Abstract
The heart is acutely sensitive to temperature in aquatic ectotherms and appears to fail before any other organ as the thermal maximum is reached, although the exact cause of this failure remains unknown. The heart is highly aerobic and therefore dependent on mitochondrial oxidative phosphorylation (OXPHOS) to meet energy requirements, but the role of cardiac mitochondria in limiting heart function at high temperatures remains unclear. We used permeabilised ventricle fibres to explore heart mitochondrial function in situ in three closely related species of small New Zealand triplefin fishes in response to temperature. We compared this to measures of whole animal respiration rates and critical oxygen tensions in these fishes. Bellapiscis medius, an intertidal species, had the greatest tolerance to hypoxia at higher temperatures and had more efficient OXPHOS at 30°C than the two subtidal species Forsterygion varium and F. malcolmi. B. medius also displayed the highest cytochrome c oxidase flux, which may in part explain how B. medius tolerates higher temperatures and hypoxia. Triplefin heart mitochondria exhibit decreased coupling to phosphorylation with increasing temperature. This most likely impairs ATP supply to the heart at elevated temperatures, potentially contributing to heart failure at ecologically relevant temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thermal tolerance limits of marine ectotherms appear to be determined by the cardiovascular system, as with rising temperature the heart appears to fail before all other organs (Farrell 1997, 2002; Pörtner et al. 2004; Somero 2002). Importantly, the upper thermal limits of many marine ectotherms appear to be only a few degrees above their respective upper habitat temperatures, and consequently it has been suggested that these species may be living on the edge of heart failure (Stillman and Somero 2000). Mitochondrial dysfunction is one potential point of failure for thermally challenged hearts.
The mitochondrion’s role in thermal adaptation has been explored in numerous studies (e.g. Guderley and St Pierre 2002; Hochachka and Somero 2002; Johnston et al. 1994). However, the role of mitochondria in determining thermal limits remains unclear. Some workers have concluded that mitochondria contribute in part to the determination of thermal limits (Pörtner et al. 2005), but are not a major limiting factor (Pörtner 2002, 2006). A direct causal role for mitochondria in setting the heart’s upper thermal limits has been discounted as in most studies the Arrhenius break-point temperatures (ABT) of maximal mitochondrial respiration (or O2 flux) appears approximately 10°C higher than either the whole animal respiration break-points (Hardewig et al. 1999; Pörtner et al. 1999; Vandenheede et al. 1973; Weinstein and Somero 1998), or the species maximum habitat temperature (Dahlhoff and Somero 1993).
However, maximal respiration may reflect only total oxygen consumption and not oxidative phosphorylation (OXPHOS), as mitochondria exhibit lower OXPHOS efficiencies at high temperatures due to an increased proportion of proton leak (Frederich and Pörtner 2000; Pörtner et al. 1999; Sommer et al. 1997). This diminishes the ATP produced for a given amount of oxygen consumed. Therefore, to maintain steady ATP production oxygen demand must increase disproportionately with increasing temperature (Pörtner 2006). Thus the fact that maximal mitochondrial oxygen consumption rates continue to rise beyond whole animal ABTs does not necessarily indicate an increase or maintenance of function, as in vivo phosphorylation efficiencies are perhaps more important for the maintenance of heart energy supply and function.
In addition, most studies reporting mitochondrial ABTs used isolated mitochondria derived from non-cardiac tissues such as liver (e.g. Hardewig et al. 1999; Weinstein and Somero 1998). Mitochondria from different tissues of model species have distinct mitochondrial proteomes (Mootha et al. 2003), dynamics (Cereghetti and Scorrano 2006), thermal sensitivities (Almeida-Val et al. 1994; Irving and Watson 1976), chemical affinities (Di Paola and Lorusso 2006), substrate requirements (Almeida-Val et al. 1994), structures, enzyme profiles (Benard et al. 2006) and respiration rates (Benard et al. 2006; Mootha et al. 2003). Most likely this holds true for non-model species also, as respiration rates of cardiac mitochondria have been shown to be 1.5–10 times greater than those from liver in sea bass (Dicentrarchus labrax) (Trigari et al. 1992), 3–4 times higher than mitochondria from red skeletal muscle in tuna (Katsuwonus pelamis) and carp (Cyprinus carpio) (Moyes et al. 1992), and two times higher than mitochondria from red skeletal muscle in trout (Oncorhynchus mykiss) (Leary et al. 2003).
Isolation of mitochondria also disrupts mitochondrial networks, contact arrangements within cells, and may select for subsarcolemmal or intermyofibrillar populations (Gnaiger 2009), which show distinct sedimentation and biochemical profiles (Chemnitius et al. 1993). Analysis of mitochondria in situ following saponin permeabilisation of muscle fibre bundles provides an alternative approach (Jüllig et al. 2008; Kuznetsov et al. 2008; Saks et al. 1998), which requires very small amounts of tissue (milligrams) and maintains mitochondrial arrangements within myofibrils (Jüllig et al. 2008; Kuznetsov et al. 1996, 2008; Saks et al. 1991, 1993, 1998). This may provide a more realistic measure of tissue-specific respiration, closer to in vivo rates, and enable measurement of very small tissue samples such as those from small ectotherms.
Here, we explore cardiac mitochondrial function in permeabilised cardiac fibres from three species of New Zealand triplefin fishes (Family Tripterygiidae). Triplefins are small, marine blennioid fishes. The New Zealand triplefin fauna makes an excellent model system for study of physiological adaptation (Hickey and Clements 2003), as the group consists of 26 endemic and closely related species (Hickey and Clements 2005; Hickey et al. 2009b) that have radiated into a variety of niches at varying depths, from thermally unstable intertidal rockpools down to deep thermally stable subtidal reefs (Clements 2003; Feary and Clements 2006; Wellenreuther et al. 2007). The three triplefin species studied here differ in their depth distribution and therefore environmental temperature exposure. Bellapiscis medius inhabits upper intertidal rockpools that range in temperature from 9 to 27°C (range ≥18°C) (Hilton et al. 2008). Forsterygion varium and F. malcolmi occur subtidally at an average depth of 7.5 and 11 m, respectively (Wellenreuther et al. 2007), where sea-surface temperatures range from 13°C to 23°C (range ≥10°C) at the capture locations (Leigh Marine Laboratory Climate Data Archives 1967–2007).
