Abstract
Adult marine mammal muscles rely upon a suite of adaptations for sustained aerobic metabolism in the absence of freely available oxygen (O2). Although the importance of these adaptations for supporting aerobic diving patterns of adults is well understood, little is known about postnatal muscle development in young marine mammals. However, the typical pattern of vertebrate muscle development, and reduced tissue O2 stores and diving ability of young marine mammals suggest that the physiological properties of harbor seal (Phoca vitulina) pup muscle will differ from those of adults. We examined myoglobin (Mb) concentration, and the activities of citrate synthase (CS), β-hydroxyacyl coA dehydrogenase (HOAD), and lactate dehydrogenase (LDH) in muscle biopsies from harbor seal pups throughout the nursing period, and compared these biochemical parameters to those of adults. Pups had reduced O2 carrying capacity ([Mb] 28–41% lower than adults) and reduced metabolically scaled catabolic enzyme activities (LDH/RMR 20–58% and CS/RMR 29–89% lower than adults), indicating that harbor seal pup muscles are biochemically immature at birth and weaning. This suggests that pup muscles do not have the ability to support either the aerobic or anaerobic performance of adult seals. This immaturity may contribute to the lower diving capacity and behavior in younger pups. In addition, the trends in myoglobin concentration and enzyme activity seen in this study appear to be developmental and/or exercise-driven responses that together work to produce the hypoxic endurance phenotype seen in adults, rather than allometric effects due to body size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In terrestrial mammals, exercise-related increases in metabolic rate are accompanied by increases in ventilation, heart rate and muscle perfusion, all of which promote continual delivery of both fuels and O2 to the working muscles (Bangsbo and Hellsten 1998; Krustrup et al. 2004; Brooks et al. 2005). In contrast, diving marine mammals rely upon a suite of adaptations that reduce O2 consumption rates while diving: these include bradycardia, vasoconstriction, decreases in body temperature, and low-cost swimming strategies (Davis et al. 1985; Kooyman 1988; Kooyman 1989; Castellini 1991; Butler and Jones 1997; Kooyman and Ponganis 1998). While these diving adaptations allow for sustained aerobic metabolism in the absence of freely available O2, at the level of the working muscle, they result in reduced blood flow and, therefore, limit O2 and fuel (glucose and/or fatty acids) delivery (Blix et al. 1983; Cherepanova et al. 1993; Davis and Kanatous 1999; Kanatous et al. 1999).
Despite this, and in contrast to initial expectations that marine mammal muscles would be adapted for anaerobic metabolism (Hochachka and Storey 1975; Elsner and Gooden 1983), multiple studies have demonstrated that diving is a highly efficient process that relies mainly on aerobic, lipid-based metabolism (Kooyman et al. 1980, 1983; Kooyman 1989; Ponganis et al. 1997a, b; Davis and Kanatous 1999). At the level of the muscle, adaptations that support muscle functioning when fuels and O2 are not being replenished by vascular supply include increased endogenous fuel and O2 stores, and increases in biochemical and histological properties that promote efficient use of said reserves (Kanatous et al. 1999; Dearolf et al. 2000; Watson et al. 2003, 2007; Polasek et al. 2006). For example, seal muscles have myoglobin concentrations ([Mb]) that are far above those in terrestrial endurance and high-altitude acclimated athletes (Reed et al. 1994; Polasek and Davis 2001; Noren et al. 2002; Polasek et al. 2006). Seals also have high densities of intramuscular lipid droplets, elevated concentrations of aerobic enzymes such as citrate synthase (CS) and β-hydroxyacyl coA dehydrogenase (HOAD), and high densities of interfibrillar mitochondria (Reed et al. 1994; Kanatous et al. 1999, 2002; Polasek et al. 2006; Watson et al. 2007). In combination with high proportions of slow oxidative (type 1) and fast oxidative-glycolytic fibers (type IIA and IID/X; Reed et al. 1994; Kanatous et al. 1999; Watson et al. 2003, 2007), these adaptations produce locomotory muscles that are specifically adapted for efficient, aerobically poised metabolism while diving (Saltin and Gollnick 1983; Kanatous et al. 1999). However, while adult seal muscles may lack purely glycolytic (type IIB) fibers (Watson et al. 2003), they do have relatively high anaerobic enzyme activity, suggesting that when necessary, diving activity can be sustained by anaerobic metabolic processes (Polasek et al. 2006).
Although the importance of these adaptations for supporting the aerobic diving patterns of adults is well understood, little is known about muscle development in young marine mammals. However, several lines of evidence suggest that the physiological properties of pup muscle will differ from those of adults. First, differences are likely because neonatal vertebrates typically have muscles with lower enzyme activities, smaller fiber diameters, and different myosin heavy chain isoforms than those of the adults (Condon et al. 1990; Dietz and Ricklefs 1997; Dearolf et al. 2000; Shea et al. 2007). As animals grow, there is very little fiber hyperplasia; rather, most muscle growth is primarily due to fiber hypertrophy (Goldspink 1970; Garry et al. 1996). In addition, fiber-type profiles and enzyme activities shift toward the adult phenotype as juveniles develop, with neonatal and slow fibers developing before faster isoforms (Condon et al. 1990; Powers et al. 1991; Schiaffino and Reggiani 1994; Garry et al. 1996; Olson 2001). However, the pattern and rate of development differ with the function of the muscle and the animals’ life history patterns, with the locomotory muscles of species in which the neonate is active early in life maturing more rapidly than in more altricial animals (Grand 1992; Choi et al. 1993; Dearolf et al. 2000; Shea et al. 2007).
