Abstract
Two groups of juvenile gilthead sea bream were kept on two different swimming regimes (Exercise, E: 1.5 body length s−1 or Control, C: voluntary activity) for 1 month. All fish were first adapted to an experimental diet low in protein and rich in digestible carbohydrates (37.2% protein, 40.4% carbohydrates, 12.5% lipid). The cellularity and capillarisation of white muscle from two selected areas (cranial (Cr), below the dorsal fin, and caudal (Ca), behind the anal fin) were compared. The body weight and specific growth rate (SGR) of group E rose significantly without an increment in feed intake, pointing to higher nutrient-use efficiency. The white muscle fibre cross-sectional area and the perimeter of cranial samples increased after sustained activity, evidencing that sustained exercise enhances hypertrophic muscle development. However, we cannot conclude or rule out the possibility of fibre recruitment because the experimental period was too short. In the control group, capillarisation, which is extremely low in gilthead sea bream white muscle, showed a significantly higher number of fibres with no surrounding capillaries (F0) in the cranial area than in the caudal area, unlike the exercise group. Sustained swimming improved muscle machinery even in tissue normally associated with short bouts of very rapid anaerobic activity. So, through its effect on the use of tissue reserves and nutrients, exercise contributes to improvements in fish growth what can contribute to reducing nitrogen losses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The locomotor strategies of animals play an important role in their response to life challenges, conditioning their nourishment, predator–prey interactions and reproduction, and determining migration capacity. Farmed fish lead a more sedentary life than their counterparts in the wild. Mechanical loads are involved in the development and maintenance of vertebrate tissue (Buchanan and Marsh 2002; Davison 1997). Comparative studies of farmed and wild fish reveal useful data about fish domestication processes and provide an insight into fish nutrition, physiology and production. Several studies have shown that wild fish have superior swimming performances than farmed fish. This is the case for brook trout (Vincent 1960), coho salmon (Brauner et al. 1994), Atlantic salmon (McDonald et al. 1998) and gilthead sea bream (Basaran et al. 2007). Consequently, wild fish show superior aerobic and anaerobic capacities. Moreover, fish-rearing conditions also affect fish quality, with cultured fish presenting a higher fat content than their wild counterparts (Vincent 1960; Thorstad et al. 1997; Grigorakis et al. 2002).
Two basic responses to exercise training can be described in humans and in some other mammals. Activities such as running, swimming and cycling increase the aerobic capacity of muscle, which improves endurance (Holloszy and Booth 1976; Hoppeler et al. 1985), whereas isometric exercise (for example, weightlifting) results in fibre hypertrophy and an increase in muscle strength (Goldspink et al. 1976; Hoppeler 1986). Fewer studies have been carried out on exercise in lower tetrapods, such as amphibians and reptiles, due to the difficulties involved in forcing them to exercise at repeatable work rates and the highly anaerobic nature of any exercise (Bennett 1978). But by their nature, fish can easily be exercised by forcing them to swim against the stream. Swimming speeds of two body lengths per second (b.l.s−1) or less are acceptable for training fish without any notable consequences for fish welfare or stress (Sänger and Stoiber 2001). Fish offer several advantages over mammalian models in exercise training studies. Many species of fish control buoyancy via their swim bladder, which limits the effects of gravitational forces compared with terrestrial animals. The locomotory musculature of fish is relatively simple: it is located around the axial skeleton, distributed in discrete myotomes, and the slow and fast muscle fibres are spatially segregated. Moreover, in contrast to mammals in which the recruitment of new skeletal muscle fibres ends soon after birth (Goldspink 1972), limiting muscle development and growth to hypertrophy, fish have the capacity to recruit new skeletal muscle fibres not only throughout larval life but also throughout juvenile and adult stages (Weatherley et al. 1988; Rowlerson and Veggetti 2001).
