Abstract
The green sturgeon is a long-lived, highly migratory species with populations that are currently listed as threatened. Their anadromous life history requires that they make osmo- and ionoregulatory adjustments in order to maintain a consistent internal milieu as they move between fresh-, brackish-, and seawater. We acclimated juvenile green sturgeon (121 ± 10.0 g) to 0 (freshwater; FW), 15 (estuarine; EST), and 24 g/l (SF Bay water; BAY) at 18°C for 2 weeks and measured the physiological and biochemical responses with respect to osmo- and ionoregulatory mechanisms. Plasma osmolality in EST- and BAY-acclimated sturgeon was elevated relative to FW-acclimated sturgeon (P < 0.01), but there was no difference in muscle water content or abundance of stress proteins. Branchial Na+, K+-ATPase (NKA) activity was also unchanged, but abundance within mitochondrion-rich cells (MRC) was greater in BAY-acclimated sturgeon (P < 0.01). FW-acclimated sturgeon had the greatest NKA abundance when assessed at the level of the entire tissue (P < 0.01), but there were no differences in v-type H+ATPase (VHA) activity or abundance between salinities. The Na+, K+, 2Cl− co-transporter (NKCC) was present in FW-acclimated sturgeon gills, but the overall abundance was lower relative to sturgeon in EST or BAY water (P < 0.01) where this enzyme is crucial to hypoosmoregulation. Branchial caspase 3/7 activity was significantly affected by acclimation salinity (P < 0.05) where the overall trend was for activity to increase with salinity as has been commonly observed in teleosts. Sturgeon of this age/size class were able to survive and acclimate following a salinity transfer with minimal signs of osmotic stress. The presence of the NKCC in FW-acclimated sturgeon may indicate the development of SW-readiness at this age/size.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The green sturgeon (Acipenser medirostris) is a rare chondrostean species with a wide distribution, inhabiting river, estuarine (EST), and marine systems along western North America (Moyle 2002). Within this range they are separated into two distinct population segments (DPS). The northern includes sturgeon that spawn within the Klamath and Rogue Rivers of California and Oregon, and the southern is comprised of sturgeon that spawn within the Sacramento River system of California (Moyle 2002). Green sturgeon hatch in freshwater (FW) where they can spend up to 3 years within river and EST habitats. As adults, they migrate to open ocean, where they are thought to spend the winter months (Lindley et al. 2008). During spring, green sturgeon adults re-enter estuaries, where they may remain for the summer or continue upstream to FW for spawning (Lindley et al. 2008). Because of their migratory patterns, they are considered to be the most anadromous of the sturgeons (Kelly et al. 2007), although few studies have investigated their osmoregulatory mechanisms (Allen and Cech 2007). Unfortunately, both green sturgeon DPS are currently in difficulty, with the northern DPS listed as a species of special concern and the southern DPS as a threatened species under the US Endangered Species Act; such classifications have made experimental investigations difficult.
The migratory nature of this species across a range of salinities requires that they be able to make physiological adjustments in order to maintain a consistent internal milieu. Although typical plasma osmolality values for various sturgeon species including the green sturgeon are lower than those reported for teleost fishes (Allen and Cech 2007; McEnroe and Cech 1985), an osmotic gradient exists with the ambient environment whether they inhabit FW or seawater (SW). There has been some previous work showing that sturgeon generally have similar morphology and biochemical mechanisms to teleosts with respect to osmo- and ionoregulation. Carmona et al. (2004) showed that mitochondrion-rich cells (MRC) were larger and more abundant in SW-acclimated Adriatic sturgeon (Acipenser naccarii) relative to those in FW, and Martinez-Alvarez et al. (2005) showed that Na+, K+-ATPase (NKA) activity increased in response to SW transfer. NKA is a basolateral membrane-bound enzyme that is found in high abundance within MRCs, where it serves as the driving force for ion excretion or absorption at the expense of ATP in SW- or FW-acclimated fishes, respectively (Marshall 2002; Perry 1997). In Adriatic sturgeon, the observed increase in MRC size was attributed to an enlargement of the basolateral tubular reticulum (Carmona et al. 2004), which has been closely associated with an increase in the capacity of NKA in other species (Ernst et al. 1980). What has been missing from the sturgeon literature however, is a characterization of other osmo- and ionoregulatory-associated proteins such as v-type H+-ATPase (VHA), an apical transporter thought to play a crucial role in the absorption of Na+ in FW-acclimated fishes, or the Na+, K+, 2Cl− co-transporter (NKCC) a basolateral membrane-bound protein within the MRCs of SW-acclimated fishes that uses the Na+ gradient generated by NKA to drive Cl− into the MRC where it can then diffuse through apical channels and into the environment (Marshall 2002). Characterization of the level of apoptosis in sturgeon would also be useful as an indicator of cellular turnover rate, which has been shown to increase with SW exposure in other species (van der Heijden et al. 1999; Wendelaar Bonga and van der Meij 1989).