The muscles of young marine mammals are also likely to have different metabolic characteristics than those of adults since pups do not possess all of the physiological adaptations to support underwater diving of the adults. For example, pups have significantly lower Mb and hemoglobin (Hb) stores (Burns and Castellini 1996; Burns et al. 2005; Noren et al. 2005; Richmond et al. 2006; Clark et al. 2007), giving them reduced O2 storage and facilitated diffusion capabilities (Davis and Kanatous 1999). In addition, young pups have also reduced cardiovascular control and higher diving and apneic heart rates than adults (Castellini 1994; Greaves et al. 2005), which, in combination, may increase muscle perfusion while diving. Enhanced perfusion would increase O2 and fuel delivery to the muscle, but is also likely associated with higher diving metabolic rates, which would limit the duration of the dive. In addition, pups have higher mass-specific metabolic rates than adults (Miller and Irving 1975; Miller et al. 1976; Worthy and Lavigne 1987; Rea and Costa 1992), which dictate higher flux rates through metabolic pathways to sustain resting levels of ATP production. Since aerobic enzyme activity levels are positively correlated with resting metabolic rates (RMRs) (Hochachka and Somero 2002), the muscles of young pups may be required to have higher enzyme activity levels than older animals, just to achieve equivalent functionality. In addition, the relatively high-fat and low-protein milk diet of nursing pups (Oftedal 2002) suggests that pup muscles will have high activities of the enzymes involved with aerobic lipid oxidation.
The objectives of this study were to examine the pattern of muscle development in harbor seal (Phoca vitulina) pups across the nursing period, and to compare the muscle biochemical parameters of pups to those of adults. Harbor seal pups are fairly unique among phocids in that they are often born in the water, and pups routinely begin diving within hours to days of birth (Lapierre et al. 2004; Greaves et al. 2005). While young pups do not dive to the depths and durations of the adults (Frost et al. 2001, 2006; Greaves et al. 2005), this early activity requires that their muscles be able to support underwater activity at a very early age despite immature [Mb], total body oxygen stores (TBO2), and cardiovascular control (Greaves et al. 2005; Clark et al. 2007). In addition, because harbor seal pups have a fairly short period of dependency prior to initiating independent foraging activity (~28 days; (Dubé et al. 2003) muscle development must either occur prenatally, or develop rapidly in the days to weeks following birth.
To determine the temporal pattern of muscle development, we focused on age-related changes in [Mb], and the activities of three enzymes: lactate dehydrogenase (LDH), CS, and HOAD. LDH supports anaerobic generation of ATP via glycolysis by regenerating NAD+ through the reduction of pyruvate to lactate (Hochachka and Somero 2002; Fluck 2006), and as such is often used as an indicator of the extent to which muscles can rely solely on glycolysis. CS is the first enzyme in the citric acid (TCA) cycle, catalyzes the conversion of oxaloacetate to citrate, and is often used as an indicator of aerobic metabolism (Emmett and Hochachka 1981; Hochachka and Somero 2002). Similarly, HOAD, an enzyme involved in the β-oxidation of lipids, is often used to indicate the relative contribution of fatty acids as fuels for aerobic respiration (Hochachka and Somero 2002). We hypothesize that as pups grow and their dives become longer and deeper (Jorgensen et al. 2001; Greaves et al. 2005; Frost et al. 2006), there will be increases in both aerobic and anaerobic enzyme activities in the muscles to support efficient underwater exercise.
Methods
Sample collection and preparation
Harbor seal pups and adult females were captured in the St. Lawrence River Estuary, Canada, throughout the 4-week nursing period from 2001 to 2003 (Clark et al. 2007). At initial capture, pup age in days (A d) was estimated based on mass and appearance (Dubé et al. 2003; Clark et al. 2007). For subsequent captures, pup age was incremented based on time since initial capture. Animals were categorized as neonates (0–4 days), early nursing (5–16 days), late nursing (17–27 days), and weaned (≥28 days). While some individuals were captured multiple times during the nursing period, only one sample from each animal was retained for analysis in this study to avoid pseudoreplication. At each handling, seals were weighed, sedated with an IV injection of Diazepam (0.3–0.8 mg kg−1, Sabex, Inc., Canada), and a muscle biopsy was collected from the main swimming muscle (longissmus dorsi) using a disposable sterile 6 mm biopsy punch or cannula. Samples were flash frozen in liquid nitrogen in the field, then transported to the University of Alaska Anchorage and stored at −80°C until analysis, 3–4 years later.
Biochemistry
Frozen muscle samples were weighed, sonicated at 0°C in 15× dilution homogenization buffer (50 mM imidazole, 1 mM EDTA, 2 mM MgCl2; (Polasek et al. 2006), and centrifuged at 10,000g for 5 min at 4°C. The supernatant was then partitioned and diluted as described below for total protein (TP), [Mb] and enzyme activity assays. TP content (mg protein g−1 wet tissue mass) of the supernatant was determined using Pierce Coomassie Blue “The Better Bradford” Total Protein Assay (Pierce Chemicals, Rockford, IL). For these assays, 10 μl of the initial supernatant was diluted to the concentration range of the assay in pH 7.0 imidazole buffer, 10 μl of the resulting 300× dilution was pipetted into three wells of a 96-well microplate, and 300 μl of the pre-diluted dye was added to each well. The plate was then incubated at room temperature for 10 min, and read at λ = 595 nm (Spectra Max 340PC, Sunnyvale, CA). TP was calculated from standard curves derived from bovine serum albumin (BSA) standards. Harbor seal tissue of known TP (as determined by “The Better Bradford” assay) was used as a tissue control.
Myoglobin concentration was determined for 24 pups (neonate, n = 1; early, n = 8; late, n = 10; weaned, n = 5). These values were combined with Mb values from an additional 100 harbor seals captured during the same period that were previously reported in Clark et al. (2007) prior to statistical analyses. The protocols of Reynafarje (1963) were modified as follows: 30 μl of the initial 15× supernatant was diluted further to a total volume of 120 μl in 0.04 M phosphate buffer, pH 6.6, a grain of sodium dithionite was added and the sample was vortexed until the sodium dithionite dissolved. Then, samples were transferred into individual wells of a 384-well microplate, the plate was placed into an airtight, CO-filled chamber for 20 min to reduce the myoglobin. This method of CO reduction was validated using harbor seal tissue of known myoglobin concentration reported in Burns et al. (2007). All assays were run in triplicate, and each run included tissue controls from an adult harbor seal of known [Mb] (Burns et al. 2007) and lyophilized horse Mb standards (Sigma–Aldrich, Allentown, PA).