In this study, we hypothesized that cardiac mitochondria in B. medius (rockpool inhabitant) would be more robust to higher temperatures than the two subtidal species, in order to cope with higher maximum temperatures experienced in thermally unstable intertidal rockpools. We predicted that OXPHOS would be more stable (more coupled) at elevated temperatures in B. medius. However, we also explored different components of the electron transport system (ETS), as little is known about its composition or thermal stability in non-model species. We measured respiration in permeabilised fibres from triplefin ventricles (0.5-1.5 mg) using sensitive high-resolution respirometers, and assayed cardiac fibres at temperatures of 15, 25 and 30°C using a substrate inhibitor titration protocol designed to test OXPHOS, and various ETS components. We tested respiratory flux and apparent coupling with both respiratory complexes I (CI) and II (CII) simultaneously, as parallel electron inputs into CI and CII have been shown to result in substantially higher and more realistic respiration rates than single complex measurements (Gnaiger 2009). We then compared mitochondrial respiration rates to whole animal respiration rates and critical oxygen tensions at 15 and 25°C.
Materials and methods
Animal collection, housing and acclimation
All fish were collected from the Hauraki Gulf, NZ (174°48′E, 36°18′S), using hand-nets. Adult B. medius were caught from intertidal rockpools, while adult F. varium and F. malcolmi were caught subtidally on SCUBA. Fish were acclimated to 15°C in aerated 30 l aquaria with recirculating seawater for 4 weeks prior to experimentation. Fish were fed daily to satiation on a mixture of krill, mysis shrimp, mussel, bloodworms and Artemia nauplii.
Mitochondrial respirometry
Preparation of intact mitochondria in heart muscle fibres
All chemicals were obtained from Sigma–Aldrich Corp., St. Louis, MO, USA. Fish were anaesthetised with clove oil (active ingredient eugenol) and the spine severed immediately at the skull. The heart was dissected immediately and immersed in 2 ml ice-cold biopsy buffer (modified from Veksler et al. 1987). Osmolarity of the biopsy and assay media were modified to equal the mean osmolarity of triplefin plasma (350 mosmol l−1, as determined using a Wescor 5500 Osmometer (Wescor Inc., Logan, UT, USA). The biopsy buffer contained (in mM) 2.77 CaK2EGTA, 7.23 K2EGTA, 5.7 Na2ATP, 6.56 MgCl2·6H2O, 20 Taurine, 15 Na2Phosphocreatine, 20 Imidazole, 0.5 Dithiothreitol, 50 MES, and 30 Sucrose, pH 7.1 at 0°C. The ventricle was dissected from the heart and teased into fibre blocks using a dissecting microscope and then placed into 1 ml of fresh ice-cold biopsy buffer. Freshly prepared saponin was added to a final concentration of 50 μg ml−1 and the fibres were gently shaken in plastic culture plates on ice for 30 min. Fibres were then removed and rinsed three times for 10 min in 1 ml ice-cold modified MiRO5 respiration medium (modified from Gnaiger et al. 2000) containing (in mM) 0.5 EGTA, 3 MgCl2·6H2O, 60 K-lactobionate, 20 Taurine, 10 KH2PO4, 20 HEPES, 140 Sucrose, and 1 g l−1 defatted BSA, pH 7.1 at 30°C.
Mitochondrial respiration assays
At least 1 mg of tissue was necessary for accurate determination of mitochondrial respiration. Therefore, for F. malcolmi and F. varium assays were conducted on fibres from single ventricles, but in the smaller B. medius assays consisted of 1–4 pooled ventricles. Most samples were between 1 and 2 mg of tissue. Fibres were blotted dry on filter paper, accurately weighed and added to 2 ml of saturated MiRO5 medium at 15, 25 or 30°C in an O2k-Oroboros™ oxygraph respirometer with 2 ml chambers (Oroboros Instruments, Innsbruck, Austria). Respiratory flux (JO 2) was calculated in real time as the negative time derivative of the oxygen concentration using Oroboros DatLab Software V 4.1.1.84 (Oroboros Instruments, Innsbruck, Austria) and expressed as pmol O2 consumed s−1 mg−1 wet weight of tissue.
The titration protocol consisted of glutamate (10 mM), malate (5 mM), and succinate (10 mM) added to measure State 2 respiration through both CI and CII in the absence of ADP (denoted “leak”). Subsequently, saturating ADP (1.25 mM) was added to induce State 3 respiration through CI and CII (denoted “OXPHOS”). To test for limitation by the F0F1-ATPase and the ETS the uncoupling agent FCCP (0.5 μM) was then added (denoted “ETS”). Subsequently rotenone (0.75 μM) was added to inhibit CI (ETSrot). Respiration was inhibited with antimycin A (10 μM), and subsequently TMPD (500 μM) and ascorbate (2 mM) were added to measure cytochrome c oxidase (Complex IV) activity (CCO). Finally, cytochrome c (10 μM) was added (CCOc). An increase in rate following cytochrome c addition provides indication of outer mitochondrial membrane damage with resultant loss of endogenous cytochrome c. As cytochrome c release to the cytosol triggers apoptosis in vertebrates, a large increase indicates potential initiation of this apoptotic trigger (Kunz et al. 2000; Kuznetsov et al. 2004). Although initial trials indicated that the fibre respiration rates were maintained down to very low oxygen concentrations, for consistency, the oxygen concentration of the respiration medium was not allowed to drop below 50% saturation. Results were corrected for background chemical oxygen consumption determined for TMPD and ascorbate at each temperature in the absence of fibres (Gnaiger et al. 1998).
We applied a simple proxy for a measure of efficiency and determined the respiratory control ratio (RCR; OXPHOS/leak (Gnaiger 2009)). This ratio provides an indication of coupling or proton leak relative to phosphorylating respiration, and thus respiration that does not contribute to phosphorylation. It is not possible to measure state 4 respiration in mitochondria respiring in situ in permeabilised muscle fibres as ATP is rapidly cycled back to ADP. Therefore, for mitochondria in permeabilised fibres the RCR is calculated as state 3:2 (Kuznetsov et al. 2008). We also determined the relative contribution of complex I to ETS (calculated as [1 − ETSrot/ETS]), as well as an alternative expression of efficiency: the proportion of oxygen consumed contributing to OXPHOS (100% − (leak/OXPHOS) × 100; %OXPHOS).
Whole animal respiration measurements
After 4 weeks of acclimation at 15°C, resting oxygen consumption rates (VO 2) and the critical oxygen concentration (O2 crit) of the fishes were measured at 15 and 25°C as described previously (Hilton et al. 2008). Briefly, fish were placed in atmospheric equilibrated water at 15°C within a Perspex® chamber with a magnetic stirrer. Respirometry chambers measured 68 ml (50 × 47 mm), 212 ml (70 × 66 mm), 420 ml (100 × 60 mm), or 880 ml (140 × 65 mm) depending on fish size. The temperature was maintained at 15°C or raised to 25°C over 2 h. After 30 min at the final measurement temperature the chamber was closed and oxygen consumption was measured using a Clark-type electrode attached to a 781 Oxygen Meter (Strathkelvin Instruments Ltd., Scotland), and the VO 2 calculated using 949 Oxygen System software (Strathkelvin Instruments Ltd., Scotland). Fish were released when they showed signs of escape behaviour (Hilton et al. 2008). The routine rate of oxygen consumption was derived from linear slopes between 80 and 60% of oxygen saturation. The transition between oxyregulation to oxyconformation (O2 crit), was approximated using the intercept of two segmented least-squares regression lines performed with ‘SegReg’ software (Oosterbaan 1994). Experiments were attempted at 30°C but this temperature appeared to be beyond the species’ upper thermal tolerance limits and was therefore not pursued.
