Abstract
We investigated molecular responses elicited by three types of dehydration (fast, slow and cryoprotective), rehydration and overhydration in larvae of the Antarctic midge, Belgica antarctica. The larvae spend most the year encased in ice but during the austral summer are vulnerable to summer storms, osmotic stress from ocean spray and drying conditions due to wind and intense sunlight. Using suppressive subtractive hybridization (SSH), we obtained clones that were potentially responsive to dehydration and then used northern blots to evaluate the gene’s responsiveness to different dehydration rates and hydration states. Among the genes most responsive to changes in the hydration state were those encoding heat shock proteins (smHsp, Hsp70, Hsp90), antioxidants (superoxide dismutase, catalase), detoxification (metallothionein, cytochrome p450), genes involved in altering cell membranes (fatty acid desaturase, phospholipase A2 activating protein, fatty acyl CoA desaturase) and the cytoskeleton (actin, muscle-specific actin), and several additional genes including a zinc-finger protein, pacifastin and VATPase. Among the three types of dehydration evaluated, fast dehydration elicited the strongest response (more genes, higher expression), followed by cryoprotective dehydration and slow dehydration. During rehydration most, but not all, genes that were expressed during dehydration continued to be expressed; fatty acid desaturase was the only gene to be uniquely upregulated in response to rehydration. All genes examined, except VATPase, were upregulated in response to overhydration. The midge larvae are thus responding quickly to water loss and gain by expressing genes that encode proteins contributing to maintenance of proper protein function, protection and overall cell homeostasis during times of osmotic flux, a challenge that is particularly acute in this Antarctic environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Antarctic midge, Belgica antarctica, is endemic to the maritime Antarctic, and in this habitat it is confronted with a wide range of environmental stressors (Gressitt 1967). The larvae spend up to 10 months each year frozen within the icy substrate, but during the austral summer when the ice melts, they resume feeding and reinitiate their growth and development (Peckham 1971). Larval development is completed after two years, and then the wingless adults emerge, reproduce and die within a 7–10 day window in late December to early January (Sugg et al. 1983). During the austral summer the larvae are exposed to rain storms, pools of melting snow and ice, ocean spray and periods of habitat drying due to wind and intense sunlight. One of the major challenges the midge larvae encounter in this environment is management of their water content.
Our previous work on water balance of this insect demonstrated that these hydrophilic larvae employ several mechanisms to both conserve water (Benoit et al. 2007) as well as lose water to enhance their cold tolerance (Hayward et al. 2007). Water conserving mechanisms include increasing levels of cryoprotectants, decreasing metabolic rate and thereby decreasing their water loss rate, and clustering (Benoit et al. 2007). The larvae tolerate the loss of up to 70% of their body water, which also enhances their freeze tolerance (Hayward et al. 2007). This tolerance for water loss is not unique to B. antarctica: a number of chironomids survive similar loses of water and employ similar water conservation adaptations (Suemoto et al. 2004). In addition to an ability to survive changes in their internal water pool, chironomids are excellent osmoregulators able to survive extreme changes in substrate salinity (Hassell et al. 2006; Nondula et al. 2004). High salinity tolerance and osmoregulation capacity are also evident in the Antarctic midge (Elnitsky et al. 2008b). Larvae may experience as many as 140 freeze–thaw cycles throughout the year (Block 1997), yet another challenge for maintenance of water balance as these cycles lead to changes in water availability.
Under natural conditions, dehydration can occur rapidly or slowly. During winter, when the larvae are directly exposed to ice, dehydration likely occurs slowly, until the vapor pressure of the midge larva matches that of the ice. This slow form of dehydration, known as cryoprotective dehydration, may be the major water management strategy used during the winter (Elnitsky et al. 2008a). Fast dehydration is experienced during the austral summer when the larvae are active and soil saturation can decrease rapidly. Thus far, little is known about the molecular changes associated with these changes in hydration state.
In this study, we used suppressive subtractive hybridization (SSH) to compare hydrated and dehydrated individuals, and from this we identified 70 genes that were putatively altered in expression. Northern blot hybridizations were used to confirm expression of those genes during dehydration. We then chose 15 candidate genes (smHsp, Hsp70, Hsp90, SOD, catalase, metallothionein, p450, FAD, PLAP, fatty acyl CoA desaturase, actin, MSA, a zinc-finger protein, pacifastin, and VATPase) to monitor expression changes during fast and slow dehydration, cryoprotective dehydration, fast and slow rehydration, and overhydration.
Materials and methods
Insects
Belgica antarctica Jacobs larvae were collected in January, February, and March 2006 and 2007, on Humble, Torgersen and Cormorant Islands and Norsel Point near Palmer Station, Anvers Island, Antarctica (64°46′S, 64°04′W). Larvae were collected with the substrate, consisting of small pieces of rock, soil, animal detritus, moss, and algae. The larvae and substrate were frozen and transported to Ohio State University (approx. −5°C for 7 days), where the genes were cloned and their expression patterns monitored. In our home laboratory, the larvae were maintained in a cold room at 4°C, a temperature that approximates that of their summer habitat. Before the larvae were subjected to the various treatments, they were sorted from the substrate in ice water (tap water) and then transferred to glass beakers containing moist paper towels at 4°C.