Fish myotomal muscles are arranged to provide the power for different swimming styles. Red muscle, composed of slow oxidative fibres, produces the force required for slower, routine and sustainable movements, including migration over long distances. White muscle is composed of fast glycolytic fibres, which are recruited to produce the force required for rapid movements, such as sprint swimming and escape responses (Gibb and Dickson 2002). In many fish species, an intermediate pink muscle is usually present between the two types of muscle. The white muscle usually represents 35%-50% of body weight, although in male salmon and tuna it can be nearly 70%, whereas red fibres comprise approximately 0.5–13% of body mass (Goolish 1989; reviewed by Dickson 1996). The pattern of fibre number and fibre size distribution and the pattern of capillaries surrounding each fibre in a particular muscle section are commonly referred to as muscle cellularity and capillarisation, respectively (Johnston 1999; Stoiber et al. 2002). Moreover, muscle cellularity is also the main determinant of both muscle growth and flesh quality (Johnston 1999).
Exercise is a powerful factor in improving growth rate and food conversion efficiency in many species (reviewed in Davison 1997). Increases in cell diameter and fibre numbers in aerobic muscle seem to be general features in teleost fish when exercise is moderate—swimming speeds below 1.5 b.l.s−1—but the effects on white muscle are controversial. Some studies have reported that swimming has no effect on white muscle cellularity (Davie et al. 1986; Sänger 1992), but others show increases in white muscle fibre diameter (Hinterleitner et al. 1992; Davison 1994). It has also been demonstrated that the aerobic capacity of the swimming muscles increases due to exercise (Farrell et al. 1991), which is also linked to the changes in the contractile machinery. The reported data regarding the effects of training upon capillarisation are also controversial; no changes were observed in salmonids (Johnston and Moon 1980; Davison 1983), whereas studies on cyprinids (Sänger 1992) and rainbow trout (Davie et al. 1986) reported that training led to higher capillarisation, mainly in red muscle.
Here we examine the effect of mechanical load on gilthead sea bream white muscle by submitting fish to a 1-month training period. During this period fish were fed on an experimental diet (40.4% CHO, 37.2% protein, 12.5% lipid). Two zones of the fish body (cranial and caudal) were selected to evaluate the effects of exercise on the reared fish. Since these variables are of crucial importance in commercial terms and are usually used as growth markers, our study focuses on white muscle cellularity and capillarisation.
Materials and methods
Fish rearing
Juvenile sea bream (Sparus aurata L.) were purchased from a commercial supplier (Cripesa, Tarragona, Spain) who reared the fish in marine cages. One hundred fish were transferred and maintained indoors in the facilities of the Faculty of Biology (University of Barcelona, Barcelona, Spain), in six 200-L seawater tanks equipped with a semi-closed recirculation system with physical and biological filters, ozone skimmers and continuous aeration at 20°C and 12L:12D, with a 35% weekly seawater renewal rate. Fish were first acclimated to a carbohydrate-rich diet (composition: 37.15% protein; 12.51% lipid; 40.04% carbohydrate; 1.77% fibre; and 8.52% ash) and fed to satiation for 2 weeks. After this period, fish were slightly anaesthetised, weighed and randomly distributed in trial tanks (initial body weight and length data are shown in Table 1). Control groups (C: 12 fish per tank, in triplicate) were kept under normal rearing conditions in 200-L circular tanks with a water flow of 350 L/h (vertical water entrance). Exercise groups (E: 12 fish per tank, in triplicate) were kept in 400-L circular tanks in the same semi-closed circuit. To obtain sustained activity, the fish were prevented from entering the central area of lower velocity by a cylindrical tube. This results in a living area corresponding to an effective space of 200 L with the same fish density as in the control tanks. Water flow was 700 L/h with a circular and uniformly distributed flow induced by a perpendicular water entrance and one additional submerged water pump (at the bottom of the tank and isolated from the fish living area). This design resulted in a swimming velocity of 1.5 body lengths per second measured at three different tank depths (at the surface, mid-tank and near the bottom). During the experimental period all groups were fed until apparent satiety twice a day (9:30 a.m. and 5:30 p.m.) and intake was recorded daily.
Sampling and histochemical procedures
After 4 weeks, 10 fish randomly sampled from both C and E groups were sacrificed by severing their spinal cord and the final body weight and body indices (hepatosomatic, muscle-somatic and perivisceral fat content) were recorded. For muscle composition, epaxial white muscle samples were obtained and immediately frozen in liquid nitrogen and then kept at –80°C until the main components were analysed. Muscle composition in water, glycogen, lipid, protein and DNA were analysed as explained elsewhere (Ibarz et al. 2007a, b).