Statistical analyses
Where assumptions were met, analysis of variance (ANOVA) with a Type III sums of squares model, and Tukey’s HSD pairwise comparisons (α = 0.05) were used to test for differences among and between species and temperatures, and for species and temperature interactions. Where variances remained unequal (Levene’s test) following transformation, a Kruskal–Wallis test was employed, followed by pairwise Mann–Whitney U tests, and a Bonferroni correction (α = 0.017) was used. All analyses were performed using SPSS V14.0 (Lead Technologies Inc., Chicago). Least squares linear regressions (LSLR) were used to test for body size effects on all parameters.
The species exhibited intraspecific scaling of whole animal VO 2 with body weight and scaling coefficients were both temperature- and species-specific. Therefore all rates were normalised to 1 g body weight using the exponents derived from LSLR of log transformed data for each species at each temperature. Normalised VO 2 was expressed as the mean mg O2 consumed h−1 g−1 ±1 standard error of the mean (SE). A significant relationship between O2 crit and body weight was observed in Bellapiscis medius at 15°C but not at 25°C or in the two subtidal species at either temperature. However, scaling of O2 crit to weight in B. medius did not affect the significance of the interspecific comparisons and therefore the un-scaled data are presented as these are the more conservative comparison. Temperature quotients (Q 10) were calculated using the Van’t Hoff equation.
Results
Morphological differences and body size
Although no allometric relationship within species was apparent between body mass and ventricle size, ventricle weight to body weight ratios differed among species, with B. medius having significantly larger ventricles relative to body mass than those of the other two species (F = 8.20, p < 0.01), which were similar to each other (p = 0.26). Ventricle: body mass ratios were B. medius 0.037 ± 0.001, n = 15; F. varium 0.026 ± 0.001, n = 20; and F. malcolmi 0.029 ± 0.002, n = 21. No significant intraspecific scaling effect of body size was found with any of the mitochondrial respiratory parameters measured. Body weight and number of animals used for each mitochondrial respiration experiment are presented in Table 1.
Mitochondrial respiration
The substrate-inhibitor titration protocol used glutamate, malate and succinate to supply substrates simultaneously to both complexes I and II (CI + CII) of the ETS to initiate State 2 respiration (Gnaiger 2009) (denoted ‘leak’ in Fig. 1). State 3 respiration (denoted OXPHOS) was initiated following ADP addition. The initial peak flux following ADP addition rapidly declined to a lower steady-state and the magnitude of this decline differed significantly between species (χ 2 = 23.56, p < 0.001). Thus, both rates are presented; the maximum flux denoted as OXPHOSmax and the ‘plateau’ steady-state flux as OXPHOSplateau (Figs. 1, 2). The plateau effect in the intertidal species, B. medius, did not differ significantly from maximum rates (average decrease from max to plateau was 8 ± 5%, paired samples t test, t = 1.89, p = 0.07). However, in the subtidal species F. varium and F. malcolmi the plateau rate was significantly lower than OXPHOSmax (overall mean decrease from max to plateau: F. varium 17 ± 2%, paired t test, t = 7.06, p < 0.001; F. malcolmi 22 ± 3%, paired t test, t = 7.91, p < 0.001). There was no significant difference in the magnitude of the plateau effect between temperatures (χ 2 = 0.23, p = 0.89).
The substrate inhibitor titration protocol used to determine respiratory flux in permeabilised ventricle fibres. Slope of declining oxygen concentration (grey line; nmol ml−1) and rate of oxygen consumption (JO 2; black line; pmol s−1 mg−1) over time (min) obtained from the oxygraph in representative substrate inhibitor titration experiments on permeabilised triplefin ventricular fibres. Arrows indicate the point of addition of each substrate/inhibitor: GMS glutamate, malate and succinate; Rot rotenone; Ant antimycin A; TMPD Asc TMPD and ascorbate; Cyt c cytochrome c. Grey boxes indicate the complex or state being measured. a Demonstrates an assay of ventricular fibres of Bellapiscis medius at 15°C. b Demonstrates an assay of ventricular fibres of Forsterygion malcolmi at 25°C showing the peak rate of OXPHOS (State 3) flux obtained ~30 s after ADP addition (denoted OXPHOSmax) declining to a lower steady state after ~2–5 min (denoted OXPHOSplateau)
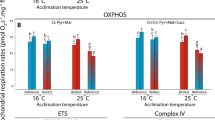
Respiration of saponin-permeabilised ventricle fibres relative to temperature. Assays were conducted at a 15°C, b 25°C, and c 30°C for Bellapiscis medius (Bm; white bars), Forsterygion varium (Fv; grey bars) and F. malcolmi (Fm; dark grey bars), in the following states and through the following complexes: CI and CII State 2 (leak); CI and CII State 3 maximum flux (OXPHOSmax); CI and CII State 3 plateau flux (OXPHOSp); CI and CII uncoupled flux (ETS); CII uncoupled flux (ETSrot); Complex IV, cytochrome c oxidase (CCO); CCO with addition of cytochrome c (CCOc). Asterisk indicates significant difference between the species and all others, or the species pairs indicated by a horizontal line (α = 0.05). Mean ± SE respiratory flux (pmol O2 s−1 mg−1 wet weight of tissue)
Overall, temperature significantly impacted net rates of respiration of all of the ETS components (p < 0.01 in all cases; Fig. 2). However, temperature did not significantly influence the ratios between components (using either OXPHOSmax or OXPHOSplateau values), with the exception of the respiratory control ratio (RCR; p < 0.01). The RCR declined significantly with increasing temperature in all species (p < 0.01, Fig. 3).
Respiratory control ratios (RCR) relative to temperature. Mitochondrial RCRs were determined in permeabilised ventricular fibres from Bellapiscis medius (Bm; white bars), Forsterygion varium (Fv; grey bars) and F. malcolmi (Fm; dark grey bars), at 15, 25 and 30°C using a OXPHOSmax (State 3) or b OXPHOSplateau. Asterisk indicates a significant difference from the other species (α = 0.05). Mean ± SE
There were no significant differences in State 2 flux (leak) between species (Fig. 2). However, state 3 (OXPHOS) flux differed significantly between species (p < 0.01) irrespective of the plateau effect. Forsterygion malcolmi and B. medius showed similar rates of maximum flux (OXPHOSmax) at 15 and 25°C, and both were higher than F. varium. However, at 30°C OXPHOSmax for B. medius was significantly higher than both F. varium (p < 0.01) and F. malcolmi (p = 0.04; Fig. 2). When OXPHOSplateau was compared, no significant differences were apparent among species at 15°C, but B. medius was significantly higher than F. varium and F. malcolmi at both 25 and 30°C (p < 0.01; Fig. 2).
