence of lower 24-h minimum body temperatures. These minima occurred during the nocturnal phase, which is when environmental temperatures were lowest and the monkeys were inactive.
While our monkeys displayed greater heterothermy (as indexed by the 24-h amplitude of the body temperature rhythm) than expected, their thermoregulatory response to the challenges they faced may have been tempered by unusually high rainfall in the year of our study. Across our study period, rainfall exceeded the annual mean for the region, and each winter month was preceded by 2 months in which more than 80 mm of rain fell (about 30 % of the average annual rainfall). A higher energy availability from increased biomass would have helped meet the energy demand in the cold better. Karoo winters characteristically are dry; our monkey groups had no access to free-standing water for up to 33 days during a drought in winter in 2009 (McDougall et al. 2010). In typical years, therefore, we would expect the monkeys to exhibit even lower minima of body temperature in the winter, and greater heterothermy. We did not quantify energy intake, but the cumulative rainfall over the two previous months, our proxy for food availability, influenced the 24-h minimum body temperature independently of environmental temperature.
Unlike the 24-h minimum body temperatures, there was no significant change in the mean 24-h maximum body temperatures between summer and winter. Baboons displayed hyperthermia when exposed to similarly high environmental temperatures as our vervet monkeys, but did so only when they were deprived of drinking water (Brain and Mitchell 1999; Mitchell et al. 2009). The absence of hyperthermia observed in our vervet monkeys in summer was likely to have been facilitated by a combination of ad libitum access to free-standing water as well as behavioural thermoregulation. Primates possess a broad array of behavioural and physiological mechanisms for coping with heat stress, including, for example, extending their tongues during the heat of the day to facilitate evaporative cooling (Campos and Fedigan 2009), sand bathing (Brain and Mitchell 1999), sweating (Stitt and Hardy 1971), employing heat dissipating postures and selecting cool microclimates (e.g. Stelzner and Hausfater 1986; Da Silva 1993; Barrett et al. 2004; Hill 2006; Kosheleff and Anderson 2009). These thermoregulatory behaviours, in addition to a constant water supply, may have buffered the effects of high environmental temperatures on the maximum daily body temperature in summer.
Behavioural mechanisms, such as sunbathing and adopting heat-conserving body postures, also play an important thermoregulatory role for primates in cold environments (Hanya 2004). Sunbathing and huddling significantly increased skin temperature in Japanese macaques, Macaca fuscata, indicating that these behaviours may help conserve energy in cold environments (Hanya et al. 2007), although, as the authors acknowledged, skin temperature may vary significantly from core body temperature. Sunbathing in non-human primates, including vervet monkeys (e.g. Morland 1993; Danzy et al. 2012), is also considered a behavioural strategy to help deal with cold stress. However, it appears that any behavioural thermoregulation by our monkeys did not compensate fully for the low environmental temperatures and food shortage experienced in winter, particularly at night, which resulted in a lowering of 24-h minimum body temperature, below summer values.
Indeed, our monkeys may not have implemented the cold defence available to them at night. The reduction in the minimum body temperature in our monkeys during winter may reflect a regulated downward setting of the thermoregulatory system (i.e. an attenuated form of torpor), whereby a circannual pattern of body temperature control has evolved to reduce energy costs in response to the seasonal fluctuations in environmental conditions (e.g. low environmental temperatures or food availability; Maloney et al. 2011). In seasonal environments, changes in photoperiod may be an important cue for such thermoregulatory adjustments. Indeed, laboratory studies have demonstrated that changes in photoperiod could affect the onset, timing and duration of torpor bouts for grey mouse lemurs, Microcebus murinus (Perrett and Aujard 2001; Genin and Perret 2003), which certainly qualify for regulated downward setting of thermoregulatory control. In our vervet monkeys, even though the species does not employ torpor, the 24-h minimum body temperature and, consequently, the 24-h amplitude of the body temperature rhythm, was linearly correlated with days between mid-winter and mid-summer (Fig. 3), and, the 24-h minimum body temperature increased significantly with increasing photoperiod independently of other variables over the duration of the study (Table 1), consistent with entrainment by photoperiod of thermoregulatory mechanisms. However, unlike 24-h minimum body temperature, and the 24-h amplitude of the body temperature rhythm, the lower modal body temperature did not change seasonally.
