Abstract
Fatty acid (FA) signature analysis has been used to study foraging ecology and food webs in marine ecosystems. This powerful method provides information about diets over an extended time period (e.g., 2–4 weeks), rather than just the most recent meal as with most traditional approaches. Using consumer FA signatures, along with a comprehensive database of diet FA signatures, and accounting for consumer FA metabolism, it is possible to estimate the proportions of diet items in the consumer’s diet using quantitative FA signature analysis (QFASA). However, before applying QFASA to free-ranging populations, ideally, controlled feeding studies are performed to determine FA deposition and turnover characteristics. We conducted feeding experiments to validate QFASA in captive spectacled eiders (Somateria fischeri) and Steller’s eiders (Polysticta stelleri) as a minimally invasive method for studying the diets of these threatened species. We determined FA deposition in eider adipose tissue relative to long-term diet, and developed calibration coefficients (CCs) to account for eider lipid metabolism. Using these CCs with subsequent diet trials, QFASA accurately indicated diet and diet switches. QFASA estimates also indicated that turnover of dietary FAs was not complete by 21 or 29 days, and confirmed that diets could be estimated over an extended period of >29 days. Thus, our understanding of diet can be backtracked to more than a month in captive feeding eiders. We conclude that applying QFASA techniques to eiders and other birds in the wild has the potential to provide valuable information about their diets at various life history stages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spectacled eider (Somateria fischeri) and Steller’s eider (Polysticta stelleri) were listed as threatened under the provisions of the U.S. Endangered Species Act in the 1990s (Federal Register 1993, 1997). Changes in the marine environment and available food resources may be limiting the recovery of both species (U.S. Fish and Wildlife Service 1996, 2002). Eiders winter and stage in marine habitats, and their breeding outcome likely depends on availability of adequate marine resources, but information about timing and sources of critical nutrient acquisition to reproduction is lacking for the threatened eider species in the North Pacific (Petersen et al. 2000; Fredrickson 2001). Determining the diet preferences and foraging habitat associations of these eiders in relation to seasonal and life history stages will provide information to help identify and characterize critical habitats of these threatened breeding populations. Given limitations of traditional methods of diet analysis such as stomach contents analyses, our overall goal was to validate quantitative fatty acid (FA) signature analysis (QFASA) to estimate the diets of spectacled and Steller’s eiders.
Fatty acid signatures have been used to study foraging ecology and food webs in marine ecosystems in three ways. First, FA signatures of consumer fat stores alone can be used to qualitatively infer spatial and temporal patterns in diets of free ranging animals. This qualitative analysis of FAs in lipids from adipose tissue, stomach oil, and blood plasma has been used in several marine bird species (Raclot et al. 1998; Dahl et al. 2003; Connan et al. 2005, 2007a, b; Käkelä et al. 2005, 2006, 2007, 2009; Iverson et al. 2007; Wang et al. 2007, 2009; Williams et al. 2008). Second, the existence of unique FAs found in a consumer can occasionally be traced to specific prey species and thus used for identification of forage items (Budge et al. 2006, 2007). Finally, using consumer FA signatures, along with a comprehensive database of prey FA signatures, and accounting for consumer FA metabolism, it is possible to estimate the proportions of different prey in the diet using QFASA (Iverson et al. 2004). QFASA estimates the diet by calculating the weighted mixture of FA signatures of diet items that most closely resembles that of the consumer’s adipose tissue after accounting for metabolism effects, and then uses the relative fat content of each diet item to translate the signature mix into a diet estimate. This approach has been validated in several marine mammals (e.g., Iverson et al. 2004, 2006; Nordstrom et al. 2008) and most recently, seabirds in the Bering Sea (Iverson et al. 2007). Additionally, Käkelä et al. (2009) showed that several FAs from plasma of herring gulls (Larus argentatus) can quantitatively reflect dietary changes. Because FAs provide information over an extended period of time (e.g., 2–4 weeks) versus the most recent meal as with most traditional methods of diet sampling (Barrett et al. 2007), it may be possible to get information about diets during non-breeding seasons (e.g., describe the diet during migration by sampling birds at arrival to breeding sites). However, in order to do this, controlled feeding studies must be performed to determine the period of time over which the diet can be back calculated. This non-lethal technique of FA signature analysis may be particularly useful in estimating the diets of species of conservation concern.
Interpretation of FA data to estimate diet is complicated by the effect of rates of biosynthesis, deposition, and metabolism of specific FAs within a consumer. As a result of consumer lipid metabolism, FA signatures of diet items will not exactly match that found in the consumer adipose tissue (i.e., the proportion of some FAs observed in the consumer may always be higher, or always lower, than that found in the diet; Iverson et al. 2004). In order to account for this metabolism, calibration coefficients (CCs) are calculated for individual FAs by feeding consumers a known diet until complete turnover of FAs is thought to have occurred, at which point consumer adipose tissue FA composition should be as similar to that of diet as it ever will be. These CCs (the ratio of a given FA in the consumer relative to that in the long-term diet) are then used in the QFASA model to weight individual FAs in subsequent diet estimation studies (Iverson et al. 2004, 2006, 2007). Related to the concept of CCs, is selecting appropriate subset(s) of FAs for use in diet estimations, as not all FAs provide equal information about diet due to consumer metabolism, FA origin (predominantly dietary versus biosynthesis), and levels found in tissue (for instance, trace levels may not be correctly identified) (Iverson et al. 2004). Finally, rates of FA turnover in adipose tissue may also be affected by consumer metabolism (e.g., Williams et al. 2009). Thus, in order to interpret the time frame of the estimated diet, turnover rates of dietary FAs in consumer adipose tissue should be estimated in a controlled experimental study. In this study, we developed CCs for individual FAs to account for eider lipid metabolism. We used simulation studies using the captive eider diet to evaluate the reliability with which diet items could be distinguished in the QFASA model. Finally, we tested the QFASA model, FA subsets, and CCs by estimating the diets of experimentally fed captive eiders.