Stress treatments
Control larvae were sorted directly from the substrate and stored at high humidity conditions at 4°C (moist paper towels in sealed glass beakers) to avoid the prolonged ice water sorting and water storage protocol we used in previous experiments (Rinehart et al. 2006; Hayward et al. 2007; Lopez-Martinez et al. 2008). This control thus represents conditions that preclude the possibility of hydration or overhydration due to storage of the larvae in water, and yet still allows for gut clearance and synchronization of the larval hydration state. Thus, none of the control larvae were stored in water, but instead were held at a high relative humidity, simulating substrate conditions during the austral summer.
Previously we investigated the water balance requirements and osmoregulatory ability of the larvae of B. antarctica (Benoit et al. 2007; Elnitsky et al. 2008b). Based on that work, we designed a dehydration and rehydration series that was not lethal and generated larvae with known amounts of water loss. Fast dehydration was achieved by exposing larvae to 75% RH (saturated NaCl; Winston and Bates 1960) for 36 h; this resulted in a loss of approximately 50% of their total water content as shown by Benoit et al. (2007). A shorter exposure (12 h) to 75% RH, resulting in a 30% water loss (Benoit et al. 2007), was also tested to mimic what occurs on summer days in the Antarctic. Slow dehydration was achieved at 98% RH (saturated K2SO4; Winston and Bates 1960) for 5 days, a treatment that resulted in approximately a 50% water loss (Benoit et al. 2007). Larvae were also subjected to cryoprotective dehydration (Elnitsky et al. 2008a), a slow dehydration over a 14 day period in the presence of ice that resulted in approximately a 35% water loss. All treatments were performed at 4°C with the exception of cryoprotective dehydration, where the larvae were slowly lowered from 0 to −5°C over the course of a week and were then maintained at that lower temperature.
Fast and slow rehydration series were also carried out following both fast (75% RH for 36 h) and slow dehydration (98% RH for 5 days). Fast rehydration was done by submerging larvae in water for 2 h, and slow rehydration was achieved by exposing larvae to 100% RH for 12 h. Both rehydration treatments were designed to restore water lost during dehydration (Benoit et al. 2007). Figure 1 shows an overview of the treatments described.
Overhydration was achieved by submerging larvae in water for 10 days; this resulted in a 10% increase in water content. To evaluate the effect of holding larvae in water, larval survival was determined after different durations of submersion. Ten larvae were placed into 1.7 ml microcentrifuge tubes containing 1 ml distilled water and held at 4°C for up to 30 days. Each day, 0.75 ml water was replaced with fresh water to prevent anoxia and build up of waste products. Every 5 days, the larvae were transferred to Petri dishes to monitor survival. The midges were observed under a dissecting microscope, and individuals were considered alive if movement was noted. After assessing survival, dead larvae were removed and stored in separate tubes for re-evaluation of mortality 5 days later. Controls consisted of groups of ten larvae held in mesh-covered tubes (N = 10) that were switched between 98 and 100% RH every 12 h, to maintain proper hydration levels (Benoit et al. 2007). Data were analyzed with ANOVA, and when significance was noted Bonferroni–Dunn multiple comparison tests were used to analyze significance over time. Percentage data were arcsin transformed prior to analysis.
Source of clones
Most clones used in this study were obtained by a suppressive subtractive hybridization (SSH) procedure in which hydrated control larvae were subtracted from a group of larvae desiccated for 12 h at 98.4% RH. The BD SMART™ PCR cDNA Synthesis kit (Clonetech Laboratories, Mountain View, CA, USA) was used to prepare the clones and a PCR-Select™ cDNA Subtraction Kit (Clonetech Laboratories) was used for the subtraction. Clones of Hsp70, Hsp90 and catalase, used in this study, were isolated previously (Rinehart et al. 2006; Lopez-Martinez et al. 2008).
Northern blot hybridization
Trizol reagent (Invitrogen, Carlsbad, California) was used to extract RNA from groups of 75 larvae, following the manufacturer’s protocol. Four micrograms of RNA per treatment were run on a 1.4% agarose, 0.41 M formaldehyde gel. The RNA was then transferred to a nylon membrane (Hybond-N+, Amersham Biosciences, Piscataway, NJ, USA) using the Schleicher & Schuell’s Turboblotter transfer system. Roche Diagnostics’s DIG-High Prime labeling kit was used to label the DNA clones for hybridization, which was done at 42°C overnight. The northern blots were treated following the protocol from the DNA Labeling and Detection Starter Kit II, also from Roche, and exposed using Blue Lite Autorad Film (ISC BioExpress, Kaysville, UT, USA) for 30 min. All membranes were stripped and re-hybridized with 28S (as a control gene). All northerns blots were confirmed by triplicate technical replication.
Results
Suppressive subtractive hybridization
Suppressive subtractive hybridization (SSH) produced 70 unique clones whose putative identity could be deduced by blasting their amino acid translations using GenBank (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Accession numbers are registered in GenBank as numbers DQ507279 through DQ507371. Northern blot hybridizations were used to confirm expression of each gene. Among the 70 clones, 42 were unaffected by fast, slow or cryoprotective dehydration and thus their expression was not monitored for rehydration or overhydration. The 15 genes that were most responsive to dehydration were also monitored by northern blot hybridization for responses to rehydration and overhydration. Clearly, many more genes are likely to respond to these stresses than were detected by SSH. This is already evident by the fact that our clones of Hsp70, Hsp90 and catalase were responsive to dehydration stress but were not among the genes identified by SSH.