The nocturnal modal body temperature for our vervet monkeys was 37.1 °C in winter, which is higher than the mean body temperature for the onset of torpor of 35.3 ± 0.4 °C for mammals weighing between 62 and 406 g (Willis 2007), and similar to mean inactive phase body temperatures for southern lesser galagos (36.8 ± 0.7 °C, ~0.2 kg; Mzilikazi et al. 2006), Cape ground squirrels, Xerus inauris (36.3 ± 0.3 °C, ~0.8 kg; Wilson et al. 2010) and rock hyrax, Procavia capensis (36.8 ± 0.1 °C, ~3.0 kg; Brown and Downs 2006), other “small” (<5 kg) free-ranging non-torpid mammals exposed to winter conditions similar to those in our study. For species that do not employ torpor, reduced body temperatures during periods of low environmental temperatures or low food availability may confer energy savings via the same mechanisms described for torpor (Angilletta et al. 2010; Glanville and Seebacher 2010; Boyles et al. 2011a). Unlike the regulated physiological adjustments proposed for torpor or hibernation, the hypothermia observed in our vervet monkeys in winter did not appear to be a regulated reduction in the “preferred” level, or set-point, at which body temperature was defended, as there was no change in the lower modal body temperature, a proxy for “preferred” body temperature (sensu Boyles et al. 2011b), between seasons. Consequently, the drop in minimum body temperature in winter is unlikely to be a regulated drop, entrained by photoperiod, but is more likely to reflect an inability to maintain homeothermy in the face of low environmental temperature and food energy shortage (Maloney et al. 2013), which happened to occur when photoperiod was short.
Our finding that the 24-h minimum body temperature significantly increased with the body mass of monkeys (Table 1) supports the idea that the low 24-h body temperatures experienced by monkeys were a consequence of a loss of thermoregulatory precision, rather than a regulated adjustment of the thermoregulatory system. That larger monkeys maintained 24-h minimum body temperature at higher levels than did smaller monkeys may be explained by the relationship between thermal conductance and body size (Aschoff 1981), as smaller monkeys would require increased heat production per unit body mass to maintain body temperature, relative to larger monkeys. The high energy inputs required to support high heat production and to replace heat lost to the environment would have been difficult to achieve when environmental temperatures were low, day length was short, and food was scarce. Body mass may therefore be the physical property explaining the increased individual variation of the 24-h body temperature rhythm, especially at night, in winter compared to summer (Fig. 4). Other factors such as social organisation (e.g. rank) or mating, reproductive status and parental requirements may also have contributed to individual variability in the channelling of resources to thermoregulation. Amongst Malagasy lemurs, for example, females exhibit deeper bouts of torpor and more prolonged periods of hibernation than do males (Schülke and Ostner 2007), perhaps because male lemurs need to remain active to acquire adequate resources to be competitive during the mating season (Schmid 1998). Determining the functional mechanism of individual variability in the body temperature rhythm of vervet monkeys requires further investigation, and may provide insight into the resource allocation of individuals within a population.
If the low 24-h minimum body temperatures reflect a loss of thermoregulatory precision, the question arises of why higher nocturnal temperature, and consequently higher mean 24-h temperature and better homeothermy, contribute to fitness. Although the maintenance of a high, stable body temperature requires significant energy expenditure, there is a net fitness gain from increased cellular function at higher body temperatures (Heinrich 1977; Somero 2004; Knies et al. 2009). But when maintenance of homeothermy becomes too costly, homeothermy can be traded off in favour of energy conservation. That trade-off may decrease fitness in the immediate term, but may ultimately increase net fitness (Angilletta et al. 2010). In our vervet monkeys the maintenance of homeothermy in the short term may be a luxury available only in well-fed monkeys exposed to mild environmental temperatures.