Materials and methods
Captive feeding trials
The feeding study took place between 19 September 2007 and 16 January 2008 with eight adult male spectacled and eight adult male Steller’s eiders housed at the Alaska SeaLife Center (ASLC) in Seward, Alaska, USA. Six of eight spectacled eiders were hatched in June 2002 in captivity and brought to the ASLC in January 2003. The other two spectacled eiders arrived from the North Slope, Alaska in 2003 and 2004 as adults. All eight Steller’s eiders were captured in the wild and brought to the ASLC as adults, six from Unalaska, Alaska between February and March 2003, and two in September 2003 from Alaska Peninsula, Alaska. Since their arrival and before the start of this feeding study, all birds were on a diet of approximately 95% Mazuri sea duck formula (Purina Mills, St Louis, MO, USA) and the remaining 5% from supplements of Antarctic krill (Euphausia superba), Atlantic silverside (Menidia menidia), Atlantic surf clam (Spisula solidissima), blue mussel (Mytilus edulis), and California market squid (Loligo opalescens). Birds were segregated by species and housed in outdoor pens at ambient conditions and natural saltwater habitats with females of the same species.
Spectacled and Steller’s eiders in this study consumed slightly different proportions of diet items offered for each experimental diet. Therefore, the experimental diet compositions “specified” here reflected the actual amounts the birds consumed. Sixty-nine days before the start of the feeding trials, the diet composition of spectacled eiders consisted of approximately 88% Mazuri, 3% krill, 4% silverside, 1% clam, and 4% mussel (Diet 1); for Steller’s eiders the diet consisted of approximately 88% Mazuri, 1% krill, 3% silverside, 1% clam, and 7% mussel (Diet 1). Mazuri was given every day while supplements of clam, krill, mussel, and silverside were fed on a weekly rotating schedule: Monday = 125 g clam, Tuesday = 200 g krill, Wednesday = 300 g mussel, Thursday = 300 g silverside, Friday = 125 g clam, Saturday no supplements, Sunday = 200 g krill etc. Feeding trials started on Day 0, and all eiders were biopsied (see below), and spectacled eiders were switched to Diet 2 which consisted of 56% krill and 44% Mazuri, while Steller’s eiders were switched to Diet 2 which consisted of 66% krill and 34% Mazuri for 21 days. After the Day 21 biopsy, spectacled eiders were switched to Diet 3 which consisted of 52% silverside and 48% Mazuri, and Steller’s eiders were switched to Diet 3 which consisted of 66% silverside and 34% Mazuri for 29 days. Final biopsies were taken on Day 50. A substantial amount of Mazuri was necessary in the experimental diets to maintain the birds on the minimum amount of required nutrients. Birds were fed in groups by species and, therefore, individual intakes were not determined. As a result, it is likely that individuals consumed different proportions of certain diet items to some degree. Krill and silverside were fed in the morning to ensure that these diet items would be consumed. Krill and silverside were sometimes hand fed to the birds for training purposes and the remaining allotment placed in feeding bowls accessible to all birds in the pen. Mazuri was placed in accessible feeding bowls in the afternoon. Daily amounts of each diet item fed and consumed per flock were recorded. Subsamples of all diet items were collected twice each week throughout the feeding study, placed in airtight plastic bags, and stored frozen until analysis.
Biopsy sampling
A live biopsy technique according to Iverson et al. (2007) with addition of a local anesthetic was used to obtain synsacral adipose tissue samples from captive eiders. The bird was held cradled breast down; feathers were parted with an alcohol sponge, which was also used to disinfect the skin. Feathers were taped back and 0.1 ml lidocaine was injected subcutaneously. The site was disinfected with a betadine swab and sprayed with lidocaine spray. A small incision of approximately 1 cm long and lateral to the midline was made (on the right side for biopsies 1 and 3, left side for biopsy 2). Between 0.02 and 0.15 g of adipose tissue was excised using forceps and a scalpel blade, and placed in aluminum foil. The site was closed with Vetbond tissue adhesive (3M, St Paul, MN, USA) and birds were returned to their pens. After weighing, samples were placed in chloroform containing 0.01% BHT (an antioxidant) in glass centrifuge tubes with Teflon-lined caps. Lipid extraction and transesterification was performed the same day the biopsy was taken.
Laboratory analysis
Lipids were quantitatively extracted from eider adipose tissue samples and homogenates of diet items according to Folch et al. (1957), as modified by (Iverson et al. 2001), using 2:1 chloroform/methanol at 20–30 parts solvent to tissue. Diet items included whole individual mussel (without shell, n = 15), whole individual silverside (n = 39), krill (n = 39), clam (n = 15), and Mazuri (n = 23). Individual krill and Mazuri pellets were pooled into samples so that the combined sample weight was approximately 1.5 g; between two and six individual krill were pooled per sample, and between 14 and 17 individual Mazuri pellets were pooled per sample. Clams were already shelled and pre-cut into 2.0–4.0 g pieces and shipped to ASLC in frozen blocks. Each pre-cut piece was analyzed as an individual sample. Fatty acid methyl esters (FAME) were prepared using an acidic transesterification (Budge et al. 2006). The presence of fatty alcohols resulting from the transesterification of wax esters in diet items was determined using thin layer chromatography. In order to account for wax esters in diets, the alcohols of which are deposited as their corresponding FA in the adipose tissue (Budge and Iverson 2003), wax ester alcohols were converted to their respective FAs according to Budge et al. (2006). FAMEs were quantified using temperature-programmed gas liquid chromatography on a Perkin Elmer Autosystem II Capillary FID gas chromatograph fitted with a 30 m × 0.25 mm id column coated with 50% cyanopropyl-methylpolysiloxane (DB-23) and linked to a computerized integration system (Varian Galaxie software) according to Iverson et al. (2002). Each chromatogram was manually assessed for correct peak identification and reintegrated, where necessary.