Fast and slow dehydration series
The identity, size and match of clones that were upregulated in response to fast dehydration (30% water loss; Benoit et al. 2007) are shown in Table 1. Table 2 shows the same information for the 8 genes that were downregulated during fast dehydration. Three heat shock proteins (a smHsp, Hsp70, Hsp90), superoxide dismutase, catalase, metallothionein, p450, fatty acid desaturase, phospholipase A2 activating protein, fatty acyl CoA desaturase, actin, muscle-specific actin, a zinc-finger protein, pacifastin, and Vacuolar (H+) ATPase were upregulated in response to fast dehydration (Fig. 2). When the duration of fast dehydration was increased to 36 h (50% water content loss; Benoit et al. 2007), most of the genes were still upregulated (Fig. 3), but expression of Mtn and ZFP decreased with the longer exposure time.
Northern blot hybridizations of the 15 genes that were upregulated in response to dehydration in B. antarctica. The treatments are controls, held in a fully hydrated state (C), fast dehydration at 75% RH for 12 h, and cryoprotective dehydration, held on ice for 14 days at −5°C(CP). 28S was used as the control. See text for names of genes
Northern blot hybridizations showing the dehydration and rehydration series for B. antarctica. The treatments are control (C), prolonged fast dehydration at 75% RH for 36 h, fast dehydration followed by fast rehydration in the presence of water (FR), fast dehydration followed by slow rehydration at 100% RH (SR) and slow dehydration at 98% RH for 5 days. 28S was used as the control. See text for names of genes
Slow dehydration (5 days at 98% RH), which led to a water content loss of 50% (Benoit et al. 2007), resulted in the upregulation of Hsp70, catalase, p450, fatty acyl CoA desaturase, actin, MSA, and pacifastin (Fig. 3). Thus, slow dehydration increased expression of some of the same genes upregulated by fast dehydration (Hsp70, catalase, p450, fatty acyl CoA desaturase, actin, MSA, and pacifastin), but there was no difference from the controls in expression levels for the smHsp, Hsp90, FAD and PLAP (Fig. 3). SOD, Mtn, ZFP and VATPase were downregulated in response to slow dehydration when compared to hydrated controls (Fig. 3).
Cryoprotective dehydration
The prolonged 14 days slow dehydration in the presence of ice resulted in a water loss of 35% of the total body water (Elnitsky et al. 2008a). The three Hsps, catalase, Mtn, FAD, PLAP, fatty acyl CoA desaturase, actin, MSA, the ZFP, and VATPase were strongly upregulated in response to cryoprotective dehydration (Fig. 2). Expression of the smHsp, fatty acyl CoA desaturase and VATPase was greater for cryoprotective dehydration than for fast dehydration. But, expression of Hsp90, Mtn, FAD, PLAP and actin was not as strong as elicited by fast dehydration. Expression of SOD, p450 and pacifastin did not differ from that of the controls (Fig. 2). Cryoprotective dehydration elicited a response that was very similar to the response to slow dehydration (98% RH for 5 days) (Fig. 3).
Fast and slow rehydration
The rehydration treatments fully restored larval water content to their initial levels (Benoit et al. 2007). Most differences in gene expression that occurred during rehydration were noted in larvae that were first subjected to fast dehydration (36 h at 75% RH). The only notable change in expression during rehydration following slow dehydration was upregulation of Hsp90, while expression of the other genes remained mostly unchanged (data not shown). Thus, the following rehydration results focus on rehydration following fast dehydration.
Following dehydration, smHsp, Hsp70, fatty acyl CoA desaturase and actin did not change in response to either fast or slow rehydration (Fig. 3). Expression was elevated by fast dehydration and it remained high during both types of rehydration. Expression of SOD, Mtn, PLAP and ZFP decreased following both types of rehydration (Fig. 3). Hsp90, catalase, MSA, pacifastin and VATPase were less expressed following fast rehydration, while P450 and FAD expression remained unchanged (Fig. 3). FAD was the only gene that was uniquely upregulated by slow rehydration (Fig. 3d). Expression of the three Hsps, catalase, fatty acyl CoA desaturase, actin, MSA, pacifastin and VATPase was not changed by slow rehydration, beyond the strong response noted during the initial fast dehydration regime (Fig. 3), whereas SOD, Mtn, p450, PLAP and the ZFP were downregulated in response to slow rehydration (Fig. 3).
Overhydration
When larvae were submerged in water, internal water content increased over the first 10 days by approximately 10% (Fig. 4); thereafter water content remained stable. Water mass increases after 5 days in water were significantly different than the controls, and this difference persisted for the duration of the experiment (P < 0.05). After 1 week in water, the larvae became less active and were clearly more turgid. This coincided with a decrease in survival from approximately 80% at the end of week 1 to nearly 50% by the end of week 2 (Fig. 4). By the end of week 4, only 30% of the larvae were still alive, and nearly all larvae died by week 5 (data not shown). This was the only treatment that caused mortality (also see Benoit et al. 2007). Only living larvae were used for our analysis of gene expression.
SmHsp, Hsp70, Hsp90, SOD, catalase, Mtn, p450, FAD, PLAP, fatty acyl CoA desaturase, actin, MSA, ZFP and pacifastin were expressed more highly when larvae were transferred from moist filter paper to water and held there for 10 days (Fig. 5). The changes in expression of these 14 genes were evident after 24 h of submersion in water, and these differences were still evident at 5 and 10 days; only day 10 data are shown. Among the genes evaluated, VATPase was the only gene that was not strongly upregulated by overhydration (Fig. 5f).