White muscles samples for histochemical examination were dissected from the cranial (Cr) and caudal (Ca) regions. Small strips of white muscle with an approximate length of 1 cm, a width of 1 cm and a thickness of 0.5 cm were obtained from each region. Each sample was immediately soaked in 3-methyl-butane pre-cooled to −160°C and stored in liquid nitrogen until subsequent sectioning (Dubowitz 1985). Serial transverse sections from each sample were cut at a thickness of 16–20 μm in a cryostat (Frigocut, Reichart-Jung, Heidelberg, Germany) at −22°C. Sections were mounted on gelatinised slides and incubated for 5 min in a buffered fixative (Viscor et al. 1992) in order to prevent shrinkage or wrinkling. After rinsing the slides thoroughly, we used the ATPase method developed by Fouces et al. (1993) in order to reveal muscle capillaries and a histochemical assay for succinate dehydrogenase to demonstrate the aerobic or anaerobic characteristics of muscle fibres (Nachlas et al. 1957).
Morpho-functional measurements
Images of the stained sections were obtained using a light microscope (BX40, Olympus, Tokyo, Japan) connected to a digital camera (KP-C550, Hitachi, Tokyo, Japan). To ensure accurate calibration of all measurements, an image of a stage micrometer was obtained each time images of samples were taken. All the parameters listed below were empirically determined from 2 × 105 μm2 windows of tissue from two different zones or muscle fields in each sample. After testing for the absence of differences between the two muscle fields from each sample (see “Statistics”), the data obtained from both fields were considered together so that the sample size was large enough. The following parameters were counted or calculated: capillary density (CD), fibre density (FD), the number of capillaries in contact with each fibre (NCF), the percentage of fibres having no capillaries in contact with them (F0) and the percentage of fibres in contact with at least one capillary (F+). Capillary and fibre counts were calculated to be expressed as capillaries and fibres per mm2. The fibre cross-sectional area (FCSA) and fibre perimeter (FPER) were determined directly using a personal computer connected to a digitiser tablet and SigmaScan software (Systat Software Inc., San Jose, CA, USA) from digital images. The total number of fibres analysed in each sample muscle ranged from 200 to 300. Two indices expressing the relationship between NCF and the FCSA: CCA = NCF × 103/FCSA or FPER: CCP = NCF × 102/FPER, were also calculated. These indices are considered a measure of the number of capillaries per 1,000 μm2 of muscle FCSA and the number of capillaries per 100 μm of muscle FPER. The maximal diffusion distance (MDD) between the capillary and the central region of the fibre was also calculated for every capillary of the region analysed. A shape factor (SF), circularity, was measured as a function of the FPER and the FCSA following the formula: SF = (4πFCSA)/FPER2. Circularity indicates the degree of adjustment of the fibre transverse section to a circular shape (SF = 1 for a perfect circle).
Statistics
Data for growth, body indices and white muscle composition under each condition, initially analysed by one-way ANOVA, did not show any differences between the three tanks. The data were then grouped and Student’s t tests were performed using n = 3 tanks for the growth study, and n = 10 animals per condition for the remaining parameters. To test for the absence of differences between both muscle fields from each muscle sample, the non-parametric Wilcoxon rank-sum test was performed. For the percentage of fibre types, the arcsine transformation was applied as a previous step. The normality of the data was tested by the Kolmogorov–Smirnov test (with Lilliefors’ correction) and the comparisons between the control and exercise groups were analysed by the Student’s t tests. Data for all the parameters are expressed as sample means ± standard error of the mean.
For FCSA histograms a dynamic fitting by nonlinear regression was performed for each section (caudal and cranial). The approximation was done by a log-normal (four parameters) equation with a dynamic fit option of 200 for both total number of fits and maximum number of iterations. Log-normal equations have been reported with R value and coefficients and standard errors for each parameter (a, b, x 0 and y 0). Individual data were linearised by an ln (x + 1) transformation, thus avoiding negative values and possible ln (0). With this linearisation, a linear regression was obtained to test for the difference in regression slope. All statistical analyses were performed using SigmaStat 4.0 (in SigmaPlot 11.0 Software, Systat Software Inc., San Jose, CA, USA) with significance at p < 0.05.