LDH, CS, and HOAD activities were determined following the methods of Polasek et al. (2006) under substrate saturating conditions, with the following assay formulae—LDH (EC 1.1.1.27): 0.3 mM NADH, 1 mM pyruvate, 50 mM imidazole buffer, pH 7.0 at 37°C, ΔA 340, millimolar extinction coefficient ε 340 = 6.22; CS (EC 4.1.3.7): 0.25 mM DTNB, 0.4 mM acetyl coA, 0.5 mM oxaloacetate, 50 mM imidazole buffer, pH 7.5 at 37°C, ΔA 412, millimolar extinction coefficient ε 412 = 13.6; and HOAD (EC 1.1.1.35): 0.3 mM NADH, 1 mM EDTA, 0.2 mM acetoacetyl coA, 50 mM imidazole buffer, pH 7.0 at 37°C, ΔA 340, millimolar extinction coefficient ε 340 = 6.22. Absolute activities (IU g−1 wet tissue mass) were calculated from the change in absorbance at the maximal linear slope of the assay. Harbor seal muscle tissue of known enzyme activities (previously verified against rat tissue, Burns, unpublished data) was used as a tissue control. Each sample was assayed in four wells, and the assay was accepted if the tissue control was within its determined enzyme activity range, had a CV of less than 10%, and if the sample CV was also less than 10%. Precision of the assays was determined from all tissue control assays and is as follows: LDH CV = 8.3%; CS CV = 16.2%; HOAD CV = 6.0%.
CS/HOAD ratios were calculated to determine if aerobic muscle metabolism was fueled primarily by lipid oxidation, or by carbohydrates and/or glycogenic amino acids in proteins, with ratios of less than 1 indicating that muscle metabolism was primarily fueled by lipid oxidation (Winder et al. 1974; Reed et al. 1994; Kanatous et al. 1999; Polasek et al. 2006). In addition, LDH/CS ratios were used as an indicator of the anaerobic scope of the muscle, with higher values indicating that the muscle had a greater ability to maintain ATP production anaerobically once oxidative pathways were saturated or O2 availability was low (Reed et al. 1994).
Statistical analysis
Differences in [Mb], enzyme activities (both absolute and metabolically scaled), and enzyme ratios between all age classes were tested using one-way ANOVA or ANCOVAs with Bonferroni post hoc tests. Mass was only included as a covariate in the examination of age effects on [Mb] because the relationship between mass and enzyme activity differed by age class (Fig. 1), thus violating the assumption of homogeneity of regression (Zar 1984). Because there was no correlation between enzyme activity and muscle protein content (LDH: r = 0.059, p = 0.784; CS: r = −0.131, p = 0.541; HOAD: r = −0.032, p = 0.883), enzyme activities were not scaled to TP prior to analysis of age effects. To account for the effect of age-specific differences in mass-specific metabolic rates on enzyme activity, absolute enzyme activities were also scaled to mass-specific RMRs (ml O2 kg−1 min−1), as reported in the literature (Table 1). These values are termed metabolically scaled enzyme activities (e.g. LDHmet). It was not possible to scale enzyme activities to muscle-specific metabolic rates, since such data do not exist for harbor seal pups. Statistical significance for all tests was set at p < 0.05 and all statistical analyses were performed using SPSS v.14.0 (Chicago, IL). Values are reported as mean ± SE unless otherwise noted.
Results
Myoglobin concentrations were determined for 124 animals (Table 1). Myoglobin concentration did not differ among the neonate, early, and late nursing pups; however, weaned pups had significantly higher myoglobin concentration than all other pups (Table 1; F 4,123 = 17.067, p < 0.001). Adult myoglobin concentrations were significantly higher than all pup age categories (Table 1; F 4,123 = 17.067, p < 0.001).
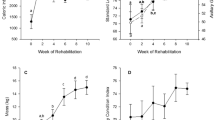
Enzyme activities were determined for 67 animals (Table 1). There were significant age-related differences in all enzyme activities on both an absolute and metabolically scaled basis. Absolute and metabolically scaled LDH (LDHmet) activity increased across the nursing period, with weaned pup and adult activity significantly higher than in the younger pups (absolute activity: F 4,62 = 6.28, p < 0.001, Table 1; LDHmet: F 4,62 = 46.104, p < 0.001, Fig. 2a). While weaned pups had similar LDH activity to adults when judged on absolute basis, LDHmet values were significantly lower than in adults. Similarly, there were increases in CS activity with pup age on both an absolute and metabolically scaled (CSmet) basis. However, in contrast to LDH, weaned pups had higher absolute CS activities as compared to adults, although levels were similar to adults when scaled to metabolic rates (absolute activity: F 4,62 = 4.963, p = 0.002, Table 1; CSmet: F 4,62 = 11.314, p < 0.001, Fig. 2b). In contrast, pups had significantly higher, and more variable, absolute HOAD activity than adults, but there was no difference among the different pup age classes (F 4,61 = 4.628, p = 0.002, Table 1). However, metabolically scaled HOAD (HOADmet) values increased with pup age with the highest levels of all age classes seen in weaned pups (F 4,61 = 5.523, p = 0.001, Fig. 2c). Adults had significantly higher CS/HOAD (F 4,60 = 14.2, p < 0.001, Fig. 3a) and LDH/CS (F 4,60 = 12.8, p < 0.001, Fig. 3b) ratios than all pup age classes, and there were no age-related trends among pup age classes.
Mean ± SE metabolically scaled (enzyme activity/ml O2 kg min−1) LDH, CS and HOAD. Different letters above bars indicate statistically significant differences between age categories within each enzyme. Sample size provided in Table 1
Mean (±SE) CS/HOAD and LDH/CS for each age category. Different letters above bars indicate statistically significant differences between age categories within an enzyme ratio. Sample sizes as in Fig. 2
Discussion
The results of this study indicate that harbor seal pup muscles are not biochemically mature at birth or at weaning, despite the precocial appearance and early swimming activity (Jorgensen et al. 2001; Greaves et al. 2005). This immaturity can be seen in both the low O2 carrying capacity (myoglobin concentration) and reduced catabolic enzyme (LDH and CS) activities. In combination, these findings suggest that pup muscle does not have the ability to support either aerobic or anaerobic performance to the same extent as adult seal muscle, and this limitation may contribute to the lower diving capacity and behavior in younger pups. In addition, the divergent effects of mass and age on enzyme activities suggest that the age-related differences in muscle biochemistry seen in this study are developmental and/or exercise-driven responses that together work to produce the hypoxic endurance phenotype seen in adults, rather than allometric effects due to body size.