Qualitative analysis of diet items and eider FA
Multivariate analyses were conducted using discriminant function analysis (DFA) followed by classification using a jack-knifing procedure (leave-one-out cross-validation) to assess how well FAs separate (1) diet items, (2) eider biopsies by species, diet, and biopsy date, and (3) diet items and eider biopsies. Given restrictions on the number of variables that can be used in DFA (n-1 of smallest sample size), we selected 14 diet item FAs which had the highest variance and overall means of the 72 total FAs identified (14:0, 16:0, 16:1n-7, 18:0, 18:1n-9, 18:1n-7, 18:2n-6, 18:3n-3, 18:4n-3, 20:1n-9, 20:1n-7, 20:5n-3, 22:5n-3, 22:6n-3), and 7 eider adipose tissue FAs with the highest observed variance across eider groupings (14:0, 16:0, 16:1n-7, 18:0, 18:1n-9, 18:2n-6, 22:6n-3), and 7 FAs with the highest observed variance and means across all diet items and eider samples (14:0, 16:0, 16:1n-7, 18:1n-9, 18:2n-6, 20:5n-3, 22:6n-3). The subsets of FAs used in these analyses are also known to be derived dominantly or solely from dietary intake (Iverson et al. 2004). Proportional representation of each FA was recalculated for this subset and arcsine square-root transformed prior to analysis. We used quadratic DFA because the within-group covariance matrices in our dataset were not homogenous (Bartlett’s test, P < 0.001), which may result in poorer separation between groups, although there is little evidence that moderate violation significantly alters cross-validated classification success (McGarigal et al. 2000).
CC and FA subset selection
We calculated eider CCs by dividing levels of individual FA in eiders from biopsy 1 (Day 0) by levels of those same FA in Diet 1, which was fed to the eiders for 69 days before this first biopsy (sensu Iverson et al. 2004). The eiders had been on a similar diet for over 3 years (consisting of almost entirely Mazuri, but with additional supplements that we incorporated into our calculations). Thus, we assumed the eiders’ FA signatures would resemble this diet as much as it ever would, and used this as a basis for calculating their CCs.
Twenty-five new FA subsets were developed based on two published subsets that include FAs that could arise from dietary origin alone (Dietary, 33 FAs) or include the Dietary subset along with 8 FAs whose levels in a consumer are also influenced by diet (Extended Dietary, 41 FAs) (Iverson et al. 2004). FAs were omitted from the published subsets in order of highest variability observed in CCs (e.g., Nordstrom et al. 2008). Twenty-seven subsets of the 72 FAs identified were tested in our model optimization exercise (see Appendix). Relative proportions of individual FA in each new subset were normalized to a sum of 100%. Subsets were used in the QFASA model and performance was evaluated using all biopsies and all diets from all eiders. The FA subset that provided the lowest overall sum of the squared errors (SSE) of diet was determined to be the optimal subset for our QFASA model, where SSE = (actual proportion of diet item−model estimate of diet item)2 summed across all diet items for Diet 1, 2, and 3.
QFASA model
To evaluate the reliability with which diet items could be distinguished in the model, we first performed a number of simulations using the captive eider diet database. These simulations were performed without CCs to assess the ability to estimate diet composition based only on differentiating and quantifying diet items by their FA signatures (sensu Iverson et al. 2004). We constructed five pseudo diets: Diets 1–3 were based on the actual Diets 1–3 that the eiders consumed. Diet 4 represented a marine diet without the commercially processed Mazuri. Diet 5 represented a diet comprised of only bivalves, which are the primary diet item for spectacled eiders wintering in the Bering Sea (Lovvorn et al. 2003). Simulations were used to evaluate how the accuracy of our estimates was affected by diets and FA subsets. Simulations were performed 1,000 times for each constructed diet. Prey-on-prey simulations were also performed to determine how well each individual diet item could be differentiated as itself from all other diet items in the diet library. These were done by taking each diet item in the library and dividing the samples into two sets: a modeling set and a simulation set. The selected diet item was modeled with the modeling set and all other diet items in the prey library. The simulation was performed 1,000 times for each diet item. Details of the simulation procedures are provided in Appendix B, Iverson et al. (2004). The diets of captive eiders were estimated using the QFASA model developed by Iverson et al. (2004). Calculated CCs were also used in the model to account for eider lipid metabolism.
Results
Qualitative separation of diet items and eider biopsies
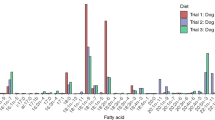
The FA signatures of the five diet items fed to captive eiders differed markedly (Fig. 1a). For example, compared to the other diet items Mazuri was extremely high in 18:2n-6, which is relatively rare in marine systems and probably reflected corn in the commercially made sea duck pellets. Mazuri was also lower in 20:5n-3 and 22:6n-3, which are relatively abundant in marine systems. Levels of 14:0, 16:0, and 18:1n-7 were higher in krill than other prey items. The two bivalves, clams and mussels, were much lower in 18:1n-9 and higher in 20:1n-7 than other diet items. Silversides had the highest levels of 22:5n-3 and 22:6n-3. These differences in levels of prey FAs were reflected in the eider biopsy signatures at Day 0, 21, and 50 (Fig. 1b, c). Although Mazuri remained a large proportion of the diet throughout the experiment, the influence of krill and then silverside FAs was evident at Days 21 and 50. For example, levels of Mazuri were relatively higher in 18:0, 18:1n-9, and 18:2n-6, all of which dropped after the introduction of krill and silverside. Likewise, the levels of krill were relatively higher in 20:5n-3 and 22:6n-3; and levels of silverside were relatively higher in 20:5n-3, 22:5n-3, and 22:6n-3, all of which increased in biopsies at Days 21 and 50 after the introduction of krill and silverside, respectively. Finally, silverside was relatively higher in 16:1n-7, 22:5n-3, 22:6n-3 and lower in 14:0, 16:0, 18:1n-7, 18:4n-3, and 20:5n-3 than krill and these changes in FA levels were reflected in the Day 50 biopsies after the birds had been switched to increased amounts of silverside. Thus, changes in dietary FAs were tracked in eider adipose tissue (Fig. 1).
Selected fatty acids (FAs, 14 out of 72 identified) with the largest overall variances and mean proportions of total FAs and that illustrate characteristic differences in patterns: a among the five diet items fed to captive eiders, b in adipose tissue of spectacled eiders at Day 0, 21, and 50, and c in adipose tissue of Steller’s eiders at Day 0, 21, and 50. Mean + 1 SE
Diet items were clearly differentiated using DFA. The 14 FAs selected for the DFA of diet items accounted for 84.4% of the total FAs identified in all diet items. Discriminant function (DF) 1 and DF 2 accounted for 90.8% of the variation (Fig. 2a). Diet samples were correctly classified to species with 100% of original grouped cases and 80% of cross-validated grouped cases (mussels were misclassified as silversides using the jack-knife procedure).