The addition of the uncoupling agent FCCP slightly inhibited respiration in F. varium (mean 0.1 ± 2%,) and F. malcolmi (mean 1.5 ± 2%) at all three temperatures, but these were not significantly different to OXPHOSplateau rates. However, flux in B. medius increased by mean 4.6 ± 2% upon addition of FCCP, which was significantly different to OXPHOSplateau flux (paired samples t test, t = 3.1, p < 0.01), indicating that the phosphorylating system may have slightly greater control of respiration in B. medius relative to the two subtidal species. FCCP is known to sometimes inhibit respiration (Park et al. 2002). The mechanism is unknown, but as FCCP is an uncoupling agent that depolarises the mitochondrial membrane potential, this may impede respiration on electrogenically imported substrates, such as glutamate and pyruvate (but not succinate).
Respiration through CII alone (following rotenone addition; ETSrot; Fig. 2) exhibited a significant interaction between species and temperature (p = 0.03). At 15°C flux rates from ventricle mitochondria of B. medius and F. malcolmi were significantly higher than F. varium (p = 0.04). At 25°C flux of B. medius was significantly higher than F. malcolmi (p < 0.01) and F. varium (p < 0.01), and at 30°C there was no significant difference between species (Fig. 2). However, the measure of the relative contribution of CI (calculated as ([1 − ETSrot/ETS) differed significantly between species at 30°C with B. medius displaying a far higher proportion of flux attributable to CI at 30°C than the other two species (p = 0.01, Fig. 4a). This suggests that B. medius demonstrates greater stability of CI function at elevated temperatures.
a The percentage contribution of complex I (CI %; calculated as 1 − [ETSrot/ETS]) and b cytochrome c oxidase (CCO) excess capacity (calculated as CCO/OXPHOSmax), of mitochondria determined in permeabilised ventricular fibres of Bellapiscis medius (Bm; white bars), Forsterygion varium (Fv; grey bars), and F. malcolmi (Fm; dark grey bars) at 15, 25 and 30°C. Asterisk indicates a significant difference from the other species (α = 0.05). Mean ± SE
Cytochrome c oxidase (CCO) activity in B. medius was significantly higher than in ventricle fibres from F. varium (p < 0.01) and F. malcolmi (p = 0.05) at all temperatures (Fig. 2). CCO activity was also greater in F. malcolmi than F. varium at 15°C (p < 0.01) and 25°C (p < 0.01) (Fig. 2). Flux through CCO was high relative to OXPHOS. The ratio CCO:OXPHOSmax provides a measure of CCO control on the ETS or the CCO ‘excess capacity’. CCO was in greater excess (provides less control) in B. medius (2.5–3-fold) than in F. varium and F. malcolmi (1.5–2-fold; Fig. 4b), however differences between species were less marked using OXPHOSplateau. Addition of cytochrome c resulted in an increase in CCO flux for all species (mean 40 ± 3%, n = 66), indicating some damage to the mitochondrial membranes and loss of cytochrome c during fibre preparations or during each assay. However, as CCO flux exceeded ETS capacity this suggests that this loss was not rate limiting for the other ETS components. This increase did not differ overall between species (F = 1.00, p = 0.42) or temperatures (F = 0.90, p = 0.41), but ANOVAs at each temperature indicated that at 30°C there was a significantly greater increase in flux with addition of cytochrome c in F. varium than in F. malcolmi (p = 0.02).
The RCR, which for fibres describes the increase from state 2 (leak) to state 3 (OXPHOS) respiration following ADP addition (Boushel et al. 2007; Gnaiger 2008, 2009; Hickey et al. 2009a; Jüllig et al. 2008), indicated that at 15°C mitochondria were tightly coupled (RCR(OXPHOSmax) = 4.5–6). Note that CII is less tightly coupled to OXPHOS relative to CI, as CII does not pump protons directly (but does so through CIII and CIV). The RCR calculated with OXPHOSplateau was lower than that calculated with OXPHOSmax, but showed a similar pattern for species and temperatures (Fig. 3). While temperature had a significant impact on the RCR in all species (p < 0.01), B. medius maintained higher RCR (OXPHOSmax) at 30°C than F. varium (p < 0.01) and F. malcolmi (p < 0.01) (Fig. 3a), and a higher RCR than both species at 25 and 30°C for RCR (OXPHOSplateau) (all p < 0.01; Fig. 3b).
An alternative expression of efficiency is to compare the proportion of oxygen consumed contributing to OXPHOS ([100% − (leak/OXPHOS × 100)]; %OXPHOS). At 15°C all species were similar, with approximately 80% of oxygen consumed contributing to OXPHOS. With an increase in temperature to 25°C the %OXPHOS declined significantly for all species (p < 0.01), and at 30°C was <50% in F. varium and F. malcolmi whilst B. medius maintained significantly higher efficiencies of 63 ± 2% at 30°C (p < 0.05).
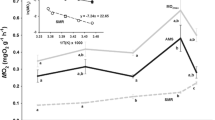
The relationship between OXPHOS and leak can also be presented as OXPHOS–leak/leak (Fig. 5). This is termed “mitochondrial phosphorylating respiration” (Pörtner et al. 1999). Although leak rates were similar among species at each temperature, neither F. varium nor F. malcolmi increased phosphorylating respiration between 15 and 25°C and both species demonstrated a decrease in phosphorylating respiration between 25 and 30°C (Fig. 5). In contrast, phosphorylating respiration of B. medius mitochondria increased between 15 and 25°C, and remained constant between 25 and 30°C (Fig. 5).
Comparison of mitochondrial phosphorylating respiration relative to leak. Phosphorylating respiration was calculated as OXPHOSmax–leak (filled circles) and leak (open circles) of mitochondria in permeabilised ventricular fibres of Bellapiscis medius (Bm), Forsterygion varium (Fv), and F. malcolmi (Fm) at 15, 25 and 30°C. Mean ± SE
Whole animal respiration rates (VO 2) and critical oxygen concentration (O2 crit)
Body weight and number of animals used for each whole animal respiration experiment are presented in Table 2. F. malcolmi showed a significantly higher mass specific VO 2 at 15°C than both F. varium (p < 0.01) and B. medius (p < 0.01), whose rates were not significantly different from each other (Fig. 6a). At 25°C VO 2 for the three species were similar (Fig. 6a). The magnitude of increase in VO 2 between 15 and 25°C in B. medius (Q 10 = 3.79) and F. varium (Q 10 = 3.55) was much greater than the increase in maximal OXPHOS flux over the same temperature range (Q 10 = 1.50 and 1.32 respectively). F. malcolmi showed a smaller magnitude of increase in VO 2 with temperature than the other two species (Q 10 = 1.06) which was similar to the temperature response of JO 2 (OXPHOSmax; Q10 = 1.20).