Results
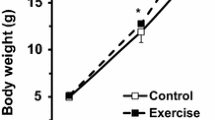
Table 1 shows the body weight and organ indices, growth rate and muscle composition of gilthead sea bream submitted to two swimming-activity regimes. Noticeably, the fish that maintained a swimming speed of 1.5 b.l.s−1 for 1 month presented a significantly higher body weight without any differences in food intake (C: 2.8 ± 0.1 vs. E: 2.6 ± 0.1 g of feed per 100 g body weight). Other body indices, such as the condition factor (CF), hepatosomatic index (HSI), body percentage of perivisceral fat and muscle-somatic index (MSI), did not alter significantly (Table 1). Epaxial white muscle samples of the exercising fish reduced lipid content by 30% compared to the control group values, but protein percentage did not change. Therefore, the increment in total body weight with similar muscle-somatic index and protein percentage implies increased muscle mass with a net gain of total protein content in white muscle. A slight decrement in the DNA content (7%) was observed in fish under sustained activity, but there was no difference in the DNA content of total white muscle mass (calculated by multiplying body weight per MSI per DNA levels) between the C and E groups (total muscle DNA ranged from 8.6 to 8.7 mg).
Muscle cellularity and capillarisation were studied in two different zones: cranial (Cr) and caudal (Ca). For both zones and conditions the histochemical assay for succinate dehydrogenase (SDH) revealed a lack of staining, indicating the absence of SDH activity and thus the anaerobic character of those muscle sections. Illustrative images from muscle section are shown in Fig. 1. Morphometrical fibre parameters and fibre density (cellularity) from both muscle samples are shown in Table 2, and capillarisation parameters in Table 3. Fish under the sustained swimming regime presented significant increases in the parameters related to muscle fibre size in the cranial zone (Table 2). Likewise, the fibre perimeter was 11% higher and the fibre area was 17% higher than the control ones. These increases took place without any change in the shape factor of the fibres. Fibre cross-sectional area (FCSA) distributions, along with adjust equations, in both sections of the gilthead sea bream white muscle are shown in Fig. 2, and linearised regression parameters of those equations are reported in Table 3. The slope for the cranial area of control group was higher (in absolute value) than those of the other groups. The number of small fibres (FCSA < 2,500 μm2) was significantly higher for the cranial area of control group, whereas white muscle fibres with FCSA > 9,000 μm2 were lower (Table 3), indicating that, in the short 1-month period of sustained activity, white muscle fibres achieved higher perimeters and areas.
Illustrative images of cross-sectional muscle of juvenile gilthead sea bream. a Cranial section of control fish, b cranial section of exercised fish, c caudal section of control fish, d caudal section of exercised fish. All images correspond to one representative serial transverse section and capillaries revealed by an ATPase detection method (see “Materials and methods” for further information). Cp capillaries, FCSA fibre cross-sectional area, FPER fibre perimeter
White muscle cross-sectional area histograms. a cranial-control, b cranial-exercise; c caudal-control, and d caudal-exercise. Muscle fibre areas were grouped in 50 × 1,000 μm2 groups and the data correspond to mean ± SEM frequency of five animals. Regression parameters are shown and analysed in Table 4. See “Materials and methods” (“Statistics”) for further information
The measures of capillarisation shown in Table 3 are the number of capillaries per fibre and distance (NCF and CD), two derived indices relating, respectively, the capillaries that surround a fibre to its cross-sectional area and perimeter (CCA and CCP), and the mean diffusion distances of the capillaries to the centre of the fibre (MDD). Moderate but sustained exercise affected the capillarisation of the caudal area by slightly increasing the surrounding capillarity of the fibre perimeter (CCP data, p < 0.05, Table 3). However, white muscle capillarisation was very low in both the cranial and caudal localisations, scarcely reaching a mean of one capillary per fibre. Unlike the exercised fish, the gilthead sea bream in the control group presented a high proportion of fibres with no capillaries (F0) in contact with them, these being significantly (p < 0.05) more abundant in the cranial zone (38.3 ± 3.4% and 27.2 ± 2.8%, CrC vs. CaC, respectively, Fig. 3). Moreover, those fibres in contact with capillaries (F+) generally only had contact with one or two, with a maximum of four capillaries observed on only a few occasions. This determines high diffusion distances, which confirms the mainly anaerobic character of the fish white muscle.