Discriminant function analysis plots for: a diet items based on 14 fatty acids (FAs) (14:0, 16:0, 16:1n-7, 18:0, 18:1n-9, 18:1n-7, 18:2n-6, 18:3n-3, 18:4n-3, 20:1n-9, 20:1n-7, 20:5n-3, 22:5n-3, 22:6n-3). Discriminant functions 1 and 2 accounted for 78.2 and 12.6% of the total variance, respectively; b eider biopsies based on seven FAs (14:0, 16:0, 16:1n-7, 18:0, 18:1n-9, 18:2n-6, 22:6n-3). Discriminant functions 1 and 2 accounted for 67.3 and 25.9% of the total variance, respectively; c diet items and eider biopsies based on seven FAs (14:0, 16:0, 16:1n-7, 18:1n-9, 18:2n-6, 20:5n-3, 22:6n-3). Discriminant functions 1 and 2 accounted for 74.4 and 14.5% of the total variance, respectively
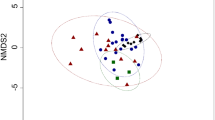
Eider adipose tissue samples were also clearly differentiated using DFA. The seven FAs selected for the eider DFA accounted for 86.2% of the total FA identified in all eider biopsies. DFs 1 and 2 accounted for 93.2% of the total variance (Fig. 2b). Eider biopsies were correctly classified to biopsy day but also to species with 97.9% of both original and cross-validated grouped cases. Thus, both species behaved in a similar manner in response to diet shifts, but additionally, the slight differences in the diet composition between the species were detected (Fig. 2b). One spectacled eider was observed to not consume much krill during Days 0–20 and consistent with this, his Day 21 biopsy was more similar to his Day 0 biopsy (Fig. 2b).
A DFA of diet items and eider biopsies combined using the seven FAs selected across all diet items and eider samples confirmed that eider biopsy FA signatures were most similar to Mazuri FA signatures, which was consistent with Mazuri being the major diet item throughout the experiment (Fig. 2c). However, after 21 days of increased krill in their diet, the Day 21 biopsy signatures had shifted towards the krill signatures. Similarly, after 29 days of increased silverside in their diet, the eider Day 50 biopsy signatures had shifted towards the silverside signatures (Fig. 2c). DFs 1 and 2 accounted for 88.9% of the variation (Fig. 2c). Diet items and eider biopsies were correctly classified to their own groups with 100% of original grouped cases and 45.5% cross-validated grouped cases (jack-knife procedure).
Calibration coefficients and FA subset selection
The CC values estimated from the eiders after 69 days on the same diet (Day 0) ranged from 0.03 for 22:2d7,13 and 22:2d7,15 to 4.26 for 21:5n-3 (Fig. 3). CC values of 1.0 indicate a 1:1 ratio of FA deposition from diet (Fig. 3). CCs were generally similar between the two eiders, except for three CCs (those for 16:1n-9, iso 17:0, 21:5n-3) that differed substantially. However, 16:1n-9 and iso 17:0 are not included in either the Dietary or Extended Dietary FA sets, and 21:5n-3 is not included in any of the modified FA subsets used in the diet modeling. CCs calculated for eiders differed from those calculated for common murre (Uria aalge) chicks. The CCs which exhibited the greatest deviance between eider and murres included the same three FAs mentioned above and most of the typical marine “dietary” FAs, that is, the long-chain mono- (20:1 and 22:1 isomers) and polyunsaturated FAs, except 20:5n-3 and 22:6n-3 (Fig. 3).
Spectacled and Steller’s eider calibration coefficients (CCs) calculated from birds fed on a constant diet for 69 days (mean ± 1 SE). Average common murre CCs determined from a previous study (Iverson et al. 2007) are presented for comparison. Asterisk in front of fatty acids (FAs) denotes FAs used in simulations and modeling. The line at 1 represents a 1:1 incorporation of FA from the diet into the eider adipose tissue
The performance of FA subsets in the QFASA model was dependent on eider species and diet (Table 1). Of the 25 other FA subsets modified from the published Dietary and Extended Dietary FA subsets, Test 18 (Reduced A) and Test 21 (Reduced B) FA subset had the lowest total SSE across all diets and were determined to be the best FA subsets for modeling diets for spectacled and Steller’s eiders, respectively (Table 1). The Dietary and Extended Dietary FA subsets from Iverson et al. (2004) were also used in the simulations and modeling for comparison.
QFASA model
The five specified pseudo diets were well estimated using QFASA simulations (Table 2). The Reduced A, B, Dietary, and Extended Dietary FA subsets produced similar results, however the Extended Dietary subset estimated proportions of individual species within each diet generally closer to the true values (Table 2). Furthermore, the prey-on-prey simulations showed that the diet items in the captive eider diet database were correctly identified as themselves 93–100% out of 1,000 simulations (Fig. 4). Mussels had the lowest identification rate with the widest distribution, while clams and Mazuri had the highest identification rate (Fig. 4). Although mussels were misclassified as other diet items (<7% as clam, krill, and Mazuri), in general the diet items in this study were reliably distinguished from one another.