Whole animal respiration rate and critical oxygen tension relative to temperature. a Whole animal resting respiration rate (VO 2; mg O2 h−1 g−1 body weight), and b critical oxygen concentration (O2 crit; mg O2 l−1), at 15°C (black bars) and 25°C (grey bars) of Bellapiscis medius (Bm), Forsterygion varium (Fv), and F. malcolmi (Fm). Mean ± SE. Bars sharing the same letter are not significantly different (α = 0.05). For comparison 1 mg O2 l−1 = 31.25 μM
The O2 crit differed significantly between all species at 15°C (all p < 0.01) with B. medius the intertidal species, showing the lowest, and F. varium the highest O2 crit (Fig. 6b). At 25°C B. medius also showed a significantly lower O2 crit than the two subtidal species (p < 0.01), but F. varium and F. malcolmi were not significantly different. For F. varium the O2 crit was not significantly different at 25°C compared to 15°C, however in B. medius and F. malcolmi the O2 crit was significantly higher at 25°C than at 15°C (p < 0.01 and p = 0.01 respectively; Fig. 6b).
Discussion
Temperature exerted profound effects on mitochondrial function in the three species of New Zealand triplefins examined in this study, and there were considerable differences between species in both mitochondrial and whole animal respiration rates, and critical oxygen tensions (O2 crit). The intertidal species B. medius demonstrated a greater overall stability and efficiency of OXPHOS at higher temperatures than the two subtidal species. In addition, the capacity to increase mitochondrial respiratory flux, and particularly flux that contributed to OXPHOS between 15 and 25°C, and to maintain this capacity between 25 and 30°C was greatest in B. medius. Notably, F. varium and F. malcolmi do not routinely encounter water temperatures as high as 25°C, whereas we have observed B. medius alive in the field at 27°C. Thus, mitochondrial phosphorylation efficiency appears well adapted to each species’ thermal range. There were also clear differences in the ETS composition and stability among species, with B. medius having a more robust CI-dependent flux at high temperatures and also a greater excess capacity of CCO at all temperatures. This was combined with a 170–230% higher tolerance to hypoxia (lower O2 crit) in B. medius compared to the two subtidal species. We also detected a morphological difference between the species, with B. medius having relatively larger ventricles than the two subtidal species. This may also influence the ability of the latter species to withstand hypoxia by increasing the circulatory capacity.
Stability of respiration
The observed ‘plateau’ effect differed across species and was virtually absent in B. medius. Such an effect is not observed in rat ventricle tissue (Jüllig et al. 2008). Although this effect may result from oxaloacetate accumulation (e.g. Chance and Hagihara 1962; Das 1937; Pardee and Potter 1948), glutamate should prevent inhibition by promoting oxaloacetate transamination to aspartate and α-ketoglutarate (Hillar et al. 1975), and ATP should also prevent inhibition (Pardee and Potter 1948). We predict that the observed ‘plateau’ effect results from differences in CI inhibition or degradation, or inner membrane instabilities. Unlike succinate, glutamate is electrogenically transported into the mitochondrial matrix and therefore this substrate is dependent on membrane potential. A loss of membrane potential with temperature may therefore account for depressed CI flux. This is also consistent with depressed RCRs at elevated temperatures in the two deeper living species.
The rates and thermal stabilities of leak respiration on combined CI and CII substrates did not differ significantly among species. However, OXPHOS flux did differ significantly between species and were most plastic in B. medius, the species which occupies thermally variable intertidal rockpools, while F. malcolmi which is the deepest living of the three species studied, had the lowest scope for change. The scope for adjustment of OXPHOS flux therefore appears to reflect the different thermal ranges of the habitats occupied by these species.
Phosphorylation capacity
The RCR is plastic and may be optimised by acclimation. Birkedal and Gesser (2003) assayed mitochondria in permeabilised ventricle fibres of cold-acclimated (12°C) cod (Gadus morhua) and trout (Oncorhynchus mykiss), and found higher RCR’s at 10°C than at 20°C. However, they found that turtles (Pseudemys scripta elegans) acclimated to 20°C had lower RCRs at 10°C than 20°C. Glanville and Seebacher (2006) demonstrated that the RCRs of isolated crocodile (Crocodylus porosus) heart mitochondria were also optimised by acclimation, and declined at temperatures above or below the acclimation temperature. The data presented here indicate that the RCRs were highest at the acclimation temperature of 15°C in all species. However, the thermal sensitivity of the RCR differed among closely related species despite 1 month of acclimation to a constant temperature.
Low RCR values with increased temperatures indicate decreased OXPHOS efficiencies. In this study the RCR values decreased in all species with increasing temperature. However, the deepest living species, F. malcolmi, had the lowest RCR values at 30°C, while the intertidal species B. medius had relatively high RCR values. This indicates that B. medius is able to maintain OXPHOS efficiency at the high temperatures experienced in intertidal rockpools. Although uncoupling at high temperatures may be beneficial by lowering free radical production (Murphy 2009), it can only be of benefit if ATP production remains adequate to meet metabolic demands.
While the mechanisms of proton leak are still poorly understood, protons may leak through uncoupling proteins (UCP) (Brand et al. 1994; Stuart et al. 2001), the dicarboxylate carrier (Wieckowski and Wojtczak 1997), the adenine nucleotide translocase (ANT) (Cadenas et al. 2000), and the aspartate/glutamate antiporter (Samartsev et al. 1997). Proton leak may also be modified by the inner mitochondrial membrane fatty acid composition (Brand et al. 1994; Brookes et al. 1998; Hulbert and Else 2000). Changes in membrane fluidities also impact membrane-associated protein function (Hazel and Williams 1990; Hulbert and Else 2000). Changes in mitochondrial membrane composition with thermal acclimation have been reported in fishes (Caldwell and Vernberg 1970; Cossins et al. 1980; Van den Thillart and De Bruin 1981), although changes may not be universal (Trigari et al. 1992; Van den Thillart and Modderkolk 1978). Such differences in lipid composition and/or membrane-associated protein function may account for the increased coupling of B. medius mitochondria at high temperatures.