Discussion
One of the biggest challenges that an insect faces is maintaining water balance (Hadley 1994). Dehydration may lead to significant physiological injury including protein denaturation, nucleic acid damage and lipid peroxidation, among others (Hansen et al. 2006) and can ultimately result in death. The severe damage that dehydration generates is better managed preventively than by attempting to repair massive cellular damage, thus it is presumably advantageous to mount an early response to dehydration (Franca et al. 2007). In this study, we have identified several genes that respond to changes in the hydration state of the Antarctic midge, B. antarctica.
Heat shock proteins
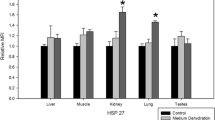
Heat shock proteins are constitutively expressed in the larvae of this midge (Rinehart et al. 2006), and our group previously found no further upregulation in response to dehydration (Hayward et al. 2007). We suspect that the discrepancy between our earlier results and the current dehydration response we note is due to the different methodologies used in our different studies. In our previous experiments, the larvae were held in water, a condition that our current experiments suggest results in overhydration. Although we still observed constitutive expression of Hsps in our current study when larvae were assayed immediately after collection from the substrate or held experimentally on moist paper towels, we now see that Hsps can be further upregulated when their hydration state is either increased (overhydration) or decreased (dehydration).
Hsps are upregulated in response to dehydration in the flesh fly, Sarcophaga crassipalpis (Tammariello et al. 1999; Hayward et al. 2004), the collembolan Folsomia candida (Bayley et al. 2001), the eutardigrade Richtersius coronifer (Jönsson and Schill 2007) and during rehydration in the flesh fly, S. crassipalpis (Hayward et al. 2004). In B. antarctica the smHsp and Hsp70 were expressed more highly during the long cryoprotective dehydration treatment than by either fast or slow dehydration. Even though cryoprotective dehydration represents a slower rate of water loss than our fast 12 h treatment, it seems to be a more stressful situation for larvae of B. antarctica, as evidenced by Hsp expression. Possibly the slow but constant loss of water during this treatment, in addition to the lower temperature, elicited a need for more chaperones. In addition, the stronger upregulation of the smHsp and Hsp70 when dehydration time increased from 12 to 36 h suggests the need for these proteins during prolonged dehydration, a result consistent with what we observed for cryoprotective dehydration. As larvae lose more water, protein and membrane damage is likely to increase, as has been observed in other organisms during water loss (Crowe et al. 1989, Franca et al. 2007), and hence the upregulation of the smHsp and Hsp70 during prolonged fast dehydration suggests that there is potentially more threat to protein integrity and function during prolonged (36 h) fast dehydration than during short (12 h) fast dehydration. Expression of Hsp90 was highest during fast dehydration and slow rehydration. Expression of different Hsps following dehydration and rehydration was previously observed in the flesh fly; a smHsp and Hsp70 were upregulated during dehydration, while Hsp90 and Hsc70 were upregulated during rehydration (Tammariello et al. 1999; Hayward et al. 2004).
Antioxidant enzymes
One of the most deleterious effects of dehydration in the cell is oxidative damage (Hermes-Lima and Zenteno-Savín 2002, Franca et al. 2007). Though the origin of the excess oxygen radicals is not fully understood, a tenfold increase in oxidation as a result of dehydration was recorded in yeast cells (Pereira et al. 2003). It is suspected that greater oxidation may play a major role in death caused by dehydration. Thus, protection against oxidation by enzymatic breakdown of oxygen radicals is crucial during water loss. A universal primary defense against oxygen toxicity is superoxide dismutase (SOD). SOD catalyzes the breakdown of the superoxide anion into hydrogen peroxide and oxygen. Hydrogen peroxide is then broken down to water and oxygen by several enzymes, the main one being catalase. Our previous work showed that SOD is highly expressed constitutively, as well as responsive to heat shock, freezing and anoxia in the larvae of B. antarctica (Lopez-Martinez et al. 2008). The upregulation of SOD in response to dehydration was greater than what we observed for freezing and anoxia, both of which lead to production of oxygen radicals during oxygen reperfusion (Storey and Storey 2009). Possibly the challenge of dehydration is stronger than the challenges of the other stresses previously tested. SOD was upregulated in response to dehydration in moss (Mayaba and Beckett 2003) and yeast (Pereira et al. 2003); in both of those systems SOD is thought to play a vital role in dehydration tolerance, and we suspect that this is the case with B. antarctica as well.
Catalase was also involved in the dehydration response of B. antarctica. Catalase is linked to dehydration in yeast (Franca et al. 2005), where a 70% increase in intracellular oxidation was observed in catalase mutants. Catalase rapidly breaks down high concentrations of hydrogen peroxide (Kranner and Birtić 2005). Thus, as water is lost and osmolality increases during dehydration in B. antarctica (Benoit et al. 2007), the increase in ROS may trigger a strong signal for catalase expression. Recently, catalase clones from the arctic collembolan, Onychiurus arcticus, were also isolated in higher numbers from desiccated individuals than from controls in an EST project (Clark et al. 2007). These results suggest that catalase plays an important role during dehydration, possibly by eliminating hydrogen peroxide molecules that can lead to lipid peroxidation. This role for catalase appears to be more critical during dehydration than during rehydration.