As has previously been seen in penguins, cetaceans, and pinnipeds (Ponganis et al. 1997a, b; Dearolf et al. 2000; Burns et al. 2005; Noren et al. 2005; Richmond et al. 2006; Burns et al. 2007; Clark et al. 2007), neonatal harbor seals had much lower [Mb] in their muscles compared to older animals, and while there was a slight increase with age, pup [Mb] did not reach adult levels by the time pups were weaned. Although [Mb] are typically low in both terrestrial and marine mammal neonates (Whipple 1926; Longo et al. 1973; Tipler et al. 1978; Weller et al. 1986; Garry et al. 1996), in marine mammals, low [Mb] carries unique consequences because the diving response involves reduction of blood flow and substrate delivery to muscles (Kanatous et al. 1999). Thus, for young divers, lower [Mb] limits the amount of time that pups can remain submerged with aerobically working muscles, thereby reducing the ability of pups to sustain diving activities. Low endogenous Mb reserves in pup muscles may also increase reliance on anaerobic metabolism when foraging success requires dives longer than the aerobic dive limit (Burns and Castellini 1996; Burns 1999) or during periods of extreme vasoconstriction.
In muscle tissue, there is generally a close match between structural and biochemical properties, with fibers that rely more heavily on oxidative metabolism typically containing higher [Mb], and glycolytic fibers containing less Mb and having higher LDH activity (Saltin and Gollnick 1983; Garry et al. 1996; Hoppeler and Fluck 2002; Fluck 2006). This pattern appears to hold for adult muscles where the high [Mb] in the longissimus dorsi is associated with a large proportion of oxidative fibers (type 1 and IIA, Watson et al. 2003) as expected based on the need to support aerobic underwater activities. However, the LDH activity measured in adult harbor seal muscles in this study is intermediate between those of terrestrial endurance athletes such as the dog, and sprinters such as the cheetah (Williams et al. 1997; Polasek et al. 2006), suggesting that adults retain significant ability to produce ATP anaerobically, as would be necessary when dives exceed their aerobic capacity (Polasek et al. 2006). The LDH values measured in this study were within the range of those reported for other phocid seals (Castellini and Somero 1981; Hochachka and Foreman 1993; Reed et al. 1994; Polasek et al. 2006) suggesting that not only our methods and results are comparable to previous studies, but also the anaerobic metabolic potential of the main locomotory muscle in phocids is fairly uniform, reflecting similar use patterns.
In contrast to LDH activity, pup muscles have much lower [Mb] in their muscles than adults. One possible explanation is that their muscles contain more glycolytic fibers than adults, perhaps due to an increased proportion of anaerobic dives (Burns 1999). However, the low LDH activity in pup muscles does not support this idea, and indeed it runs counter to the prevailing pattern of muscle fiber development, where fast glycolytic fibers typically mature after the more oxidative forms (Condon et al. 1990; Powers et al. 1991; Schiaffino and Reggiani 1994; Garry et al. 1996; Olson 2001). Taken together, the lower absolute LDH and LDHmet activity indicates that pup muscles have a decreased ability both to convert lactic acid to pyruvate, and to maintain the redox balance necessary to sustain glycolytic pathways when O2 is limited. Similarly, the significantly lower LDH/CS ratio suggests that pup muscles have reduced ability to switch to anaerobic metabolism when aerobic stores are exhausted (Reed et al. 1994; Polasek et al. 2006). Thus, pup muscles do not appear to be as adapted for anaerobic underwater activities as adults.
Instead, the combination of low [Mb] and LDH activities in pup muscles may reflect both developmental constraints and use patterns. Postnatal development in many vertebrates is characterized by an increase in LDH activity (Blaise Smith 1980; Bishop et al. 1995; Agüera et al. 2001; Gondret et al. 2004), perhaps in association with increases in the proportion and/or size of type II fibers, and the increase in LDH activity with age (and mass) observed here suggests that harbor seal muscle development follows a broadly similar pattern. In addition, behavioral patterns suggest that LDH levels would likely not need to be high because the short and shallow dives made by pups are rarely long enough to exhaust circulating O2 stores (Burns et al. 2005; Clark et al. 2007). Similarly, since harbor seal pups are unable to sustain low heart rates while diving (Greaves et al. 2005), pup muscles may be better perfused during dives than adult seal muscles. Since Mb can only serve as an endogenous O2 reserve if tissue pO2 levels drop significantly due to vasoconstriction, under higher perfusion rates myoglobin would serve primarily to facilitate O2 delivery to mitochondria, and muscle pO2 levels would remain high as long as did vascular levels (Davis and Kanatous 1999). This would favor oxidative metabolism and likely reduce lactate production.
The low [Mb] in the pups are also helpful in interpreting the aerobic capacities of their muscles. For example, given the high absolute enzyme activities one might expect that pup muscles are capable of supporting sustained aerobic activities, and consist of largely oxidative fibers with high mitochondrial densities, as seen in adults (Kanatous et al. 1999; Watson et al. 2003, 2007). However, since pups have relatively poor cardiovascular control (Greaves et al. 2005), low blood and muscle O2 stores (Burns et al. 2005; Clark et al. 2007), and high mass-specific metabolic rates, it is unlikely that they can function long in the absence of freely available O2. Instead, the relatively high absolute enzyme activities likely result from a need to match enzyme activity to metabolic rate (Hochachka and Somero 2002), as elevated CS and HOAD levels have been documented in a variety of precocial mammals and birds, but are less common in altricial species (Blaise Smith 1980; Glatz and Veerkamp 1982; Agüera et al. 2001; Krijgsveld et al. 2001; Shea et al. 2007; Kanatous et al. 2008). Despite these elevated levels, in harbor seals, when the high absolute enzyme activities are scaled to the mass-specific RMR of each age class, it becomes clear that younger pups have less ability to support aerobic metabolism than older animals.