Excess capacity of cytochrome c oxidase
Bellapiscis medius also had a higher CCO flux with less CCO control (CCO/OXPHOS) than the two subtidal species. Reasons for the excess capacity of CCO above that of maximal respiration appear to be complex (Gnaiger et al. 1998), particularly in heart tissue where CCO is high (Gnaiger et al. 1998). The higher relative flux of CCO in B. medius may result from either elevated amounts of CCO, or from altered kinetics, i.e. higher affinities for substrates (O2, cytochrome c). In either context, an elevated CCO flux may aid O2 binding at low O2 tensions (Gnaiger 2003). This is consistent with B. medius having a considerably greater hypoxia tolerance (lower O2 crit) than the other species tested. Rockpools can become hypoxic, due to respiration and elevated temperatures, or hyperoxic, due to algal photosynthesis. Elevated CCO may also be an advantage for B. medius in the case of hyperoxia, as elevated CCO activities have been associated with an increase in hyperoxia tolerance in cultured cells through reduced ROS production (Campian et al. 2007).
CCO is also regulated by nitric oxide (NO) (Antunes et al. 2004), in particular in pathological settings (Borutaite and Brown 1996; Davidson and Duchen 2006). At least in mammalian heart, NO is a vasodilator that is produced by nitric oxide synthase (NOS) under hypoxia, and NO plays a substantial role in cardiac regulation (Davidson and Duchen 2006; Paulus and Bronzwaer 2004). Although it is a vasodilator it also inhibits respiration at CCO at low O2 tensions, while in normoxia CCO metabolises NO (Borutaite and Brown 1996). An elevated number of CCO enzymes can also act to increase the binding affinity of CCO for O2 (Gnaiger et al. 1998). Therefore an elevated CCO pool may provide B. medius with a means to maintain adequate function during periods of hypoxia despite elevated NO inhibition. It may also aid NO clearance on return to normoxia. Mammalian cardiac mitochondria have a greater excess capacity of CCO relative to other tissues such as liver (Benard et al. 2006), and this may be to accommodate NO inhibition and to increase oxygen binding affinities.
Bellapiscis medius has the highest CCO flux and also displays the greatest tolerance to hypoxia among the three species tested. These data suggest that selection has occurred at CCO in B. medius, and may support the proposal that limitations on oxygen supply may mediate cardiac failure in ectotherms at elevated temperatures (Pörtner 2001; Pörtner 2002; Pörtner and Knust 2007; Pörtner et al. 2004), as enhanced O2 binding in B. medius may compensate for decreased O2 delivery to the heart.
Summary
We have demonstrated that heart mitochondrial respiratory efficiency declines markedly within ecologically relevant temperatures in these fishes, despite full oxygenation. Mitochondrial dysfunction at high temperatures can limit ATP supply and is often accompanied by elevated reactive oxygen species formation (Abele et al. 2007; Turrens 2003). Both can result in a drive towards necrosis or apoptosis, and these processes may ultimately lead to heart failure even in the presence of adequate oxygen (Sheeran and Pepe 2006). Therefore mitochondrial dysfunction with rising temperatures may at least in part drive temperature-induced heart failure in ectotherms.
Overall, this study has shown clear differences in mitochondrial function between three closely related species of fishes that appear to reflect differences in the species range and maximal habitat temperatures, and likely exposure to hypoxia. The intertidal species B. medius shows a greater efficiency of OXPHOS at high temperatures than the two subtidal species and a greater ability to increase and maintain OXPHOS at higher temperatures than its subtidal relatives. A much higher tolerance to hypoxia in B. medius is also associated with a more stable CI flux and higher CCO flux at all temperatures, indicating that differences in mitochondrial performance may be important in allowing this species to occupy a more extreme and variable habitat.
Abbreviations
- ADP:
-
Adenosine-5′-diphosphate
- ATP:
-
Adenosine-5′-triphosphate
- BSA:
-
Bovine serum albumin
- CCO:
-
Cytochrome c oxidase
- CCOc :
-
Cytochrome c oxidase in the presence of additional cytochrome c
- EGTA:
-
Ethylene glycol tetraacetic acid
- ETS:
-
Electron transport system
- FADH2 :
-
Flavin adenine dinucleotide (reduced)
- FCCP:
-
Carbonyl cyanide p-(trifluoro-methoxy) phenyl-hydrazone
- HEPES:
-
Na N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- JO 2 :
-
Rate of mitochondrial oxygen consumption
- LSLR:
-
Least squares linear regression
- MES:
-
2-(N-morpholino) ethanesulfonic acid
- NADH:
-
Nicotinamide adenine dinucleotide (reduced)
- OXPHOS:
-
Oxidative phosphorylation
- RCR:
-
Respiratory control ratio
- ROS:
-
Reactive oxygen species
- TMPD:
-
N,N,N′,N′-tetramethyl-p-phenyldiamine
- VO 2 :
-
Rate of oxygen consumption
References
Abele D, Philipp E, González PM, Puntarulo S (2007) Marine invertebrate mitochondria and oxidative stress. Front Biosci 12:933–946
Almeida-Val VMF, Buck LT, Hochachka PW (1994) Substrate and acute temperature effects on turtle heart and liver mitochondria. Am J Physiol 266R:858–862
Antunes F, Boveris A, Cadenas E (2004) On the mechanism and biology of cytochrome oxidase inhibition by nitric oxide. PNAS 101:16774–16779
Benard G, Faustin B, Passerieux E, Galinier A, Rocher C, Bellance N, Delage J-P, Casteilla L, Letellier T, Rossignol R (2006) Physiological diversity of mitochondrial oxidative phosphorylation. Am J Physiol 291C:1172–1182
Birkedal R, Gesser H (2003) Creatine kinase and mitochondrial respiration in hearts of trout, cod and freshwater turtle. J Comp Physiol 173B:493–499
Borutaite V, Brown G (1996) Rapid reduction of nitric oxide by mitochondria, and reversible inhibition of mitochondrial respiration by nitric oxide. Biochem J 315:295–299
Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsoe R, Dela F (2007) Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetalogia 50:790–796
Brand MD, Chien L-F, Ainscow EK, Rolfe DFS, Porter RK (1994) The causes and functions of mitochondrial proton leak. Biochim Biophys Acta 1187:132–139
Brookes PS, Buckingham JA, Tenreiro AM, Hulbert AJ, Brand MD (1998) The proton permeability of the inner membrane of liver mitochondria from ectothermic and endothermic vertebrates and from obese rats: correlations with standard metabolic rate and phospholipid fatty acid composition. Comp Biochem Physiol 119B:325–334