Detoxification
Metallothioneins (MTs) are a group of cystine-rich small proteins involved in heavy metal detoxification and cellular homeostasis. These proteins sequester heavy metals, sustain the balance of essential trace metals and maintain a reservoir for copper and zinc (Sato and Bremner 1993). MTs also have antioxidant capabilities by rapidly reacting with hydroxyl radicals with a higher affinity than to the superoxide anion (Thornalley and Vasäk 1985). This is especially important because there is no specific enzymatic defense against the most potent reactive oxygen species, the hydroxyl radicals (–OH). In addition to the response to dehydration that we report here, we have recorded elevated expression of Mtn2 in B. antarctica in response to freezing and anoxia (unpublished observation), stresses known to generate oxygen radicals. Thus, the upregulation of Mtn2 in response to dehydration is consistent with a role for Mtn2 in scavenging oxygen radicals during the process of water loss and maintaining homeostasis of trace metals as osmolality in the cell increases.
Cytochrome P450 monooxygenases, a huge family of enzymes involved in the metabolism of endogenous compounds and xenobiotics (Scott and Wen 2001), have been studied extensively (Feyereisen 1999; Li et al. 2007), but the complete extent of their function is still not fully known. The main reactions they catalyze can lead to reduction of harmful substances, including oxygen radicals, and this is possibly their role in response to fast dehydration.
Membrane restructuring
Fatty acid desaturases catalyze the synthesis of mono- and polyunsaturated fatty acids by introducing double bonds at specific locations in the saturated fatty acid molecules (Macartney et al. 1994). The Δ9 FAD that we cloned from B. antarctica introduces a double bond at the Δ9 position. In insects, changes in fatty acid composition of the membrane have been observed most frequently in response to low temperature and in relation to homeoviscous adaptation (Koštál et al. 2003; Kayukawa et al. 2007). However, in the soil collembolan Folsomia candida, a large increase in the proportion of mono-unsaturated fatty acids was also recorded in response to drought tolerance (Bayley et al. 2001). Thus the strong upregulation we see in response to fast dehydration and the consistent expression during rehydration indicates that membrane re-structuring likely occurs during dehydration. Given that oxygen radicals are increased by dehydration and that they target polyunsaturated fatty acids in the membrane, the activity of FAD, i.e., the increase in mono-unsaturated fatty acids in the membrane, may be important during dehydration to maintain membrane integrity.
Fatty Acyl CoA Δ9 desaturases are also involved in restructuring membranes, and these enzymes are essential for regulating membrane fluidity in eukaryotes (Eigenheer et al. 2002). Most functional studies of these enzymes suggest they play an important role in maintaining homeoviscous adaptation during low temperature exposure and acclimation by assisting in the membrane transition from the liquid crystalline to gel phase (Drobnis et al. 1993; Koštál et al. 2003). These desaturases also play a prominent role in pheromone synthesis (Rosenfield et al. 2001). However, their role during dehydration has not been explored previously; the most likely scenario is that upregulation of this gene during dehydration is related to homeoviscous adaptation or other forms of membrane reorganization. Membrane restructuring during water loss is crucial because, as oxygen radicals increase, the cell requires both a mechanism to prevent lipid peroxidation and the ability to restructure its membrane to prevent rupture.
Phospholipase A2 activating protein is the enzyme that activates phospholipase A2 (PLA2). Once activated this enzyme cleaves fatty acids at the sn2 position of phospholipids (Oliver et al. 1995). Normally it is polyunsaturated fatty acids, such as arachidonic acid, that are cleaved and then replaced with more saturated fatty acids (Clark et al. 1991). This is beneficial not only because it allows another fatty acid to enter the membrane to change its fluidity but also because it can prevent lipid peroxidation. One of the reactive oxygen species, singlet oxygen, can directly interact with polyunsaturated fatty acid side chains and initiate lipid peroxidation (Halliwell 1987). A membrane that has already been compromised by lipid peroxidation is more likely to undergo further damage by oxygen radicals (Hermes-Lima and Zenteno-Savín 2002, Franca et al. 2007). Therefore, the cleaving of side chains that will react with oxygen radicals is likely to be a key component of the dehydration survival mechanism used by this insect. Hence the upregulation of phospholipase A2 activating protein (PLAP), along with the desaturases, suggest that some membrane restructuring occurs in response to water loss. In such a scenario, we would not expect the upregulation of PLAP to occur in response to rehydration because the membrane may not need to again change its confirmation until the fully hydrated state is achieved. The expression of FAD, PLAP and fatty acyl CoA desaturase in response to dehydration suggests that a suite of enzymes is involved in protecting the cell and its membrane from damage due to water loss.
Cytoskeletal genes
Actin is a major component of the cytoskeleton; this ubiquitous protein is involved in filament formation and is crucial for cell motility and locomotion (Lovato et al. 2001). Like vertebrates, insects have muscle-specific actins (MSAs), and in larvae these MSAs form filaments that are found along the abdominal walls, in the head, and in the alimentary canal (Mounier and Prudhomme 1991). Actin is upregulated in response to dehydration in the nematode Steinernema feltiae, where it maintains the cell skeleton and rapidly forms filaments as the nematode shrinks (Chen et al. 2005). Thus, the high expression of actin and muscle-specific actin that we observe in this study of B. antarctica suggests that restructuring of both the cellular cytoskeleton and the locomotory muscles of the larvae occur in response to dehydration, rehydration and overhydration. A proteomics study recently completed by our group also shows increased abundance of actin and several other contractile proteins in response to dehydration and rehydration (Li et al. 2008), thus further validating the mRNA results reported here.