That this is an age- and not mass-driven effect is evident by the fact that pups have much higher aerobic enzyme levels than expected based on their body mass and that the correlation between enzyme activity and body mass is negative for weaned pups (see Fig. 1), where older animals are lighter than younger ones (Muelbert and Bowen 1993). Indeed, the increases in enzyme activities observed during the nursing period run counter to the pattern predicted based on mass alone. Among adult animals, larger body size is associated with lower aerobic enzyme activities (Emmett and Hochachka 1981), likely due to the decline in mass-specific metabolic rates. In this study, the CS and HOAD activities measured in adult muscles were similar to those previously reported for harbor seals (Reed et al. 1994; Polasek et al. 2006), but higher than those reported for the larger Weddell and gray seals (Kanatous et al. 2002; Reed et al. 1994). One exception was the enzyme activities of fasting/lactating females, which had higher HOAD levels than would be expected based on body size alone (Burns et al. 2010; Kanatous et al. 2008).
In pups, the age-related increases in enzyme activity are consistent with the developmental patterns seen in other vertebrates, where hypertrophy is frequently accompanied by increases in catabolic enzyme activity and thermoregulatory abilities (Dearolf et al. 2000; Olson 2001; Shea et al. 2007). However, increases in Mb and enzyme activity during the nursing period may also be due to increases in the depth, duration, and frequency of dives (Jorgensen et al. 2001; Baechler et al. 2002; Greaves et al. 2005), as endurance exercise training and intermittent tissue hypoxia both increase the oxidative potential of muscle tissue by increasing mitochondrial densities and aerobic enzyme activities (Kayar et al. 1986; Hoppeler and Fluck 2002; Fluck 2006). Therefore, increased diving activity, in combination with exercise-induced hypoxia (due to low muscle Mb), likely augments developmental mechanisms to increase the endurance capabilities of pup muscles.
Age-related differences in enzyme activities also likely reflect dietary changes. In adult animals, diets high in fat are associated with increases in aerobic capacities of muscle due to elevated aerobic enzyme activities, and mitochondrial and lipid droplet densities (Miller et al. 1984; Reynolds et al. 2005; Garcia-Roves et al. 2007). For adult harbor seals, the high reliance on lipid-based aerobic respiration to fuel efficient underwater diving activities is reflected in CS/HOAD ratios much lower than in most terrestrial animals (Hochachka et al. 1983; Reed et al. 1994; Polasek et al. 2006) Yet, even these values are higher than observed in pups. However, during the nursing period, harbor seal pups subsist on milk that is much higher in fat (40–50%), and lower in both protein (9%) and carbohydrates (Oftedal 2002; Lang et al. 2005) than the adult diet of fish and invertebrates (Bowen and Harrison 1996). The increased reliance on milk lipid as a fuel source is reflected in the much lower CS/HOAD ratio for nursing pups as compared to adults, while the low CS/HOAD ratio in weaned pups likely reflects their heavy reliance on the mobilization of blubber lipids to support metabolism as they learn to forage successfully (Muelbert and Bowen 1993; Lesage et al. 1999; Burns et al. 2005). Thus, provided that the vascular supply of O2 is sufficient to compensate for their lower [Mb] content, pup muscles appear to be as metabolically primed for lipid-based aerobic respiration as adults.
The rate and extent of muscle development may depend on muscle function (Choi et al. 1993; Bishop et al. 1995; Agbulut et al. 2003), in which case the patterns observed here for the major locomotory muscle in harbor seals may not apply to all species and muscle. However, several lines of evidence suggest that the overall pattern is broadly applicable across marine mammal species. Age-related increases in enzyme activities in both locomotory and non-locomotory skeletal muscles, and in cardiac muscles, have been documented in harbor seals (this study), harp and hooded seals (Burns et al. 2010; Lestyk, unpublished data), Weddell seals (Kanatous et al. 2008), and Steller sea lions (Richmond, unpublished data), just as have been seen in many terrestrial mammals and birds (Gondret et al. 2004; Griffiths et al. 1994; Olson 2001; Shea et al. 2007). However, in both terrestrial and marine species, there are differences in the rate and magnitude of the developmental increases based on muscle use (Grand 1992), so behavior and morphology should be considered when making comparisons across species. In addition, when comparing enzyme activities across studies, it may also be necessary to consider the impact of season, diet, and animal condition, as all these factors are known to influence muscle metabolic activity in terrestrial species (Hoppeler and Fluck 2002; Kelsen et al. 1985).
In summary, in the context of a young, swimming and diving seal, neonatal harbor seals have low O2 stores and decreased capacities to produce ATP both aerobically and anaerobically as compared to the adults, particularly when examined relative to metabolic rates. These immature parameters may not have much functional significance early in the nursing period when diving activity is limited, but become more important once pups are weaned and must forage on their own. By weaning, pup muscles have developed slightly, but are likely still constrained in their ability to sustain underwater aerobic metabolism by lower Mb content and LDH activity. In combination with lower TBO2 stores, higher mass-specific RMRs, and poor cardiovascular control (Burns et al. 2005; Greaves et al. 2005; Clark et al. 2007), this study suggests that the diving behavior of weaned pups may be further limited by their underdeveloped muscle metabolic capacities.