White muscle capillary surrounding. Bar chart showing the percentage of fibres with no surrounding capillaries (F0) and the percentage of fibres contacting at least one capillary (F+). CrC cranial-control, CrE cranial-exercise, CaC caudal-control, and CaE caudal-exercise. Data are mean ± SEM of five animals. Significant differences between groups are indicated on the graph
Discussion
The present work is the first attempt to study the muscle cellularity of gilthead sea bream using sustained activity as a rearing condition to enhance the growth of fish on a rich carbohydrate diet. During recent years, new extrusion feed techniques permit improved availability of highly digestible carbohydrates and recent studies of sea bream have focused on this source to diminish dietary protein levels and waste nitrogen (Georgopoulos and Conides 1999; Venou et al. 2003; Fernández et al. 2007). The aim of replacing dietary protein and lipid by using a carbohydrate source also aims to increase the quality of meat by reducing lipid deposition in the sedentary fish. In the present study, the reduction of dietary protein did not limit energy availability as fish were fed to apparent satiety and thus, the levels of dietary protein proposed (37.2%) should be not considered as a limiting factor for growth. In studies on other species, mainly salmonids, differences in fibre size and number along the length of the body (Stickland 1983; Mascarello et al. 1995) or in ventral and dorsal regions (Kiessling et al. 1991) have been reported. Our data show that untrained gilthead sea bream (with body weight of approximately 100 g) had cranial fibres with lower cross-sectional areas and perimeters than caudal fibres. However, Abdel et al. (2005) did not show significant differences in white muscle size distribution when measuring muscle cellularity in the cranial and caudal areas of sea bass (D. labrax) with body weights of over 350 g. Although there are few published data, this disparity could be related to the fish size and age, which would mean that muscle growth potential is not the same in early stages of live as in adults. In fact, fish muscle plasticity has been strongly linked to the existence of seasonal cycles (reviewed by Johnston 1999).
Our results also show that the dynamics of gilthead sea bream growth are highly sensitive to swimming regimes. Thus, induced swimming at 1.5 b.l.s−1 affected white muscle fibres at cranial level, with increases in both area and perimeter values, but it did not modify the caudal fibres. Larger fibre sizes led to increased muscle masses and a higher total body weight, supporting the theory that normal sedentary rearing conditions under-exploit the growth potential of fish muscle. Indeed, exercise is a powerful stimulus in muscle hypertrophy, although the effects of muscle recruitment on hyperplasia have not been determined (Johnston 1999). Little is known about the origin of new fibres produced during the post-larval growth phase, and hyperplasic and hypertrophic growth usually occurs simultaneously in fish (reviewed by Johnston 1999). Nevertheless, in fast-growing fish, including cultured species that reach larger sizes, hyperplasia usually continues for longer than in small fish, in which hyperplasia stops earlier and hypertrophy is a more effective growth mechanism (Kiessling et al. 1991; Valente et al. 1999; Rowlerson and Veggetti 2001; Aguiar et al. 2005). In the current trial, the changes observed in the distribution of the muscle fibre cross-sectional area were interpreted as markers of changes in the relative contribution of hyperplasic and hypertrophic growth processes. Muscle protein percentage and total muscle DNA content remained invariable, which indicates that hypertrophy is the main muscle-growth mechanism in this short-term exercise, although hyperplasic effects cannot be ruled out, since the test period was short (1 month).