Understanding whether relaxation of body temperature control represents an adaptive response or inability to cope with environmental stress will be important in predicting species responses to future climate change scenarios, which project a ~2 °C increase in mean ambient temperatures and reduced precipitation for the Karoo (Rutherford et al. 1999; van Jaarsveld and Chown 2001; Hoffman et al. 2009). If the relaxation reflects an involuntary loss of precision, an increase in winter minimum environmental temperatures may alleviate one thermoregulatory challenge for vervet monkeys, but periods of food scarcity are likely to be exacerbated by a reduction in precipitation in an already arid ecosystem. If the reduced precipitation limits access to free-standing water, and vervet monkeys are unable to behaviourally compensate for the high environmental temperatures they may exhibit greater heterothermy in summer, and consequently throughout the year. The survival of vervet monkeys and the role of physiological plasticity through future climate change remain to be elucidated. We require long-term studies monitoring physiological parameters (e.g. body temperature) of individual animals in conjunction with direct measures of fitness (e.g. reproduction, growth, and survival), and with environmental parameters.
References
Angilletta MJ, Cooper BS, Schuler MS, Boyles JG (2010) The evolution of thermal physiology in endotherms. Front Biosci 2:861–881
Arnold W, Ruf T, Reimoser S, Tataruch F, Onderscheka K, Schober F (2004) Nocturnal hypometabolism as an overwintering strategy of red deer (Cervus elaphus). Am J Physiol 286:174–181
Aschoff J (1981) Thermal conductance in mammals and birds: its dependence on body size and circadian phase. Comp Biochem Physiol A 69:611–619
Barclay RMR, Lausen CL, Hollis L (2001) What’s hot and what’s not: defining torpor in free-ranging birds and mammals. Can J Zool 79:1885–1890
Barrett L, Gaynor D, Rendall D, Mitchell D, Henzi SP (2004) Habitual cave use and thermoregulation in chacma baboons (Papio hamadryas ursinus). J Hum Evol 46:215–222
Barton RA, Whitten A, Strum SC, Byrne RW, Simpson AJ (1992) Habitat use and resource availability in baboons. Anim Behav 43:831–844
Boyles JG, Seebacher F, Smit B, McKechnie AE (2011a) Adaptive thermoregulation in endotherms may alter responses to climate change. Int Comp Biol 51:676–690
Boyles JG, Smit B, McKechnie AE (2011b) A new comparative metric for estimating heterothermy in endotherms. Physiol Biochem Zool 84:115–123
Boyles JG, Thompson AB, McKechnie AE, Malan E, Humphries MM, Careau V (2013) A global heterothermic continuum in mammals. Glob Ecol Biogeogr 22:1029–1039
Brain C, Mitchell D (1999) Body temperature changes in free-ranging baboons (Papio hamadryas ursinus) in the Namib Desert, Namibia. Int J Primatol 20:585–598
Brown KJ, Downs CT (2006) Seasonal patterns in body temperature of free-living rock hyrax (Procavia capensis). Comp Biochem Physiol A 143:42–49
Campos FA, Fedigan LM (2009) Behavioural adaptations to heat stress and water scarcity in white-faced capuchins (Cebus capucinus) in Santa Rosa National Park, Costa Rica. Am J Phys Anthropol 138:101–111
Da Silva GL (1993) Postural changes and behavioural thermoregulation in Colobus polykomos: the effect of climate and diet. Afr J Ecol 31:226–241
Danzy J, Grobler JP, Freimer N, Turner TR (2012) Sunbathing: a behavioural response to seasonal climatic change among South African vervet monkeys (Chlorocebus aethiops). Afr Prim 7:230–237
Dausmann KH, Glos J, Ganzhorn JU, Heldmaier G (2004) Hibernation in a tropical primate. Nature 429:825–826
Fuller A, Dawson T, Helmuth B, Hetem RS, Mitchell D, Maloney SK (2010) Physiological mechanisms in coping with climate change. Physiol Biochem Zool 83:713–720
Geiser F (2004) Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu Rev Physiol 66:239–274
Genin F, Perret M (2003) Daily hypothermia in captive grey mouse lemurs (Microcebus murinus): effects of photoperiod and food restriction. Comp Biochem Physiol A 136:71–81