Cadenas S, Buckingham J, St Pierre J, Dickinson K, Jones R, Brand M (2000) AMP decreases the efficiency of skeletal-muscle mitochondria. Biochem J 351:307–311
Caldwell RS, Vernberg FJ (1970) The influence of acclimation temperature on the lipid composition of fish gill mitochondria. Comp Biochem Physiol 34:179–191
Campian JL, Gao X, Qian M, Eaton JW (2007) Cytochrome c oxidase activity and oxygen tolerance. J Biol Chem 282:12430–12438
Cereghetti GM, Scorrano L (2006) The many shapes of mitochondrial death. Oncogene 25:4717–4724
Chance B, Hagihara B (1962) Activation and inhibition of succinate oxidation following adenosine diphosphate supplements to pigeon heart mitochondria. J Biol Chem 237:3540–3545
Chemnitius J, Manglitz T, Kloeppel M, Doenst T, Schwartz P, Kreuzer H, Zech R (1993) Rapid preparation of subsarcolemmal and interfibrillar mitochondrial subpopulations from cardiac muscle. Int J Biochem 25:589–596
Clements KD (2003) Triplefins. In: Andrew N, Francis MP (eds) The living reef: the ecology of New Zealand’s rocky reefs. Craig Potton Publishing, Nelson, pp 160–167
Cossins AR, Kent J, Prosser CL (1980) A steady state and differential polarised phase fluorimetric study of the liver microsomal and mitochondrial membranes of thermally acclimated green sunfish (Lepomis cyanellus). Biochim Biophys Acta 599:341–358
Dahlhoff E, Somero GN (1993) Effects of temperature on mitochondria from abalone (Genus Haliotis): adaptive plasticity and its limits. J Exp Biol 185:151–168
Das NB (1937) Studies on the inhibition of the succinic and lactic-malic dehydrogenases. Biochem J 31:1124–1130
Davidson S, Duchen M (2006) Effects of NO on mitochondrial function in cardiomyocytes: pathophysiological relevance. Cardiovasc Res 71:10–21
Di Paola M, Lorusso M (2006) Interaction of free fatty acids with mitochondria: coupling, uncoupling and permeability transition. Biochim Biophys Acta 1757:1330–1337
Farrell AP (1997) Effects of temperature on cardiovascular performance. In: Wood CM, Mc Donald DG (eds) Global warming: implications for freshwater and marine fish. Cambridge University Press, Cambridge, pp 135–158
Farrell AP (2002) Cardiorespiratory performance in salmonids during exercise at high temperature: insights into cardiovascular design limitations in fishes. Comp Biochem Physiol 132A:797–810
Feary DA, Clements KD (2006) Habitat use by triplefin fishes (Family Trypterygiidae) on rocky reefs in New Zealand. J Fish Biol 69:1031–1046
Frederich M, Pörtner HO (2000) Oxygen limitation of thermal tolerance defined by cardiac and ventilatory performance in spider crab, Maja squinado. Am J Physiol 279R:1531–1538
Glanville EJ, Seebacher F (2006) Compensation for environmental change by complementary shifts of thermal sensitivity and thermoregulatory behaviour in an ectotherm. J Exp Biol 209:4869–4877
Gnaiger E (2003) Oxygen conformity of cellular respiration; a perspective of mitochondrial physiology. Kluwer Academic/Plenum Publishers, New York
Gnaiger E (2008) Polarographic oxygen sensors, the oxygraph, and high-resolution respirometry to assess mitochondrial function. In: Dykens J, Will Y (eds) Drug-induced mitochondrial dysfunction. John Wiley & Sons, Inc
Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837–1845
Gnaiger E, Lassnig B, Kuznetsov AV, Rieger G, Raimund M (1998) Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol 201:1129–1139
Gnaiger E, Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Steurer W, Margreiter R (2000) Mitochondria in the cold. In: Heldmaier M, Klingenspor M (eds) Life in the cold. Springer, Heidelberg, pp 431–442
Guderley H, St Pierre J (2002) Going with the flow or life in the fast lane: contrasting mitochondrial responses to thermal change. J Exp Biol 205:2237–2249
Hardewig I, Peck LS, Pörtner HO (1999) Thermal sensitivity of mitochondrial function in the Antarctic Notothenioid Lepidonotothen nudifrons. J Comp Physiol 169B:597–604
Hazel JR, Williams EE (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–227
Hickey AJR, Clements KD (2003) Key metabolic enzymes and muscle structure in triplefin fishes (Tripterygiidae): a phylogenetic comparison. J Comp Physiol 173B:113–123
Hickey AJR, Clements KD (2005) Genome size evolution in New Zealand triplefin fishes. J Hered 96:356–362
Hickey AJR, Chai CC, Choong SY, de Freitas Costa S, Skea GL, Phillips ARJ, Cooper GJS (2009a) Impaired ATP turnover and ADP supply depress cardiac mitochondrial respiration and elevate superoxide in nonfailing spontaneously hypertensive rat hearts. Am J Physiol 297:C766–C774
Hickey AJR, Lavery SD, Hannan DA, Baker SC, Clements KD (2009b) New Zealand triplefin fishes (Family Tripterygiidae): contrasting population structure and mtDNA diversity within a marine species flock. Mol Ecol 18:680–696
Hillar M, Lott V, Lennox B (1975) Correlation of the effects of citric acid cycle metabolites on succinate oxidation by rat liver mitochondria and submitochondrial particles. Bioenergetics 7:1–16
Hilton Z, Wellenreuther M, Clements KD (2008) Physiology underpins habitat partitioning in a sympatric sister-species pair of intertidal fishes. Funct Ecol 22:1108–1117
Hochachka PW, Somero GN (2002) Biochemical adaptation. Mechanism and process in physiological evolution. Oxford University Press, New York
Hulbert AJ, Else PL (2000) Mechanisms underlying the cost of living in animals. Annu Rev Physiol 62:207–235
Irving DO, Watson K (1976) Mitochondrial enzymes of tropical fish: a comparison with fish from cold waters. Comp Biochem Physiol 54B:81–92
Johnston IA, Guderley H, Franklin CE, Crockford T, Kamunde C (1994) Are mitochondria subject to evolutionary temperature adaptation? J Exp Biol 195:293–306
Jüllig M, Hickey AJR, Chai CC, Skea GL, Middleditch MJ, Costa S, Choong SY, Philips AJC, Cooper GJS (2008) Is the failing heart out of fuel or a worn engine running rich? A study of mitochondria in old spontaneously hypertensive rats. Proteomics 8:2556–2572
Kunz WS, Kudin A, Vielhaber S, Elger CE, Attardi G, Villani G (2000) Flux control of cytochrome c oxidase in human skeletal muscle. J Biol Chem 275:27741–27745
Kuznetsov A, Tiivel T, Sikk P, Kaambre T, Kay L, Daneshrad Z, Rossi A, Kadaja L, Peet N, Seppet E, Saks V (1996) Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur J Biochem 241:909–915