Few studies exist on muscle capillarity in fish, but all show that fish white muscle is poorly capillarised compared with mammal muscles, reflecting the low O2 fluxes that fish white muscle needs. Only highly aerobic fish muscle presents high capillary-to-fibre number ratios. In carp, it was 2.2 at 28°C, but increased to 4.8 as the fibre size increased with acclimation to 2°C (Johnston 1982a); in tuna red muscle it was below 2, but with a relatively small fibre size (Mathieu-Costello et al. 1996), and it was 12.9 in the highly aerobic muscle of anchovy (Johnston 1982b). Therefore, the fact that 30–40% of the fibres in the white muscle of gilthead sea bream had no surrounding capillaries (F0) should be not surprising. Apart from escape bouts or prey capture, the low capillarisation of fish white muscle paralleled very low tissue metabolic activity rates and slow oxygen and nutrient supplies, these being lower in the cranial location of untrained fish than in exercised. The induced sustained activity, however, increased the number of capillaries in contact with each fibre (NCF) in the caudal area. Capillarisation in this zone relates to a higher number of pink fibres in the caudal area and, as a consequence, a greater need for oxygen.
In general, fish appetite is stimulated by activity and exercised fish generally consume more food. Nevertheless, a unit mass of growth is achieved when a lower amount of food is consumed, and the mass gain is achieved faster (Davison 1997). Another important aspect of the present study is that for gilthead sea bream under the conditions studied, muscle growth, and consequently whole-body growth, was achieved without significant increases in food consumption. Moreover, the lipid content of white muscle diminished in trained fish, an important aspect that can contribute to increased muscle quality, thus avoiding the excessive fat deposition of many cultured fish species (Cakli et al. 2007; Grigorakis 2007).
Conclusions
All the present results lead to the conclusion that firstly, moderate sustained activity improves whole-body growth through hypertrophic white muscle growth, and secondly, through its effects on the use of tissue reserves and nutrients, the exercise would contribute to a reduction in water contamination by lowering nitrogen waste from dietary protein. The markedly higher white muscle development was seen in the cranial region.
References
Abdel I, López-Albors O, Ayala MD, García-Alcazar A, Abellán E, Latorre R, Gil F (2005) Muscle cellularity at cranial and caudal levels of the trunk musculature of commercial size sea sass, Dicentrarchus labrax (Linnaeus, 1758). Anat Histol Embryol 34:280–285
Aguiar DH, Barros MM, Padovani CR, Pezzato LE, Pai-Silva MD (2005) Growth characteristics of skeletal muscle tissue in Oreochromis niloticus larvae fed on a lysine supplemented diet. J Fish Biol 67:1287–1298
Basaran F, Ozbilgin H, Ozbilgin YD (2007) Comparison of the swimming performance of farmed and wild gilthead sea bream, Sparus aurata. Aquacult Res 38:452–456
Bennett AF (1978) Activity metabolism of the lower vertebrates. Annu Rev Physiol 40:447–469
Brauner CJ, Iwama GK, Randall DJ (1994) The effect of short-duration seawater exposure on the swimming performance of wild and hatchery-reared juvenile coho salmon (Oncorhyncus kisutch) during smoltification. Can Special Publ Fisheries Aquatic Sci 51:2188–2194
Buchanan CI, Marsh RL (2002) Effects of exercise on the biomechanical, biochemical and structural properties of tendons. Comp Biochem Physiol 133 A:1101–1107
Cakli S, Kilinc B, Cadun A, Dincer T, Tolasa S (2007) Quality differences of whole ungutted sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) while stored in ice. Food Control 18:391–397
Davie PS, Wells RMG, Tetens V (1986) Effects of sustained swimming on rainbow trout muscle structure, blood oxygen transport, and lactate dehydrogenase isozymes: evidence for increased aerobic capacity of white muscle. J Exp Zool 237:159–171
Davison W (1983) Changes in muscle cell ultrastructure following exercise in Salmo trutta. Experientia 39:1017–1018