References
Agbulut O, Noirez P, Beaumont F, Butler-Browne G (2003) Myosin heavy chain isoforms in postnatal development of mice. Biol Cell 95:399–406
Agüera E, Muñoz A, Castejón F, Essén-Gustavsson B (2001) Skeletal muscle fibre characteristics in young and old bulls and metabolic response after a bullfight. J Vet Med 48:313–319. doi:10.1046/j.1439-0442.2001.00362.x
Baechler J, Beck CA, Bowen WD (2002) Dive shapes reveal temporal changes in the foraging behaviour of different age and sex classes of harbour seals (Phoca vitulina). Can J Zool 80:1569–1577. doi:10.1139/z02-150
Bangsbo J, Hellsten Y (1998) Muscle blood flow and oxygen uptake in recovery from exercise. Acta Physiol Scand 162:305–312. doi:10.1046/j.1365-201X.1998.0331e.x
Bishop C, Butler P, Egginton S, El Haj A, Gabrielsen G (1995) Development of metabolic enzyme activity in locomotor and cardiac muscles of the migratory barnacle goose. Am J Physiol 269:R64–R72
Blaise Smith P (1980) Postnatal development of glycogen- and cyclic AMP-metabolizing enzymes in mammalian skeletal muscle. Biochim Biophys Acta 628:19–25
Blix AS, Elsner RW, Kjekshus JK (1983) Cardiac output and its distribution through capillaries and A-V shunts in diving seals. Acta Physiol Scand 118:109–116. doi:10.1111/j.1748-1716.1983.tb07250.x
Bowen WD, Harrison GD (1996) Offshore diet of grey seals Halichoerus grypus near Sable Island, Canada. Mar Ecol Prog Ser 112:1–11
Brooks GA, Fahey TD, Baldwin KM (2005) Exercise physiology. McGraw-Hill, New York
Burns JM (1999) The development of diving behavior in juvenile Weddell seals: pushing physiological limits in order to survive. Can J Zool 77:737–747. doi:10.1139/cjz-77-5-737
Burns JM, Castellini MA (1996) Physiological and behavioral determinants of the aerobic dive limit in Weddell seal (Leptonychotes weddellii) pups. J Comp Phys B 166:473–483. doi:10.1007/BF02338290
Burns JM, Costa DP, Frost K, Harvey JT (2005) Development of body oxygen stores in harbor seals: effects of age, mass, and body composition. Physiol Biochem Zool 78:1057–1068. doi:10.1086/432922
Burns JM, Lestyk KC, Folkow LP, Hammill MO, Blix AS (2007) Size and distribution of oxygen stores in harp and hooded seals from birth to maturity. J Comp Phys B 177:687–700. doi:10.1007/s00360-007-0167-2
Burns JM, Skomp N, Bishop N, Lestyk K, Hammill MO (2010) Development of aerobic and anaerobic metabolism in cardiac and skeletal muscles from harp and hooded seals. J Exp Biol (in press)
Butler PJ, Jones DR (1997) Physiology of diving of birds and mammals. Physiol Rev 77:837–899
Castellini MA (1991) The biology of diving mammals: behavioral, physiological and biochemical limits. In: Gilles R (ed) Advances in comparative and environmental physiology, 8th edn. Springer, Berlin, pp 105–134
Castellini MA (1994) Apnea tolerance in the elephant seal during sleeping and diving: physiological mechanisms and correlations. In: LeBoeuf BJ, Laws RM (eds) Elephant seals: population ecology behavior and physiology. University of California Press, Berkeley, pp 343–353
Castellini MA, Somero GN (1981) Buffering capacity of vertebrate muscle: correlations with potential for anaerobic function. J Comp Phys B 143:191–198
Cherepanova V, Neshumova T, Elsner RW (1993) Muscle blood flow in diving mammals. Comp Biochem Physiol A 106:1–6
Choi IH, Ricklefs RE, Shea RE (1993) Skeletal muscle growth, enzyme activities, and the development of thermogenesis: a comparison between altricial and precocial birds. Physiol Zool 66:455–473
Clark CA, Burns JM, Schreer JF, Hammill MO (2007) A longitudinal and cross-sectional analysis of total body oxygen store development in nursing harbor seals (Phoca vitulina). J Comp Phys B 177:217–227. doi:10.1007/s00360-006-0123-6
Condon K, Silberstein L, Blau HM, Thompson WJ (1990) Development of muscle fiber types in the prenatal rat hindlimb. Dev Biol 138:256–274
Davis RW, Kanatous SB (1999) Convective oxygen transport and tissue oxygen consumption in Weddell seals during aerobic dives. J Exp Biol 202:1091–1113
Davis RW, Williams TM, Kooyman GL (1985) Swimming metabolism of yearling and adult harbor seals, Phoca vitulina. Physiol Zool 58:590–596
Dearolf JL, McClelland BR, Dillaman RM, Frierson D, Pabst DA (2000) Precocial development of axial locomotor muscle in bottlenose dolphin (Tursiops truncatus). J Morphol 244:203–215. doi:10.1002/(SICI)1097-4687(200006)244:3<203:AID-JMOR5>3.0.CO;2-V
Dietz MW, Ricklefs RE (1997) Growth rate and maturation of skeletal muscles over a size range of Galliform birds. Physiol Zool 70:502–510
Dubé Y, Hammill MO, Barrette C (2003) Pup development and timing of pupping in harbour seals (Phoca vitulina) in the St Lawrence River Estuary, Canada. Can J Zool 81:188–194. doi:10.1139/z02-231
Elsner RW, Gooden B (1983) Diving and asphyxia: a comparative study of animals and man. Monogr Physiol Soc 40:1–168
Emmett B, Hochachka PW (1981) Scaling of oxidative and glycolytic enzymes in mammals. Respir Physiol 45:261–272
Fluck M (2006) Functional, structural and molecular plasticity skeletal muscle in response to exercise stimuli. J Exp Biol 209:2239–2248. doi:10.1242/jeb.02149
Frost KJ, Simpkins MA, Lowry LF (2001) Diving behavior of subadult and adult harbor seals in Prince William Sound, Alaska. Mar Mamm Sci 17:813–834. doi:10.1111/j.1748-7692.2001.tb01300.x
Frost KJ, Simpkins M, Small RJ, Lowry LF (2006) Development of diving by harbor seal pups in two regions of Alaska: use of the water column. Mar Mamm Sci 22:617–643. doi:10.1111/j.1748-7692.2006.00056.x
Garcia-Roves P, Huss JM, Han DH, Hancock CR, Iglesias-Gutierrez E, Chen M, Holloszy JO (2007) Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci 104:10709–10713. doi:10.1073/pnas.0704024104