Kuznetsov AV, Schneeberger S, Seiler R, Brandacher G, Mark W, Steurer W, Saks V, Usson Y, Margreiter R, Gnaiger E (2004) Mitochondrial defects and heterogenous cytochrome c release after cardiac cold ischemia and reperfusion. Am J Physiol 286H:1633–1641
Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS (2008) Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc 3:965–976
Leary SC, Lyons CN, Rosenberger AG, Ballantyne JS, Stillman J, Moyes CD (2003) Fiber-type differences in muscle mitochondrial profiles. Am J Physiol 285R:817–826
Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, Bolouri MS, Ray HN, Sihag S, Kamal M (2003) Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115:629–640
Moyes CD, Mathieu-Costello OA, Brill RW, Hochachka PW (1992) Mitochondrial metabolism of cardiac and skeletal muscles from a fast (Katsuwonus pelamis) and a slow (Cyprinus carpio) fish. Can J Zool 70:1246–1253
Murphy M (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Oosterbaan RJ (1994) Frequency and regression analysis. In: Ritzema HP (ed) Drainage principles and applications. ILRI Publication 16, Wageningen, The Netherlands, pp 175–223
Pardee AB, Potter VR (1948) Inhibition of succinate dehydrogenase by oxaloacetate. J Biol Chem 176:1085–1094
Park K-S, Jo I, Pak Y, Bae S-W, Rhim H, Suh S-H, Park S, Zhu M, So I, Kim K (2002) FCCP depolarizes plasma membrane potential by activating proton and Na+ currents in bovine aortic endothelial cells. Pflügers Arch Eur J Physiol 443:344–352
Paulus W, Bronzwaer J (2004) Nitric oxide’s role in the heart: control of beating or breathing? Am J Physiol 287:H8–H13
Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol 132A:739–761
Pörtner HO (2006) Climate-dependent evolution of Antarctic ectotherms: an integrative analysis. Deep Sea Res Part II: Top Stud Oceanogr 53:1071–1104
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97
Pörtner HO, Hardewig I, Peck LS (1999) Mitochondrial function and critical temperature in the Antarctic bivalve, Laternula elliptica. Comp Biochem Physiol 124A:179–189
Pörtner HO, Mark FC, Bock C (2004) Oxygen limited thermal tolerance in fish? Answers obtained by nuclear magnetic resonance techniques. Respir Physiol Neurobiol 141:243–260
Pörtner HO, Lucassen M, Storch D (2005) Metabolic biochemistry: it’s role in thermal tolerance and the capacities of physiological and ecological function. The physiology of polar fishes. Elsevier, New York, pp 79–154
Saks V, Belikova Y, Kuznetsov A (1991) In vivo regulation of mitochondrial respiration in cardiomyocytes: specific restrictions for intracellular diffusion of ADP. Biochim Biophys Acta 1074:302–311
Saks V, Vasil’eva E, Belikova Y, Kuznetsov A, Lyapina S, Petrova L (1993) Retarded diffusion of ADP in cardiomyocytes: possible role of mitochondrial outer membrane and creatine kinase in cellular regulation of oxidative phosphorylation. Biochim Biophys Acta 1144:134–148
Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, Tranqui L, Olivares J, Winkler K, Wiedemann F, Kunz WS (1998) Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem 184:81–100
Samartsev VN, Smirnov AV, Zeldi IP, Markova OV, Mokhova EN, Skulachev VP (1997) Involvement of aspartate/glutamate antiporter in fatty-acid-induced uncoupling of liver mitochondria. Biochim Biophys Acta 1319:251–257
Sheeran FL, Pepe S (2006) Energy deficiency in the failing heart: linking increased reactive oxygen species and disruption of oxidative phosphorylation rate. Biochim Biophys Acta 1757:543–552
Somero GN (2002) Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integ Comp Physiol 42:780–789
Sommer A, Klein B, Pörtner HO (1997) Temperature induced anaerobiosis in two populations of the polychaete worm Arenicola marina (L). J Comp Physiol 167B:25–35
Stillman J, Somero GN (2000) A comparative analysis of the upper thermal tolerance limits of eastern pacific porcelain crabs, genus Petrolisthes: influences of latitude, vertical zonation, acclimation, and phylogeny. Physiol Biochem Zool 73:200–208
Stuart JA, Cadenas S, Jekabsons MB, Roussel D, Brand MD (2001) Mitochondrial proton leak and the uncoupling protein 1 homologues. Biochim Biophys Acta 1504:144–158
Trigari G, Pirini M, Ventrella V, Pagliarani A, Trombetti F, Borgatti AR (1992) Lipid composition and mitochondrial respiration in warm- and cold-adapted sea bass. Lipids 27:371–377
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Van den Thillart G, De Bruin G (1981) Influence of environmental temperature on mitochondrial membranes. Biochim Biophys Acta 640:439–447
Van den Thillart G, Modderkolk J (1978) The effect of acclimation temperature on the activation energies of state III respiration and on the unsaturation of membrane lipids of goldfish mitochondria. Biochim Biophys Acta 510:38–51
Vandenheede JR, Criddle RC, Ahmed AI, Feeney RE (1973) Heart mitochondrial oxidations of an Antarctic fish. Comp Biochem Physiol 46B:313–319
Veksler VI, Kuznetsov AV, Sharov VG, Kapelko VI, Saks VA (1987) Mitochondrial respirometry parameters in cardiac tissue: a novel method of assessment by using saponin-skinned fibers. Biochim Biophys Acta 892:191–196
Weinstein RB, Somero GN (1998) Effects of temperature on mitochondrial function in the Antarctic fish Trematomus bernacchii. J Comp Physiol 168B:190–196
Wellenreuther M, Barrett PT, Clements KD (2007) Ecological diversification in habitat use by subtidal triplefin fishes (Tripterygiidae). Mar Ecol Prog Ser 330:235–246
Wieckowski MR, Wojtczak L (1997) Involvement of the dicarboxylate carrier in the protonophoric action of long-chain fatty acids in mitochondria. Biochem Biophys Res Commun 232:414–417
Acknowledgments
We are very grateful to Ms K Ruggiero for advice with statistical analyses, Brady Doak and Murray Birch as skippers of the R.V. Hawere, Ryan Chai for assistance in the laboratory, and three anonymous reviewers for comments that greatly improved the manuscript. This work was supported by a Royal Society of New Zealand Marsden Fund grant (UOA218) to KDC. All experiments and procedures met with the ethical requirements of the University of Auckland, New Zealand (NZ) (Approval AEC/03/2006/R456) and with the current laws of NZ.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Rights and permissions
About this article
Cite this article
Hilton, Z., Clements, K.D. & Hickey, A.J.R. Temperature sensitivity of cardiac mitochondria in intertidal and subtidal triplefin fishes. J Comp Physiol B 180, 979–990 (2010). https://doi.org/10.1007/s00360-010-0477-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0477-7