Davison W (1994) Exercise training in the banded wrasse Notolabrus fucicola affects muscle fibre diameter but not muscle mitochondrial morphology. N Z Nat Sci 21:11–16
Davison W (1997) The effects of exercise training on teleost fish, a review of recent literature. Comp Biochem Physiol 117A:67–75
Dickson KA (1996) Locomotor muscle of high-performance fishes: what do comparisons of tunas with ectothermic sister taxa reveal? Comp Biochem Physiol 113A:39–49
Dubowitz V (1985) Muscle biopsy: a practical approach. Baillière Tindall, London
Farrell AP, Johansen JA, Suarez RK (1991) Effects of exercise training on cardiac performance and muscle enzymes in rainbow trout, Oncorhynchus mykiss. Fish Physiol Biochem 9:303–312
Fernández F, Miquel AG, Córdoba M, Varas M, Metón I, Caseras A, Baanante IV (2007) Effects of diets with distinct protein-to-carbohydrate ratios on nutrient digestibility, growth performance, body composition and liver intermediary enzyme activities in gilthead sea bream (Sparus aurata.L.) fingerlings. J Exp Mar Biol Ecol 343:1–10
Fouces V, Torrella JR, Palomeque J, Viscor G (1993) A histochemical ATPase method for the demonstration of the muscle capillary network. J Histochem Cytochem 41:283–289
Georgopoulos GB, Conides AJ (1999) Study on the effects of dietary starch on growth, food conversion and digestibility of adult gilthead sea bream (Sparus aurata L., 1758). J Aquac Trop 14:143–152
Gibb AC, Dickson KA (2002) Functional morphology and biochemical indices of performance: is there a correlation between metabolic enzyme activity and swimming performance? Integrative Comp Biol 42(2):199–207
Goldspink G (1972) Postembryonic growth and differentiation of striated skeletal muscle. In: Bourne GH (ed) The structure and function of muscle. Academic Press, New York, pp 179–236
Goldspink G, Howells KF, Ward PS (1976) Effects of exercise on muscle fibre size. In: Jokl E (ed) Medicine sport 9. Advances in exercise physiology. Karger, Basel, pp 103–l13
Goolish EM (1989) The scaling of aerobic and anaerobic muscle power in rainbow trout (Salmo gairdneri). J Exp Biol 147:493–505
Grigorakis K (2007) Compositional and organoleptic quality of farmed and wild gilthead sea bream (S. aurata) and sea bass (D. labrax) and factors affecting it: a review. Aquaculture 272:55–75
Grigorakis K, Alexis MN, Taylor KDA, Hole M (2002) Comparison of wild and cultured gilthead sea bream (Sparus aurata); composition, appearance and seasonal variations. Int J Food Sci Technol 37:477–484
Hinterleitner S, Huber M, Lackner R, Wieser W (1992) Systemic and enzymatic responses to endurance training in two cyprinid species with different life styles (Teleostei: Cyprinidae). Can J Fish Aquat Sci 49:110–115
Holloszy JO, Booth FW (1976) Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol 38:273–291
Hoppeler H (1986) Exercise-induced ultrastructural changes in skeletal muscle. Int J Sports Med 7:187–204
Hoppeler H, Howald H, Conley KE, Lindstedt SL, Claassen H, Vock P, Weibel ER (1985) Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol 59:320–327
Ibarz A, Beltrán M, Fernández-Borràs J, Gallardo MA, Sánchez J, Blasco J (2007a) Alteration in lipids metabolism and use of energy depots of gilthead sea bream (Sparus aurata) at low temperatures. Aquaculture 262:470–480
Ibarz A, Blasco J, Sala-Rabanal M, Gallardo MA, Redondo A, Fernández-Borràs J (2007b) Metabolic rate and tissue reserves in gilthead sea bream (Sparus aurata) under thermal fluctuations and fasting, and their capacity for recovery. Can J Fish Aquat Sci 64:1034–1042
Johnston IA (1982a) Capillarization, oxygen diffusion distances and mitochondrial content of carp muscles following acclimation to summer and winter temperatures. Cell Tissue Res 222:325–337
Johnston IA (1982b) Quantitative analyses of ultrastructure and vascularization of the slow muscle fibres of the anchovy. Tissue Cell 14:319–328
Johnston IA (1999) Muscle development and growth: potential implications for flesh quality in fish. Aquaculture 177:99–l15