Other genes
The zinc-finger protein (ZFP) we found in B. antarctica may be responsive to stress in a similar fashion as other zinc-finger proteins reported in desiccation-tolerant plants (Sugano et al. 2003; Mukhopadhyay et al. 2004). The high expression of a zinc finger protein in petunias leads to an increase in drought tolerance and thus survival (Sugano et al. 2003). We also found that ZFP was upregulated in response to heat shock (1 h at 30°C), freezing (−5°C for 2 days) and anoxia (unpublished observation), thus it is possible that this particular ZFP gene is responsive to a wide variety of stresses and that it is involved in maintaining cellular homeostasis.
A relatively new family of serine protease inhibitors, the pacifastin family, has been found in arthropods (Simonet et al. 2003). The function of this family of protease inhibitors is unknown, but they have been implicated in the prophenoloxidase activating system (ProPO-AS), which is part of the immune system of arthropods and is involved in wound healing and melanotic encapsulation. Even though no activation of the ProPO-AS was observed in Schistocerca gregaria, the pacifastin peptides appear to contribute to the immune system (Franssens et al. 2008). Pacifastin peptides also have been found in the corpora cardiaca of Locusta migratoria, suggesting a possible neurosecretory function (Clynen et al. 2001). The upregulation of this peptide in response to desiccation has not been reported previously.
Vacuolar (H+) ATPases are proton pumps with a highly conserved structure (Kane 2006). Their main function is the acidification of certain organelles such as lysosomes, early and late endosomes, and the late Golgi apparatus (Mellman et al. 1986). This acidification is crucial for a range of cellular processes such as ion homeostasis, protein sorting and degradation (Kane 2006). More recently a link between oxidative stress and V-ATPase has been found. In vma (V-ATPase subunits) mutants an increase in the levels of reactive oxygen species was found, accompanied by protein damage (Kane 2007). This dual function of VATPases may be important during dehydration as they not only maintain acidification of the cell but also help counter the mass increase in reactive oxygen species that occurs during dehydration. As water re-enters the cell after dehydration, these pumps would presumably be active to maintain regular cellular function. Previously we found that this VATPase was upregulated in response to freezing and anoxia in B. antarctica (unpublished observation).
Overhydration
We are aware of no other studies examining molecular responses to overhydration in insects. We are not yet certain whether this elevation in water content represents an intracellular or extracellular increase, but we suspect that both are likely. Regardless of the location of this “extra water”, the larvae can tolerate this condition for up to a month. It is evident that starvation was not the cause of mortality because control larvae were unfed for the same period of time and did not die. Possible scenarios are that mortality was caused by ion depletion or the high energy expenditure of attempting to maintain water balance in distilled water through osmoregulation or the comprehensive gene expression profile seen here. The strong upregulation of many of the genes we evaluated suggests a massive defense strategy against an increase in body water. Many of these genes are likely to be instrumental in maintaining cellular homeostasis after the 10% increase in water content caused by submersion in water. Given that larvae of B. antarctica are commonly encountered in pools of rain water and ocean spray, their ability to defend themselves during events of overhydration is likely an important component of their survival strategy in the constantly changing environment of the Antarctic peninsula during the austral summer.
Conclusions
In summary, the fifteen genes that were monitored after dehydration, rehydration and overhydration define an array of processes that occur during changes in the hydration state and the cellular defenses that are mounted against water loss. As water rushes out of the cell as it does during dehydration (Hayward et al. 2007; Elnitsky et al. 2008a), maintaining normal cellular function is key to survival. The ability to sequester molecular chaperones such as Hsps may be important for maintaining proper functions of other cellular proteins. The cell membrane likely becomes re-structured and unsaturated by FAD, PLAP and fatty acyl CoA desaturases, while the antioxidant enzymes and metallothioneins scavenge oxygen radicals to prevent protein damage and lipid peroxidation in the membrane. Maintaining cellular integrity requires the rapid production of actin filaments. It is important that, during this process, the normal ionic balance of the cell is maintained by controlling cell acidity (VATPase) and metal homeostasis (Mtn). Collectively, these genes we have identified are likely to enable the midge larvae to respond to changes in hydration state that are prevalent during the short austral summer when the larvae are active and during the long Antarctic winter when larvae are encased in an icy matrix.