Results for prey-on-prey simulation using the Reduced A, B, Dietary, and Extended Dietary fatty acid (FA) subsets which shows the ability of the QFASA procedure to identify the FA profile for a given diet item from the FA profiles of the other diet items in the diet library. Dietary and Extended Dietary subsets from Iverson et al. (2004). Estimates are represented in box plots, as the median (middle horizontal bar), the 25% percentile (lower bar), and the 75th percentile (top bar) of the data distribution (i.e., the box contains 50% of the data). Dots represent outliers defined as being any value greater (or less) than 1.5 times the interquartile range (75th percentile–25th percentile) above the 75th (or below the 25th) percentile. Results are presented as the proportion of prey item correctly identified as itself from all other prey items in 1,000 trials. For Reduced A FA subset: 100% of Mazuri, 99% of krill, 98% of silversides, 100% of clams, and 94% of mussels were correctly identified as themselves. For Reduced B FA subset: 100% of Mazuri, 98% of krill, 98% of silversides, 99% of clams, and 93% of mussels were correctly identified as themselves. For Dietary FA subset: 100% of Mazuri, 99% of krill, 98% of silversides, 100% of clams, and 96% of mussels were correctly identified as themselves. For Extended Dietary FA subset: 100% of Mazuri, 99% of krill, 98% of silversides, 100% of clams, and 97% of mussels were correctly identified as themselves
We used the Reduced A, B, Dietary, and Extended Dietary FA subsets in the model along with species-specific CCs calculated for spectacled and Steller’s eiders. For comparison, we also modeled eider diets using the Extended Dietary subset and the common murre chick CCs from Iverson et al. (2007). Our results showed that the QFASA model estimated the relative proportion of diet items in Diet 1 at Day 0 for both eider species generally very well no matter which subset and CCs were used, although exact levels varied among subsets and CCs (Fig. 5). From Day 0 to 20, spectacled eiders consumed a diet of 56% krill and 44% Mazuri, and Steller’s eiders consumed a diet of 66% krill and 34% Mazuri. The model correctly predicted a substantial proportion of krill coming into the diet by Day 21 (Fig. 5), with the predicted dietary percentages of krill averaging 24% in spectacled eiders and about 33% in Steller’s eiders. From Day 21 to 49, spectacled eiders consumed a diet of 52% silverside and 48% Mazuri, and Steller’s eiders consumed a diet of 66% silverside and 34% Mazuri. Again, the model accurately predicted a substantial proportion of silverside coming into the diet at Day 50, with some residual krill remaining from Diet 2. The percentage of silverside predicted in diet averaged 21% in spectacled eiders and 36% in Steller’s eiders. Commensurate with the increases in krill or silverside at both Day 21 and 50, the model correctly estimated a predictable decrease in Mazuri in the Diets 2 and 3. Compared to the actual diets fed, the lower levels of krill predicted in Diet 2 and the lower levels of silverside along with residual krill in Diet 3, suggests that complete turnover of FA from a switch in diet (Diet 2 and Diet 3) was not complete at either 21 or 29 days.
Quantitative fatty acid signature analysis estimates using the Reduced A, B, Dietary, Extended Dietary fatty acid (FA) subsets for Diets 1, 2, and 3 at Days 0, 21, and 50 for spectacled and Steller’s eiders. Species-specific calibration coefficients (CCs) were used in the model. Common murre (COMU) chick CCs were also used with the Extended Dietary FA subset for comparison. Dietary and Extended Dietary FA subsets from Iverson et al. (2004) and COMU chick CCs from Iverson et al. (2007). Actual diets consumed for spectacled eiders Diet 1 (Day 0): 88% Mazuri, 3% krill, 4% silverside, 1% clam, 4% mussel; Diet 2 (Day 21): 44% Mazuri, 56% krill; Diet 3 (Day 50): 48% Mazuri, 52% silverside. Actual diets consumed for Steller’s eiders Diet 1: 88% Mazuri, 1% krill, 3% silverside 1% clam, 7% mussel; Diet 2: 34% Mazuri, 66% krill; Diet 3: 34% Mazuri, 66% silverside. Mean + 1 SE
The Extended Dietary FA subset and eider CCs overestimated the proportion of mussel in Diet 2 (Day 21) and Diet 3 (Day 50) (Fig. 5). Birds were not fed mussel in Diets 2 or 3 and the amount of mussel fed in Diet 1 was relatively lower than the other prey items (4% in spectacled, 7% in Steller’s eiders). However, the amount of mussel predicted using the Extended Dietary FA subset and eider CCs, especially in Diet 3 (Day 50) was much greater than the amount that was consumed by either eider species (Fig. 5). Additionally, the Extended Dietary FA subset and common murre chick CCs overestimated the amount of silverside in Diet 2 (Day 21) (Fig. 5).
Discussion
The results from this captive feeding study demonstrated that for both species of eiders, changes in diet were clearly discriminated using adipose tissue FAs. Captive eider diet items were reliably differentiated from each other using both multivariate DFA and more rigorous QFASA simulations. Our results also demonstrated that the response of adipose tissue FAs in both species of eiders to dietary intake was highly predictable. Relative dietary percentages of these five diet items were accurately quantified by QFASA when those percentages had been constant for 10 weeks. Three weeks after a shift between diets with very distinct FA profiles, the shift was clearly reflected in tissue FA but the estimated dietary percentages were lower from the true percentages in diet, reflecting an integration of previous diet in the estimations and confirming that turnover was not complete within the 21–29 day study periods. Most dietary subsets used in QFASA yielded very similar estimates of dietary percentages, although one subset appreciably underestimated the percentages of Mazuri and krill and overestimated the percentage for mussels. Comparison of results for a suite of dietary subsets should identify such problems. CCs for the two eider species fed a diet of 88% Mazuri with supplements of krill, silversides, and bivalves were generally very similar and yielded similar results in QFASA estimates. The few instances where CCs differed substantially between the two species (16:1n-9, iso 17:0, 21:5n-3), were cases where the FAs were present in only trace amounts in diet items and eider adipose tissue and thus CCs were variably estimated. CCs for common murre chicks fed entirely Atlantic silversides appeared to differ from those of the eiders, however, when the murre CCs were applied to eiders, dietary estimates remained similar except for slower loss of silverside at Day 21 and more rapid incorporation of FAs from silversides at Day 50.
Diet items were correctly classified with 80–100% accuracy in the DFA using only 14 out of the 72 FAs identified. The DFA demonstrated that multivariate techniques are an important precursor to using the QFASA model in determining which potential diet items have overlapping FA signatures. In the QFASA simulations, FA profiles of mussels and silversides also had the lowest rate of discrimination from other diet items in the prey library, but were still well identified at a rate of 93–98%. The eider biopsies were correctly classified with 97.9% accuracy using only 7 out of the 72 FAs identified in the DFA and showed that there were changes in FA signatures among biopsies and therefore that they reflected changes in diet, which was confirmed in the QFASA model.
Model estimates were significantly influenced by the FA subset and CC set. The Reduced A and B FA subsets were modified from the published Dietary FA subset (Iverson et al. 2004) and provided the best overall diet estimates for spectacled and Steller’s eiders, respectively. The Extended Dietary subset and subsets modified from it provided poorer diet estimates. FAs were omitted from the published subsets in order of highest variability observed in eider CCs (sensu Nordstrom et al. 2008), which tended to provide better diet estimates. In contrast, Iverson et al. (2007) removed FAs with large CCs, which produced poorer estimates in the model. However, diets, CCs, and bird species differed between studies and likely influenced model results. The Extended Dietary subset includes all FAs in the Dietary subset and eight FAs that could be biosynthesized by consumers, but whose levels in a consumer are also influenced by consumption of specific diet items. This may have influenced the diet estimates because eiders were on a diet heavily influenced by carbohydrates from the corn-based Mazuri (approximately 50% carbohydrate), which could have led to more biosynthesis of these FAs than with the experimental diets contributing less carbohydrate. Although 27 subsets were evaluated, the possibility remains for different combinations of FA to provide more accurate diet estimates, and we stress that the method for selecting FA subsets for use in the QFASA model requires further development.