Johnston IA, Moon TW (1980) Exercise training in skeletal muscle of the brook trout (Salvelinus fontinalis). J Exp Biol 87:177–194
Kiessling A, Storebakken T, Asgard T, Kiessling K-H (1991) Changes in the structure and function of the epaxial muscle of rainbow trout Oncorhynchus mykiss in relation to ration and age. I. Growth dynamics. Aquaculture 93:335–356
Mascarello F, Rowlerson A, Radaelli G, Scapolo PA, Veggetti A (1995) Differentiation and growth of muscle in the fish Sparus aurata L. I. Myosin expression and organization of fibre types in lateral muscle from hatching to adult. J Muscle Res Cell Motil 16:213–222
Mathieu-Costello O, Brill RW, Hochachka PW (1996) Structural basis for oxygen delivery: muscle capillaries and manifolds in tuna red muscle. Comp Biochem Physiol 113A:25–31
McDonald DG, Milligan CL, McFarlane WJ, Croke S, Currie S, Hooke B, Angus RB, Tufts BL, Davidson K (1998) Condition and performance of juvenile Atlantic salmon (Salmo salar): effects of rearing practices on hatchery fish and comparison with wild fish. Can J Fish Aquat Sci 55:476–488
Nachlas MM, Tsou KC, De Souza E, Cheng CS, Seligman AM (1957) Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem 5:420–436
Rowlerson A, Veggetti A (2001) Cellular mechanisms of post-embryonic muscle growth in aquaculture species. In: Johnston IA (ed) Muscle development and growth. Academic Press, London, pp 103–140
Sänger AM (1992) Effects of training on axial muscle of two cyprinid species: Chondrostoma nasus (L.) and Leuciscus cephalus (L.). J Fish Biol 40:637–646
Sänger AM, Stoiber W (2001) Muscle fibre diversity and plasticity. In: Johnston IA (ed) Muscle development and growth. Academic Press, London, pp 187–237
Stickland NC (1983) Growth and development of the muscle fibres in the rainbow trout (Salmo gairdneri). J Anat 137:323–333
Stoiber W, Haslett JR, Wenk R, Steinbacher P, Gollman H-P, Sänger AM (2002) Cellularity changes in developing red and white fish muscle at different temperatures: simulating natural environmental conditions for a temperate freshwater cyprinid. J Exp Biol 205:2349–2364
Thorstad EV, Finstad B, Okland F, McKinley RS, Booth RK (1997) Endurance of farmed and sea-ranched Atlantic salmon Salmo salar L. at spawning. Aquac Res 28:635–640
Valente LMP, Rocha E, Gomes EFS, Silva MW, Oliveira MH, Monteiro RAF, Fauconneau B (1999) Growth dynamics of white and red muscles in fast and slow growing strains of rainbow trout. J Fish Biol 55:675–691
Venou B, Alexis MN, Fountoulaki E, Nengas I, Apostolopoulou M, Castritsi-Cathariou I (2003) Effect of extrusion of wheat and corn on gilthead sea bream (Sparus aurata) growth, nutrient, utilization efficiency, rates of gastric evacuation and digestive enzymes activities. Aquaculture 25:207–223
Vincent RE (1960) Some in£uences of domestication upon three stocks of brook trout (Salvelinus fontinalis). Trans Am Fisheries Soc 89:3–14
Viscor G, Torrella JR, Fouces V, Palomeque J (1992) Skeletal muscle capillarization and fiber types in urban and homing pigeons (Columba livia). Comp Biochem Physiol 101A:751–757
Weatherley AH, Gill HS, Lobo AE (1988) Recruitment and maximal diameter of axial muscle fibres in teleosts and their relationship to somatic growth and ultimate size. J. Fish Biol 33:851–859
Acknowledgments
This study was supported by grants from the Catalan government and the Spanish government Ministry of Culture. O. Felip and M. Martín-Pérez received fellowships from FPI-2007 and FI-2007 of the Spanish government and the Catalan government, respectively. The authors thank David Rizo for his technical assistance in processing muscle samples and Dr. Ginés Viscor and Dr. Martín Rios for their valuable contribution in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Ibarz, A., Felip, O., Fernández-Borràs, J. et al. Sustained swimming improves muscle growth and cellularity in gilthead sea bream. J Comp Physiol B 181, 209–217 (2011). https://doi.org/10.1007/s00360-010-0516-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0516-4