References
Bayley M, Petersen SO, Knigge T, Köhler HR, Holmstrup M (2001) Drought acclimation confers cold tolerance in the soil collembolan, Folsomia candida. J Insect Physiol 47:1197–1204
Benoit JB, Lopez-Martinez G, Elnitsky MA, Lee RE Jr, Denlinger DL (2007) Mechanisms to reduce dehydration stress in the Antarctic midge, Belgica antarctica. J Insect Physiol 53:656–667
Block W (1997) Ecophysiological strategies of terrestrial arthropods in the maritime Antarctic. In: Battaglia B, Valencia J, Walton DWH (eds) Antarctic communities: species, structure and survival. Cambridge University Press, Cambridge, pp 316–320
Chen S, Gollop N, Glazer I (2005) Cross-stress tolerance and expression of stress-related proteins in osmotically desiccated entomopathogenic Steinernema feltiae IS-6. Parasitology 131:695–703
Clark MA, Özgür LE, Conway TM, Dispoto J, Crooke ST, Bomalaski JS (1991) Cloning of a phospholipase A2-activating protein. Proc Natl Acad Sci USA 88:5418–5422
Clark MS, Thorne MA, Purać J, Grubor-Lajśić G, Kube M, Reinhardt R, Worland MR (2007) Surviving extreme polar winters by desiccation: clues from Arctic springtail (Onychiurus arcticus) EST libraries. BMC Genomics 8:475
Clynen E, Baggerman E, Veelaert D, Cerstiaens A, van der Horst D, Harthoorn L, Derua R, Waelkens E, de Loof A, Schoofs L (2001) Peptidomics of the pars intercerebralis-corpus cardiacum complex of the migratory locust, Locusta migratoria. Eur J Biochem 268:1929–1939
Crowe JH, McKersie BD, Crowe LM (1989) Effects of free fatty acids and transition temperature on the stability of dry liposomes. Biochim Biophys Acta 979:7–10
Drobnis EZ, Crowe LM, Berger T, Anchordoguy TJ, Overstreet JW, Crowe JH (1993) Cold shock damage is due to lipid phase transitions in cell membranes: a demonstration using sperm as a model. J Exp Zool 265:432–437
Eigenheer AL, Young S, Blomquist GJ, Borgeron CE, Tillman JA, Tittiger C (2002) Isolation and molecular characterization of Musca domestica delta-9 desaturase sequences. Insect Mol Biol 11:533–542
Elnitsky MA, Hayward SAL, Rinehart JP, Denlinger DL, Lee RE Jr (2008a) Cryoprotective dehydration and the resistance to inoculative freezing in the Antarctic midge, Belgica antarctica. J Exp Biol 211:524–530
Elnitsky MA, Benoit JB, Lopez-Martinez G, Denlinger DL, Lee RE Jr (2008b) Osmoregulation and salinity tolerance in the Antarctic midge, Belgica antarctica: seawater acclimation confers cross tolerance to freezing and dehydration (submitted)
Feyereisen R (1999) Insect P450 enzymes. Annu Rev Entomol 44:507–533
Franca MB, Panek AD, Eleutherio ECA (2005) The role of cytoplasmic catalase in dehydration tolerance of Saccharomyces cerevisiae. Cell Stress Chaperon 10:167–170
Franca MB, Panek AD, Eleutherio ECA (2007) Oxidative stress and its effect during dehydration. Comp Biochem Physiol A 146:621–631
Franssens V, Simonet G, Breuglemans B, Van Soest S, Van Hoef V, Vanden Broeck J (2008) The role of hemocytes, serine protease inhibitors and pathogen-associated patterns in prophenoloxidase activation in the desert locust, Schistocerca gregaria. Peptides 29:235–241
Gressitt JL (1967) Notes on arthropod populations in the Antarctic Peninsula–South Shetland Islands–South Orkney Islands area. In: Gressitt JL (ed) Entomology of Antarctica. The Horn-Shafer Company, Maryland, pp 373–392
Hadley NF (1994) Water relations of terrestrial arthropods. Academic Press, New York
Halliwell B (1987) Oxidative damage, lipid peroxidation and antioxidant protection in chloroplasts. Chem Phys Lipids 44:327–340
Hansen JM, Go YM, Jones DP (2006) Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol 46:215–234
Hassell KL, Kefford BJ, Nugegoda D (2006) Sub-lethal and chronic salinity tolerances of three freshwater insects: Cloeon sp. and Centroptilum sp. (Ephemeroptera: Baetidae) and Chironomus sp. (Diptera: Chironimidae). J Exp Biol 209:4024–4032
Hayward SAL, Rinehart JP, Denlinger DL (2004) Desiccation and rehydration elicit distinct heat shock protein transcript response in flesh fly pupae. J Exp Biol 207:963–971
Hayward SAL, Rinehart JP, Sandro LH, Lee RE Jr, Denlinger DL (2007) Slow dehydration promotes desiccation and freeze tolerance in the Antarctic midge Belgica antarctica. J Exp Biol 210:836–844
Hermes-Lima M, Zenteno-Savín T (2002) Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp Biochem Physiol C 133:537–556
Jönsson KI, Schill RO (2007) Induction of Hsp70 by desiccation, ionizing radiation and heat-shock in the eutardigrade Richtersius coronifer. Comp Biochem Physiol B 146:456–460
Kane PM (2006) The where, when, and how of organelle acidification by the yeast vacuolar H+-ATPase. Microbiol Mol Biol Rev 70:177–191
Kane PM (2007) The long physiological reach of the yeast vacuolar H+-ATPase. J Bioenerg Biomembr 39:415–421
Kayukawa T, Chen B, Hoshizaki S, Ishikawa I (2007) Upregulation of a desaturase is associated with the enhancement of cold hardiness in the onion maggot, Delia antiqua. Insect Biochem Mol Biol 37:1160–1167
Koštál V, Berková P, Šimek P (2003) Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae of Chymomyza costata (Drosophilidae). Comp Biochem Physiol B 135:407–419