The CCs estimated for spectacled and Steller’s eiders were relatively similar to each other but differed from those estimated for the common murre chicks CCs from Iverson et al. (2007). The explanation for the FAs that exhibited the greatest differences between eider and murre CCs (16:1n-9, iso 17:0, 21:5n-3, also mentioned above, and most of the typical marine long-chain (≥20 C) “dietary” FAs; Fig. 3) may at least in part be attributed to the diets fed. The eiders in this study had been fed a long-term diet of primarily Mazuri, a high carbohydrate corn-based feed. Although listed as a “sea duck formula”, Mazuri contained generally only trace levels of typical marine long-chain mono- and polyunsaturated FAs (except 20:5n-3 and 22:6n-3), and hence so also did eider adipose tissue, leading to variable estimation of CCs for these FAs in eiders and differences from murre CCs estimated from a fish (silverside) diet. Thus, in addition to possibly greater contribution in eiders from FA biosynthesis on a low-fat high-carbohydrate diet, estimation of CCs for these FAs may have been different had eiders been fed a more marine diet. The single exception was for 20:5n-3 and 22:6n-3, both of which were present at relatively abundant levels in both Mazuri and silverside, and hence in eider and murre adipose tissue, resulting in relatively similar estimated CCs for these FAs among the three species (Fig. 3). Additionally, differences in CCs could also be influenced by differences in age classes (adults versus chicks). That is, growing chicks may differentially use or mobilize essential and other FAs for tissue growth. To our knowledge, this is the first study to calculate CCs for adult birds from adipose tissue. It should be remembered that the only means we currently have for accounting for predator metabolism is the use of CCs (Iverson et al. 2004), which although have been shown to result in accurate diet estimation using QFASA, remain a fairly basic mathematical attempt to describe somewhat complex biochemistry. Interestingly, despite the apparent differences in CCs, the QFASA model provided similar diet estimates when using the Reduced A, B, and Dietary subsets and eider CCs as estimates using the Extended Dietary subset and common murre CCs.
We have demonstrated that QFASA can be a powerful technique for estimating diets of captive eiders, potentially providing a minimally invasive method for obtaining information about their diets in the wild. The issues and requirements for using QFASA and assembling a prey library have previously been reviewed (Iverson et al. 2004, 2007; Budge et al. 2006; Iverson 2009). In regards to marine birds, there remain several areas for further investigation. The development of species-specific or diet-specific CCs is a necessary component of QFASA. We are aware of only two other studies that have calculated CCs for marine birds from adipose tissue: common murre chicks (Iverson et al. 2007) and tufted puffin (Fratercula cirrhata) chicks (Williams et al. 2009). The common murre chicks were fed a diet composed entirely of Atlantic silversides for 45 days. The tufted puffin chicks were fed Pacific herring (Clupea pallasi) in two groups for 27 days: high calorie (120 g herring/day) and low calorie (restricted to 60 g herring/day) and the CCs of low and high-calorie diets were highly correlated (Williams et al. 2009). In our captive study, although the diet was only strictly regulated for 69 days prior to the biopsy for CCs, the eiders had been on a similar diet for over 3 years (consisting of almost entirely Mazuri, but with additional supplements that we incorporated into our calculations). Thus, we assumed the eiders’ FA signatures would look as much like the diet as it ever would, and used this as a basis for weighting FAs. Because of the length of time of the long-term diet, we are confident we have good estimates for CCs for the eiders used in the experiment. However, given the differences between the eider and common murre chick CCs, and some differences in model estimates using different CCs and FA subsets, the influence of FA composition and dietary fat content, as well as growing juveniles versus adults, on estimation of CCs in both birds and mammals requires further investigation.
Quantitative fatty acid signature analysis estimates for Diet 2 and Diet 3 confirmed that complete FA turnover of the new introduced diets was not complete by 21 or 29 days. Model estimates for Diet 1 were accurately quantified by QFASA, which suggests that turnover is almost, if not complete by 69 days. Nordstrom et al. (2008) suggested that in captive harbor seals (Phoca vitulina) turnover of FA may be dependent on quantity of prey consumed, diet composition, and growth pattern, and may not be a strictly linear process. However, Williams et al. (2009) found that the rates of FA turnover of tufted puffin chicks were not different between high (120 g herring/day) and low-calorie (60 g herring/day) diets, and turnover was close to, but not entirely complete, after 27 days on both high and low-calorie diets.
Turnover rates give insight to the time period of dietary history and are necessary for interpretation of the integration period of FA data. There may be a large range for complete rate of turnover depending on intake and metabolism rates. This makes interpretation of FA data from wild individuals complicated and results from our captive study to estimated diets of wild eiders should be applied with caution. For example, from our study we estimate that complete turnover of dietary FAs occurred between 29 (~4 weeks) and 69 days (~10 weeks) in a captive eider feeding on Mazuri, krill, silverside, clam, and mussel. If eiders consumed only krill on their wintering grounds for 3 months, and then migrated to their staging areas and fed on silversides for 4 weeks, and turnover was complete at 4 weeks, their biopsies at the end of those 4 weeks would accurately estimate a diet of silversides for the past 4 weeks but would likely not estimate any krill in the diet from their wintering grounds. If turnover was complete at 10 weeks, their biopsies at the end of those 4 weeks would estimate a mixed a diet of krill and silverside. However, if there is no prior knowledge of what the birds were eating at the different habitats, whether the krill and/or silverside were consumed on the wintering grounds and staging areas cannot be determined. Additionally, Day 0 eider biopsies indicate that if the diet had been constant for 10 weeks, the estimated relative proportion of diet items using QFASA are very accurate. However, without knowing that the diet had been constant for 10 weeks, a dietary percentage of 20% could mean that (1) the diet item was truly 20% of a constant diet, (2) the proportion of that diet item was much greater (perhaps 50%) up until 3–4 weeks prior to biopsy sampling and had not been consumed at all after that, or (3) the proportion of that diet item increased over the last 3–4 weeks but was not consumed before then. Thus, the exact quantitative proportions in diet do indeed represent an unknown integrated period and may be less meaningful than detecting shifts in diet. That is, sampling birds at arrival on breeding grounds over time can detect shifts in diets at staging areas and possibly wintering areas over time, which would provide meaningful information about changes in relative abundance of prey at these areas over time. Complete turnover rates of dietary FAs have not been calculated in marine birds and the effect of types of diet (e.g., benthic versus pelagic), and different metabolic rates (e.g., different seabirds species, captive versus wild birds) on FA metabolism and rates of turnover requires further investigation.