Garry DJ, Bassel-Dubay R, Richarson JG, Neufer PD, Williams RS (1996) Postnatal development and plasticity of specialized fiber characteristics in the hindlimb. Dev Genet 19:146–156. doi:10.1002/(SICI)1520-6408(1996)19:2<146:AID-DVG6>3.0.CO;2-9
Glatz J, Veerkamp J (1982) Postnatal development of palmitate oxidation and mitochondrial enzyme activities in rat cardiac and skeletal muscle. Biochim Biophys Acta 711:327–335
Goldspink G (1970) The proliferation of myofibrils during muscle fiber growth. J Cell Sci 6:593–603
Gondret F, Damon M, Jadhau S, Houdebine L, Herpin P, Hocquette J (2004) Age-related changes in glucose utilization and fatty acid oxidation in a muscle-specific manner during rabbit growth. J Muscle Res Cell Motil 25:405–410. doi:10.1007/s10974-004-2768-7
Grand TI (1992) Altricial and precocial mammals: a model of neural and muscular development. Zoo Biol 11:3–15. doi:10.1002/zoo.1430110103
Greaves DK, Schreer JF, Hammill MO, Burns JM (2005) Diving heart rate development in postnatal harbour seals, Phoca vitulina. Physiol Biochem Zool 78:9–17. doi:10.1086/425201
Griffiths RI, Baldwin J, Berger PJ (1994) Metabolic development of the sheep diaphragm during fetal and newborn life. Respir Phys 95:334–347
Hochachka PW, Foreman RA (1993) Phocid and cetacean blueprints of muscle metabolism. Can J Zool 71:2089–2098. doi:10.1139/z93-294
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, New York
Hochachka PW, Storey KB (1975) Metabolic consequences of diving in animals and man. Science 187:613–621. doi:10.1126/science.163485
Hochachka P, Stanley C, Merkt J, Sumar-Kalinowski J (1983) Metabolic meaning of elevated levels of oxidative enzymes in high-altitude adapted animals: an interpretive hypothesis. Respir Physiol 52:303–313
Hoppeler H, Fluck M (2002) Normal mammalian skeletal muscle and its phenotypic plasticity. J Exp Biol 205:2143–2152
Jorgensen C, Lydersen C, Kovacs KM (2001) Diving development in nursing harbour seal pups. J Exp Biol 204:3993–4004
Kanatous SB, DiMichele LV, Cowan DF, Davis RW (1999) High aerobic capacities in skeletal muscles of pinnipeds: adaptations to diving hypoxia. J Appl Physiol 86:1247–1256
Kanatous SB, Davis RW, Watson R, Polasek L, Williams TM, Mathieu-Costello O (2002) Aerobic capacities in the skeletal muscles of Weddell seals: key to longer dive durations? J Exp Biol 205:3601–3608
Kanatous SB, Hawke T, Trumble S, Pearson L, Watson R, Garry D, Williams T, Davis R (2008) The ontogeny of aerobic and diving capacity in the skeletal muscles of Weddell seals. J Exp Biol 211:2559–2565
Kayar SR, Hoppeler H, Howald H, Claassen H, Oberholzer F (1986) Acute effects of endurance exercise on mitochondrial distribution and skeletal muscle morphology. Eur J Appl Physiol 54:578–584. doi:10.1007/BF00943344
Kelsen SG, Ference M, Kapoor S (1985) Effects of prolonged undernutrition on the structure and function of the diaphragm. J Appl Physiol 58:1354–1359
Kooyman GL (1988) Diving physiology. Marine mammals. In: Wood SF (ed) Comparative pulmonary physiology: current concepts. Marcel-Dekker Inc, New York, pp 721–734
Kooyman GL (1989) Diverse divers: physiology and behavior. Springer, Berlin
Kooyman GL, Ponganis PJ (1998) The physiological basis of diving to depth: birds and mammals. Ann Rev Physiol 60:19–32. doi:10.1146/annurev.physiol.60.1.19
Kooyman GL, Wahrenbrock EA, Castellini MA, Davis RW, Sinnett EE (1980) Aerobic and anaerobic metabolism during voluntary diving in Weddell seals: evidence of preferred pathways from blood chemistry and behavior. J Comp Phys B 138:335–346. doi:10.1007/BF00691568
Kooyman GL, Castellini MA, Davis RW, Maue RA (1983) Aerobic diving limits of immature Weddell seals. J Comp Phys B 151:171–174. doi:10.1007/BF00689915
Krijgsveld K, Olson J, Ricklefs R (2001) Catabolic capacity of the muscles of shorebird chicks: maturation of function in relation to body size. Physiol Biochem Zool 74:250–260. doi:10.1086/319655
Krustrup P, Söderlund K, Mohr M, Bangsbo J (2004) The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflüg Arch Eur J Phys 447:855–866. doi:10.1007/s00424-003-1203-z
Lang SLC, Iverson SJ, Bowen WD (2005) Individual variation in milk composition over lactation in harbour seals (Phoca vitulina) and the potential consequences of intermittent attendance. Can J Zool 83:1525–1531. doi:10.1139/z05-149
Lapierre JL, Schreer JF, Burns JM, Hammill MO (2004) Developmental changes in cardiorespiratory patterns associated with terrestrial apnoeas in harbour seal pups. J Exp Biol 207:3891–3896. doi:10.1242/jeb.01222
Lesage V, Hammill MO, Kovacs KM (1999) Functional classification of harbor seal (Phoca vitulina) dives using depth profiles, swim velocity, and an index of foraging success. Can J Zool 77:74–87. doi:10.1139/cjz-77-1-74
Longo LD, Koos BJ, Power GG (1973) Fetal myoglobin: quantitative determination and importance for oxygenation. Am J Physiol 224:1032–1036
Miller K, Irving L (1975) Metabolism and temperature regulation in young harbor seals, Phoca vitulina richardsi. Am J Physiol 229:506–511
Miller K, Rosenmann M, Morrison PR (1976) Oxygen uptake and temperature regulation of young harbor seals (Phoca vitulina richardsi) in water. Comp Biochem Physiol 54A:105–107
Miller WC, Bryce GR, Conlee RK (1984) Adaptations to a high fat diet that increase exercise endurance in male rats. J Appl Physiol 56:78–83
Muelbert MMC, Bowen WD (1993) Duration of lactation and postweaning changes in mass and body composition of harbour seal, Phoca vitulina, pups. Can J Zool 71:1405–1414. doi:10.1139/z93-194
Noren SR, Lacave G, Wells RS, Williams TM (2002) The development of blood oxygen stores in bottlenose dolphins (Tursiops truncatus): implications for diving capacity. J Zool 258:105–113. doi:10.1017/S0952836902001243