Kranner I, Birtić S (2005) A modulating role for antioxidants in desiccation tolerance. Integr Comp Biol 45:734–740
Li X, Schuler MA, Berenbaum MR (2007) Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol 52:231–253
Li A, Benoit JB, Lopez-Martinez G, Elnitsky MA, Lee RE Jr, Denlinger DL (2008) Distinct contractile and cytoskeletal protein patterns in the Antarctic midge are elicited by desiccation and rehydration (submitted)
Lopez-Martinez G, Elnitsky MA, Benoit JB, Lee RE Jr, Denlinger DL (2008) Elevated expression of the gene encoding superoxide dismutase and high resistance to oxidative damage in the Antarctic midge, Belgica antarctica. Insect Biochem Mol Biol 38:796–804
Lovato TL, Meadows SM, Baker PW, Sparrow JC, Cripps RM (2001) Characterization of muscle specific actin genes in Drosophila virilis reveals significant molecular complexity in skeletal muscle types. Insect Mol Biol 10:333–340
Macartney A, Maresca B, Cossins AR (1994) Acyl-CoA desaturases and the adaptive regulation of membrane lipid composition. In: Cossins AR (ed) Temperature adaptation of biological membranes. Portland Press, London, pp 129–139
Mayaba N, Beckett RP (2003) Increased activities of superoxide dismutase and catalase are not the mechanism of desiccation tolerance induced by hardening in the moss Atrichum androgynum. J Bryol 25:281–286
Mellman I, Fuchs R, Helenius A (1986) Acidification of the endocytic and exocytic pathways. Annu Rev Biochem 55:663–700
Mounier N, Prudhomme JC (1991) Differential expression of muscle and cytoplasmic actin genes during development of Bombyx mori. Insect Biochem 21:523–533
Mukhopadhyay A, Vij S, Tyagi AK (2004) Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc Natl Acad Sci USA 101:6309–6314
Nondula N, Marshall DJ, Baxter R, Sinclair BJ, Chown SL (2004) Life history and osmoregulatory ability of Telmatogeton amphibious (Diptera: Chironomidae) at Marion Island. Polar Biol 27:629–635
Oliver AE, Fisk E, Crowe LM, de Araujo PS, Crowe JH (1995) Phospholipase A2 activity in dehydrated systems: effect of physical state of substrate. Biochim Biophys Acta 1267:92–100
Peckham V (1971) Notes on the chironomid midge Belgica antarctica Jacobs at Anvers Island in the maritime Antarctic. Pac Insects 25:145–166
Pereira ED, Panek AD, Eleutherio ECA (2003) Protection against oxidation during dehydration of yeast. Cell Stress Chaperon 8:120–124
Rinehart JP, Hayward SAL, Elnitsky MA, Sandro LH, Lee RE Jr, Denlinger DL (2006) Continuous up-regulation of heat shock proteins in larvae, but not adults, of a polar insect. Proc Natl Acad Sci USA 103:14223–14227
Rosenfield C, Man You K, Marsella-Herrick P, Roelofs WL, Knipple DC (2001) Structural and functional conservation and divergence among acyl-CoA desaturases of two noctuid species, the corn earworm, Helicoverpa zea, and the cabbage looper, Trichoplusia ni. Insect Biochem Mol Biol 31:949–964
Sato M, Bremner I (1993) Oxygen free radicals and metallothionein. Free Radical Bio Med 14:325–337
Scott JG, Wen Z (2001) Cytochromes P450 of insects: tip of the iceberg. Pest Manag Sci 57:958–967
Simonet G, Claets I, Franssens V, De Loof A, Vanden Boeck J (2003) Genomics, evolution and biological functions of the pacifastin peptide family: a conserved serine protease inhibitor family in arthropods. Peptides 24:1633–1644
Storey KB, Storey JM (2009) Oxygen: stress and adaptation in cold hardy insects. In: Denlinger DL, Lee RE Jr (eds) Low temperature biology of insects. Cambridge University Press, Cambridge (in press)
Suemoto T, Kawai K, Imabayashi H (2004) A comparison of desiccation tolerance among 12 species of chironomid larvae. Hydrobiologia 515:107–114
Sugano S, Kaminaka H, Rybka Z, Catala R, Salinas J, Matsui K, Ohme-Takagi M, Takatsuji H (2003) Stress-responsive zinc finger protein ZPT2–3 plays a role in drought tolerance in petunia. Plant J 36:830–841
Sugg P, Edwards JS, Baust J (1983) Phenology and life history of Belgica antarctica, an Antarctic midge (Diptera: Chironomidae). Ecol Entomol 8:105–113
Tammariello SP, Rinehart JP, Denlinger DL (1999) Desiccation elicits heat shock protein transcription in the flesh fly, Sarcophaga crassipalpis, but does not enhance tolerance to high or low temperatures. J Insect Physiol 45:933–938
Thornalley PJ, Vasäk M (1985) Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanisms of its reaction with superoxide and hydroxyl radicals. Biochim Biophys Acta 827:36–44
Winston PW, Bates DS (1960) Saturated salt solutions for the control of humidity in biological research. Ecology 41:232–237
Acknowledgments
This research was funded by NSF grants OPP-0337656 and OPP-0413786. We thank the staff at Palmer Station, Antarctica whose support was deeply valued.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. V. Carey.
Rights and permissions
About this article
Cite this article
Lopez-Martinez, G., Benoit, J.B., Rinehart, J.P. et al. Dehydration, rehydration, and overhydration alter patterns of gene expression in the Antarctic midge, Belgica antarctica . J Comp Physiol B 179, 481–491 (2009). https://doi.org/10.1007/s00360-008-0334-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-008-0334-0