Ideally, captive studies should mimic the natural environment as much as possible. Experimental diets in this study did not mimic the true natural diets of eiders; nonetheless, the experimental diets demonstrated that tracking diet changes in eiders using FAs is possible. Spectacled and Steller’s eiders are benthic-feeding birds and do not consume Atlantic krill, silversides, or Mazuri in the wild. Spectacled eiders wintering in the Bering Sea forage on a variety of food items, including clams, mussels, amphipods, and polychaetes (Petersen et al. 1998; Lovvorn et al. 2003). Steller’s eiders have been reported to consume a diverse diet of invertebrates, suggesting they are non-selective foragers (Petersen 1980; Petersen 1981; Bustnes and Systad 2001). During the nesting season, eiders feed on insects, insect larvae, seeds, and plant materials in tundra ponds resulting in a diet higher in carbohydrates (Petersen et al. 2000; Fredrickson 2001). In our experiment, the simulated pseudo diet 5 consisted of 50% clam and 50% mussel and was well estimated using the model and FA subsets indicating that two species of bivalves can be distinguished from each other. However, the simulated pseudo diets and actual diets were comprised of only five diet items. Thus, further work is needed to examine the FA profiles of clams, amphipod, polychaetes, and other diet items of wild eiders.
We conclude that applying minimally invasive QFASA techniques to determine the diets of spectacled and Steller’s eiders during different life history stages will provide more useful information about their diets than other approaches and help identify critical habitats to support conservation of these threatened breeding populations. Nevertheless, we advise researchers to apply our QFASA results with some caution in that the captive eider CCs, turnover rates, and FA subsets may require further consideration in application to wild birds. We stress that further work is required to calculate complete turnover rates of FAs from a natural diet, investigate the sensitivity of CCs and FA subsets to diets, and of the QFASA model to the CCs and FA subsets used. However, despite this sensitivity our study showed that QFASA accurately estimated Diet 1 and accurately indicated diet switches in captive eiders, and that diets can be estimated over an extended time period. Thus, our understanding of diet can be back-tracked to more than a month in a captive feeding eider. Regardless of model limitations, this study provides an important basis for further validation studies and interpretation of adipose tissue FA data from wild populations.
References
Barrett RT, Camphuysen KCJ, Anker-Nilssen T, Chardine JW, Furness RW, Garthe S, Hüppop O, Leopold MF, Montevecchi WA, Veit RR (2007) Diet studies of seabirds: a review and recommendations. ICES J Mar Sci 64:1675–1691. doi:10.1093/icesjms/fsm152
Budge SM, Iverson SJ (2003) Quantitative analysis of fatty acid precursors in marine samples: direct conversion of wax ester alcohols and dimethylacetals to FAMEs. J Lipid Res 44:1802–1807. doi:10.1194/jlr.D300009-JLR200
Budge SM, Iverson SJ, Koopman HN (2006) Studying trophic ecology in marine ecosystems using fatty acids: a primer on analysis and interpretation. Mar Mamm Sci 22:759–801. doi:10.1111/j.1748-7692.2006.00079.x
Budge SM, Springer AM, Iverson SJ, Sheffield G (2007) Fatty acid biomarkers reveal niche separation in an Arctic benthic food web. Mar Ecol Prog Ser 336:305–309. doi:10.3354/meps336305
Bustnes JO, Systad GH (2001) Comparative feeding ecology of Steller’s eider and long-tailed ducks in winter. Waterbirds 24(3):407–412
Connan M, Mayzaud P, Boutoute M, Weimerskirch H, Cherel Y (2005) Lipid composition of stomach oil in a procellariiform seabird Puffinus tenuirostris: implications for food web studies. Mar Ecol Prog Ser 290:277–290. doi:10.3354/meps290277
Connan M, Cherel Y, Mabille G, Mayzaud P (2007a) Trophic relationships of white-chinned petrels from Crozet Islands: combined stomach oil and conventional dietary analyses. Mar Biol 152:95–107. doi:10.1007/s00227-007-0664-6
Connan M, Cherel Y, Mayzaud P (2007b) Lipids from stomach oil of procellariiform seabirds document the importance of myctophid fish in the Southern Ocean. Limnol Oceanogr 52:2445–2455
Dahl TM, Falk-Petersen S, Gabrielsen GW, Sargent JR, Hop H, Millar RM (2003) Lipids and stable isotopes in common eider, black-legged kittiwake and northern fulmar: a trophic study from an Arctic fjord. Mar Ecol Prog Ser 256:257–269. doi:10.3354/meps256257
Federal Register (1993) Final rule to list the spectacled eider as threatened. Fed Regist 58:27474–27480
Federal Register (1997) Endangered and threatened wildlife and plants; threatened status for the Alaska breeding population of the Steller’s eider. Fed Regist 62:31748–31757
Folch J, Lees M, Sloane-Stanly GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Fredrickson LH (2001) Steller’s eider (Polysticta stelleri). In: Poole A, Gill F (eds) The birds of North America, vol 571. The Birds of North America Inc, Philadelphia
Iverson SJ (2009) Tracing aquatic food webs using fatty acids: from qualitative indicators to quantitative determination. In: Arts MT, Brett MT, Kainz M (eds) Lipids in aquatic ecosystems. Springer, New York
Iverson SJ, Lang SLC, Cooper M (2001) Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissues. Lipids 36:1283–1287
Iverson SJ, Frost KJ, Lang SLC (2002) Fat content and fatty acid composition of forage fish and invertebrates in Prince William Sound, Alaska: factors contributing to among and within species variability. Mar Ecol Prog Ser 241:161–181. doi:10.3354/meps241161