Glanville EJ, Seebacher F (2010) Advantage to lower body temperatures for small mammal (Rattus fuscipes) experiencing chronic cold. J Mammal 91:1197–1204
Grigg G, Beard L, Dorges B, Heucke J, Coventry J, Coppock A, Blomberg S (2009) Strategic (adaptive) hypothermia in bull dromedary camels during rut; could it increase reproductive success? Biol Lett 5:853–856
Hanya G (2004) Seasonal variations in the activity budget of Japanese macaques in the coniferous forest of Yakushime: effects of food and temperature. Am J Primatol 63:165–177
Hanya G, Kiyono M, Hayaishi S (2007) Behavioural thermoregulation of wild Japanese macaques: comparisons between two subpopulations. Am J Primatol 69:802–815
Heinrich B (1977) Why have some animals evolved to regulate a high body temperature? Am Nat 111:623–640
Hetem RS, Maloney SK, Fuller A, Meyer LCR, Mitchell D (2007) Validation of a biotelemetric technique, using ambulatory miniature black globe thermometers, to quantify thermoregulatory behaviour in ungulates. J Exp Biol 307A:342–356
Hetem RS, Strauss WM, Fick LG, Maloney SK, Meyer LCR, Shobrak M, Fuller A, Mitchell D (2010) Variation in the daily rhythm of body temperature of free-living Arabian oryx (Oryx leucoryx): does water limitation drive heterothermy? J Comp Physiol B 180:1111–1119
Hill RA (2006) Thermal constraints on activity scheduling and habitat choice in baboons. Am J Phys Anthropol 129:242–249
Hoffman MT, Carrick PJ, Gillson L, West AG (2009) Drought, climate change and vegetation response in the succulent karoo, South Africa. S Afr J Sci 105:54–60
IUPS Thermal Commission (2001) Glossary of terms for thermal physiology. Jpn J Physiol 51:245–280
Knies JL, Kingsolver JG, Burch CL (2009) Hotter is better and broader: thermal sensitivity of fitness in a population of bacteriophages. Am Nat 173:419–430
Kosheleff VP, Anderson CNK (2009) Temperature’s influence on the activity budget, terrestriality, and sun exposure of chimpanzees in the Budongo Forest, Uganda. Am J Phys Anthropol 139:172–181
Le Houerou HN (1984) Rain use efficiency: a unifying concept in arid-land ecology. J Arid Envir 7:213–247
Maloney SK, Fuller A, Meyer LCR, Kamerman PR, Mitchell G, Mitchell D (2011) Minimum daily core body temperature in western grey kangaroos decreases as summer advances: a seasonal pattern, or a direct response to water, heat or energy supply? J Exp Biol 214:1813–1820
Maloney SK, Meyer LCR, Blache D, Fuller A (2013) Energy intake and the circadian rhythm of core body temperature in sheep. Physiol Rep 1:e00118
McDougall P, Forshaw N, Barrett L, Henzi SP (2010) Leaving home: responses to water depletion by vervet monkeys. J Arid Environ 74:924–927
McFarland R, Hetem R, Fuller A, Mitchell D, Henzi SP, Barrett L (2013) Assessing the reliability of biologger techniques to measure activity in a free-ranging primate. Anim Behav 85:861–866
Mitchell D, Maloney SK, Jessen C, Laburn HP, Kamerman PR, Mitchell G, Fuller A (2002) Adaptive heterothermy and selective brain cooling in arid-zone mammals. Comp Biochem Physiol B 131:571–585
Mitchell D, Fuller A, Maloney SK (2009) Homeothermy and primate bipedalism: is water shortage or solar radiation the main threat to baboon (Papio hamadryas) homeothermy? J Hum Evol 56:439–446
Morland HS (1993) Seasonal behavioral variation and its relationship to thermoregulation in ruffed lemurs (Varecia variegata variegata). In: Kappeler PM, Ganzhorn JU (eds) Lemur social systems and their ecological basis. Plenum Press, New York, pp 193–204
Mortola JP, Lanthier C (2004) Scaling the amplitudes of the circadian pattern of resting oxygen consumption, body temperature and heart rate in mammals. Comp Biochem Physiol A 139:83–95
Mzilikazi N, Master JC, Lovegrove BG (2006) Lack of torpor in free-ranging southern lesser galagos, Galago moholi: ecological and physiological considerations. Folia Primatol 77:465–476
Nowack J, Mzilikazi N, Dausmann KH (2010) Torpor on demand: heterothermy in the non-lemur primate Galago moholi. PLoS One 5:e10797