Noren SR, Iverson SJ, Boness DJ (2005) Development of the blood and muscle oxygen stores in gray seals (Halichoerus grypus): implications for juvenile diving capacity and the necessity of a terrestrial postweaning fast. Physiol Biochem Zool 78(4):482–490. doi:10.1086/430228
Oftedal OT (2002) Use of maternal reserves as a lactation strategy in large mammals. Proc Nutr Soc 59:99–106. doi:10.1017/S0029665100000124
Olson JM (2001) Ontogeny of catabolic and morphological properties of skeletal muscle of the red-winged blackbird (Agelaius phoeniceus). J Comp Phys B 171:527–542. doi:10.1007/s003600100202
Polasek LK, Davis RW (2001) Heterogeneity of myoglobin distribution in the locomotory muscles of five cetacean species. J Exp Biol 204:209–215
Polasek LK, Dickson KA, Davis RW (2006) Metabolic indicators in the skeletal muscles of harbor seals (Phoca vitulina). Am J Phys: Regul Int Comp Phys 290:R1720–R1727. doi:10.1152/ajpregu.00080.2005
Ponganis PJ, Costello ML, Starke LN, Mathieu-Costello O, Kooyman GL (1997a) Structural and biochemical characteristics of locomotory muscles of emperor penguins, Aptenodytes forsteri. Respir Physiol 109:73–80. doi:10.1016/S0034-5687(97)84031-5
Ponganis PJ, Kooyman GL, Winter LM, Starke LN (1997b) Heart rate and plasma lactate responses during submerged swimming and trained diving in California sea lions, Zalophus californianus. J Comp Phys B 167:9–16
Powers LV, Kandarian SC, Kunz TH (1991) Ontogeny of flight in the little brown bat, Myotis lucifugus: behavior, morphology, and muscle histochemistry. J Comp Physiol A 168:675–685. doi:10.1007/BF00224357
Rea LD, Costa DP (1992) Changes in standard metabolism during long-term fasting in northern elephant seal pups (Mirounga angustirostris). Physiol Zool 65:97–111
Reed JZ, Butler PJ, Fedak MA (1994) The metabolic characteristics of the locomotory muscles of grey seals (Halichoerus grypus), harbour seals (Phoca vitulina), and Antarctic fur seals (Arctocephalus gazelle). J Exp Biol 194:33–46
Reynafarje B (1963) Simplified method for the determination of myoglobin. J Lab Clin Med 61:138–145
Reynolds AJ, Taylor CR, Hoppeler H, Weibel ER, Weyand P, Roberts TJ, Reinhart G (2005) The effect of diet on sled dog performance, oxidative capacity, skeletal muscle microstructure, and muscle glycogen metabolism. In: Carey DP, Norton SA, Bolser SM (eds) Recent advances in canine and feline nutritional research. Orange Frazer Press, Wilmington, OH, pp 181–198
Richmond JP, Burns JM, Rea LD (2006) Ontogeny of total body oxygen stores and aerobic dive potential in Steller sea lions (Eumetopias jubatus). J Comp Phys B 176:535–545
Saltin B, Gollnick P (1983) Skeletal muscle adaptability: significance for metabolism and performance. In: Peachey AR, Adrian RH (eds) Handbook of physiology, Section 10: skeletal muscle. American Physiological Society, Baltimore, pp 555–631
Schiaffino S, Reggiani C (1994) Myosin isoforms in mammalian skeletal muscle. J Appl Physiol 77:493–501
Shea RE, Olson JM, Ricklefs RE (2007) Growth rate, protein accumulation, and catabolic enzyme activity of skeletal muscles of Galliform birds. Physiol Biochem Zool 80:306–316. doi:10.1086/512984
Tipler TD, Edwards YH, Hopkinson DA (1978) Developmental changes in protein profiles of human cardiac and skeletal muscle. Ann Hum Genet 41:409–418. doi:10.1111/j.1469-1809.1978.tb00911.x
Watson R, Miller TA, Davis RW (2003) Immunohistochemical fiber typing of harbor seal skeletal muscle. J Exp Biol 206:4105–4111
Watson R, Kanatous SB, Cowan DF, Wen JW, Han VC, Davis RW (2007) Volume density and distribution of mitochondria in harbor seal (Phoca vitulina) skeletal muscle. J Comp Phys B 177:89–98. doi:10.1007/s00360-006-0111-x
Weller PA, Price M, Isenberg H, Edwards YH, Jeffreys AJ (1986) Myoglobin expression: early induction and subsequent modulation of myoglobin and myoglobin mRNA during myogenesis. Mol Cell Biochem 6:4539–4547
Whipple GH (1926) The hemoglobin of striated muscle. Am J Physiol 76:693–707
Williams TM, Dobson GP, Mathieu-Costello O, Morsbach D, Worley MB, Phillips JA (1997) Skeletal muscle histology and biochemistry of an elite sprinter, the African cheetah. J Comp Phys B 167:527–535. doi:10.1007/s003600050105
Winder W, Baldwin K, Holloszy J (1974) Enzymes involved in ketone utilization in different types of muscle: adaptation to exercise. Eur J Biochem 47:461–467. doi:10.1111/j.1432-1033.1974.tb03713.x
Worthy GAJ, Lavigne DM (1987) Mass loss, metabolic rate, and energy utilization by harp and gray seal pups during the postweaning fast. Physiol Zool 60:352–364
Zar JH (1984) Biostatistical analysis. Prentice-Hall, Englewood Cliffs, CA
Acknowledgments
Many thanks to the 2000–2002 field crews for their hardwork and enthusiasm, most notably: P. Carter, D. Dion, Y. Dubé, J. Gosselin, D. Greaves, J. Greig, J. Lapierre, S. Turgeon, and G. Yunker. Thanks to L. Polasek for laboratory assistance. Funding for this work was provided by the Department of Fisheries and Oceans, Canada, the Natural Sciences and Engineering Research Council of Canada through an Operating Grant to JFS, and contributions from Alaska EPSCoR (NSF EPS-0346770) to JMB and JSP. Research protocols were approved by the Department of Fisheries and Oceans Canada, and the University of Alaska Anchorage Institutional Animal Care and Use Committee. Samples were imported under MMPA Permit # 1003-1646-00.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Prewitt, J.S., Freistroffer, D.V., Schreer, J.F. et al. Postnatal development of muscle biochemistry in nursing harbor seal (Phoca vitulina) pups: limitations to diving behavior?. J Comp Physiol B 180, 757–766 (2010). https://doi.org/10.1007/s00360-010-0448-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0448-z