Iverson SJ, Field C, Bowen WD, Blanchard W (2004) Quantitative fatty acid signature analysis: A new method of estimating predator diet. Ecol Monogr 74:11–235
Iverson SJ, Stirling I, Lang SLC (2006) Spatial and temporal variation in the diets of polar bears across the Canadian Arctic: indicators of changes in prey populations and environment. In: Boyd IL, Wanless S, Camphuysen CJ (eds) Top predators in marine ecosystems: their role in monitoring and management. Cambridge University Press, Cambridge, pp 98–117
Iverson SJ, Springer AM, Kitaysky AS (2007) Seabirds as indicators of food web structure and ecosystem variability: qualitative and quantitative diet analyses using fatty acids. Mar Ecol Prog Ser 352:235–244. doi:10.3354/meps07073
Käkelä R, Käkelä A, Kahle S, Becker PH, Kelly A, Furness R (2005) Fatty acid signatures in plasma of captive herring gulls as indicators of demersal or pelagic fish diet. Mar Ecol Prog Ser 293:191–200. doi:10.3354/meps293191
Käkelä A, Crane J, Votier SC, Furness RW, Käkelä R (2006) Fatty acid signatures as indicators of diet in great skuas Stercorarius skua, Shetland. Mar Ecol Prog Ser 319:297–310. doi:10.3354/meps319297
Käkelä A, Furness RW, Kelly A, Strandberg U, Waldron S, Käkelä R (2007) Fatty acid signatures and stable isotopes as dietary indicators in North Sea seabirds. Mar Ecol Prog Ser 342:291–301. doi:10.3354/meps342291
Käkelä R, Furness RW, Kahle S, Becker PH, Käkelä A (2009) Fatty acid signatures in seabird plasma are a complex function of diet composition: a captive feeding trial with herring gulls. Funct Ecol 23:141–149. doi:10.1111/j.1365-2435.2008.01475.x
Lovvorn JR, Richman SE, Grebmeier JM, Cooper LW (2003) Diet and body condition of spectacled eiders wintering in pack ice of the Bering Sea. Polar Biol 26:259–267. doi:10.1007/s00300-003-0477-0
McGarigal K, Cushman S, Stafford S (2000) Multivariate statistics for wildlife and ecology research. Springer, New York
Nordstrom CA, Wilson LJ, Iverson SJ, Tollit DJ (2008) Evaluating quantitative fatty acid signature analysis (QFASA) using harbour seals Phoca vitulina richardsi in captive feeding studies. Mar Ecol Prog Ser 360:245–263. doi:10.3354/meps07378
Petersen MR (1980) Observations of wing-feather moult and summer feeding ecology of Steller’s eiders at Nelson Lagoon, Alaska. Wildfowl 31:99–106
Petersen MR (1981) Populations, feeding ecology and molt of Steller’s eiders. Condor 83:256–262
Petersen MR, Piatt JF, Trust KA (1998) Foods of spectacled eiders Somateria fischeri in the Bering Sea, Alaska. Wildfowl 49:124–128
Petersen MR, Grand JB, Dau CP (2000) Spectacled eider (Somateria fischeri). In: Poole A, Gill F (eds) The birds of North America. The Birds of North America Inc, Philadelphia
Raclot T, Groscolas R, Cherel Y (1998) Fatty acid evidence for the importance of myctophid fishes in the diet of king penguins, Aptenodytes patagonicus. Mar Biol 132:523–533
U.S. Fish and Wildlife Service (1996) Spectacled eider recovery plan. U.S. Fish and Wildlife Service, Anchorage
U.S. Fish and Wildlife Service (2002) Steller’s eider recovery plan. U.S. Fish and Wildlife Service, Fairbanks
Wang SW, Iverson SJ, Springer AM, Hatch SA (2007) Fatty acid signatures of stomach oil and adipose tissue of northern fulmars (Fulmarus glacialis) in Alaska: implications for diet analysis of Procellariiform birds. J Comp Physiol B 177:893–903. doi:10.1007/s00360-007-0187-y
Wang SW, Iverson SJ, Springer AM, Hatch SA (2009) Spatial and temporal diet segregation in northern fulmars (Fulmarus glacialis) breeding in Alaska: insights from fatty acid signatures. Mar Ecol Prog Ser 377:299–307. doi:10.3354/meps07863
Williams CT, Iverson SJ, Buck CL (2008) Stable isotopes and fatty acid signatures reveal age- and stage-dependent foraging niches in tufted puffin. Mar Ecol Prog Ser 363:287–298. doi:10.3354/meps07477
Williams CT, Iverson SJ, Buck CL (2009) The effects of diet and caloric restriction on adipose tissue fatty acid signatures of tufted puffin (Fratercula cirrhata) nestlings. J Comp Physiol B. doi:10.1007/s00360-009-0354-4
Acknowledgments
Financial support was provided by the North Pacific Research Board (NPRB Project 724), U.S. Fish and Wildlife Service, National Park Service-Ocean Alaska Science and Learning Center and the Natural Sciences and Engineering Research Council (NSERC) Canada. P. Flint, J. Lovvorn, and two anonymous reviewers provided very helpful comments on an earlier draft. We thank H. Cline, T. DiMarzio, M. Grue, G. Gerdsen, and N. Brandt for the care and maintenance of the eiders in this study. We also thank D. Mulcahy, C. Goertz, P. Tuomi, M. Gray, S. Skeba, D. Safine, A. Riddle, and R. Federer for assistance with biopsies. We are grateful to S. Al-Shaghay for assistance with lab analyses. The collection of samples for this project was carried under permits from Institutional Animal Care and Use Committee (No. 07-011) at the Alaska SeaLife Center, the Alaska Department of Fish and Game (No. 07-045), and the Federal Fish and Wildlife Permit (No. 065912). This is NPRB publication No. 216.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Appendix
Rights and permissions
About this article
Cite this article
Wang, S.W., Hollmén, T.E. & Iverson, S.J. Validating quantitative fatty acid signature analysis to estimate diets of spectacled and Steller’s eiders (Somateria fischeri and Polysticta stelleri). J Comp Physiol B 180, 125–139 (2010). https://doi.org/10.1007/s00360-009-0393-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-009-0393-x