Ostrowski S, Williams JB, Ismael K (2003) Heterothermy and the water economy of free-living Arabian oryx (Oryx leucoryx). J Exp Biol 206:1471–1478
Pasternak G, Brown LR, Kienzle S, Fuller A, Barrett L, Henzi SP (2013) Population ecology of vervet monkeys in a high latitude, semi-arid riparian woodland. Koedoe 55:1–9
Pereira MA, Aines J, Scheckter JL (2002) Tactics of heterothermy in eastern grey squirrels (Sciurus carolinensis). J Mammal 83:467–477
Perrett M, Aujard F (2001) Daily hypothermia and torpor in a tropical primate: synchronization by 24-h light-dark cycle. Am J Physiol Regul Integr Comp Physiol 281:1925–1933
Pinheiro JC, Bates DM (2000) Mixed effects models in S and S-PLUS. Springer, New York
Refinetti R, Menaker M (1992) The circadian rhythm of body temperature. Physiol Behav 51:613–637
Rutherford MC, Midgley GF, Bond WJ, Powrie LW, Roberts R, Allsopp J (1999) Plant biodiversity: vulnerability and adaptation assessment. South African country study on climate change. National Botanical Institute, Cape Town
Schmid J, Speakman JR (2000) Daily energy expenditure of the grey mouse lemur (Microcebus murinus): a small primate that uses torpor. J Comp Physiol B 170:633–641
Schmidt-Nielsen K, Schmidt-Nielsen B, Jarnum SA, Houpt TR (1956) Body temperature of the camel and its relation to water economy. Am J Physiol 188:103–112
Schmid J (1998) Tree holes used for resting by gray mouse lemurs (Microcebus murinus) in Madagascar: insulation capacities and energetic consequences. Int J Primatol 19:797–809
Schülke O, Ostner J (2007) Physiological ecology of cheirogaleid primates: variation in hibernation and torpor. Acta Ethol 10:3–21
Signer C, Ruf T, Arnold W (2011) Hypometabolism and basking: the strategies of Alpine ibex to endure harsh over-wintering conditions. Funct Ecol 25:537–547
Skinner JD, Chimimba CT (2005) The mammals of the Southern African subregion, 3rd edn. Cambridge University Press, Cambridge
Somero GN (2004) Adaptation of enzymes to temperature: searching for basic “strategies”. Comp Biochem Physiol B 139:321–333
StataCorp (2007) Stata statistical software: release 10. Stata Press, College Station
Stelzner JK, Hausfater G (1986) Posture, microclimates, and thermoregulation in yellow baboons. Primates 27:449–463
Stitt JT, Hardy JD (1971) Thermoregulation in the squirrel monkey (Saimiri sciureus). J Appl Physiol 31:48–54
Turbill C, Bieber C, Ruf R (2011) Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc R Soc Lond B 278:3355–3363
van Jaarsveld AS, Chown SL (2001) Climate change and its impacts in South Africa. Trends Ecol Evol 16:13–14
Willis CKR (2007) An energy-based body temperature threshold between torpor and normothermia for small mammals. Physiol Biochem Zool 80:643–651
Wilson WA, O’Riain MJ, Hetem RS, Fuller A, Fick LG (2010) Winter body temperature patterns in free-ranging Cape ground squirrel, Xerus inauris: no evidence for torpor. J Comp Physiol B 180:1099–1110
Acknowledgments
We thank Mark and Sarah Tompkins for permission to work on Samara Private Game Reserve. We are grateful to Anna Haw for undertaking the explant surgery, and to Mary-Ann Costello, Natalie Freeman, Jessica Gohnert, Tina Lavin, Hilary Lease, Aarti Mistry, Graham Pasternak, Karel Rabe, Tom Peschak, Jess Smart, Brittany Thomas, Alena Matlock, Eric Matlock and Benjamin Rey for assistance in the field. This work was supported by a Carnegie large research grant to AF, a National Research Foundation (NRF, South Africa) grant to DM, AF, SKM, RH and SPH, a Harry Oppenheimer Fellowship Award to DM, a Claude Leon Fellowship awarded to RM, an Open Society Institute bursary and an NRF bursary awarded to AL, and Natural Sciences and Engineering Research Council (Canada) Discovery grants to LB and SPH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Lubbe, A., Hetem, R.S., McFarland, R. et al. Thermoregulatory plasticity in free-ranging vervet monkeys, Chlorocebus pygerythrus . J Comp Physiol B 184, 799–809 (2014). https://doi.org/10.1007/s00360-014-0835-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-014-0835-y