The purpose of this study was to investigate the osmo- and ionoregulatory abilities of the green sturgeon using a comprehensive characterization of physiological and biochemical variables. We measured plasma osmolality and muscle hydration to assess the level of osmotic stress resulting from salinity acclimation, as well as the activities of NKA and VHA and caspase 3/7. NKA and VHA are well-documented enzymes involved in the mechanisms of FW and SW acclimation, and caspase 3/7 was used as a characteristic enzyme of cellular apoptosis. Finally we used tissue microarrays (TMA) to assess the abundance of several proteins in the branchial epithelium, such as NKA, VHA, NKCC, heat shock proteins (HSP) 60, 70, and 90, and ubiquitin. HSPs and ubiquitin are good indicators of cellular stress and were used as indicators for damaged proteins being salvaged or destroyed, respectively. The measurement of these variables using TMAs offered a more rapid, higher throughput, method for protein quantification, while reducing the overall variation from sample to sample. The benefits of this technique have previously been described by Lima and Kültz (2004) and Sardella et al. (2008).
Materials and methods
Fish
Adult green sturgeon that had been housed at the UC Davis Center for Aquatic Biology and Aquaculture were artificially spawned in the spring of 2007. To our knowledge this was the first successful spawning effort of this species from a captive brood stock. The resulting progeny were reared in FW and grown to a size of 121 ± 10.0 g (approximately 6 months in age) before experiments were conducted. Eighteen individuals were removed from the flow-through rearing tanks and divided equally into three 425 l FW recirculation systems at the start of the experiment. Fish were acclimated to three salinities FW (0 g/l), estuary water (EST; 15 g/l), and San Francisco Bay water (24 g/l; BAY) in stepwise fashion. Salinity was increased via 50% water change by 4 g/l per day until reaching target salinity, and then held for 2 weeks to allow sturgeon to acclimate (Altinok et al. 1998). Recirculating systems were filtered by mechanical, chemical, and biological filters so ammonia accumulation was minimal. Sturgeon were fed by slow-timed automatic feeders that kept small quantities of food constantly available. Salinity was manipulated using Deep Ocean synthetic sea salt and measured with a light refractometer. After 2 weeks of acclimation, sturgeon were killed in accordance with UCD IUCAC-approved methods (# 12649) by lethal dose of MS-222 and spinal transaction.
Sampling protocol
Blood was quickly collected directly into heparinized capillary tubes after severing the tail. After blood collection, we perfused the ventral aorta with ice-cold phosphate buffered saline (PBS) in order to clear blood from the gills. Gills were then dissected, the second arch was rapidly frozen in liquid nitrogen, and the third arch was fixed with PBS-buffered paraformaldehyde (4%). To determine muscle hydration, a piece of the dorsal epaxial muscle (approximately 0.5 g) was dissected and the skin was removed. The muscle was washed with distilled water quickly, patted dry and placed into pre-weighed aluminum foil to dry for 96 h. The difference in weight before and after drying was expressed as percent water as described by Sardella et al. (2004a).
Enzyme assays
Gill tissue was homogenized in 1 ml of SEID buffer (250 mM sucrose, 10 mM EDTA Na2, 50 mM imidazole, pH 7.3, deoxycholic acid (0.05%)). NKA activity was measured as the ouabain-inhibited fraction of total ATP hydrolysis as described by McCormick (1993) and expressed as μmol of ADP per hour. VHA activity was measured in similar fashion to NKA, with ouabain present in all VHA assays and the activity measured as the fraction of ATP hydrolysis inhibited by N-ethylmaleimide (NEM) as described by Lin and Randall (1993). Gill caspase 3/7 activity was quantified using the Promega CaspaseGlo 3/7 luminescent assay. Crude homogenates were diluted 100× with buffer (10 mM Tris–HCl 7.5, 100 mM NaCl, 0.1 mM EDTA, and 0.2% Triton-X), and mixed 50:50 with CaspaseGlo reagent in triplicate using a 96-well microplate. Assays were incubated at room temperature (~20°C) for 1 h before measuring the relative luminescence. All enzyme assays were standardized by total protein, which was measured as absorbance at 560 nm following 30 min of incubation at 37°C with a 50:1 solution of bicinchoninic acid to CuSO4.
Tissue microarray construction
Using the method originally described by Lima and Kültz (2004), fixed gill tissue was embedded in paraffin using a Tissue Tek vacuum infiltration processor (Sakura Finetek, Torrance, CA, USA). Paraffin blocks were constructed with a Tissue Tek tissue embedding center (Sakura Finetek, Torrance, CA, USA). One millimeter wax cores were removed from an empty paraffin block using an MTA-1 tissue microarrayer (Beecher Instruments, Sun Prairie WI, USA), and filled with cores taken from embedded gill tissue. Gill tissue cores from all treatment groups were inserted into recipient block, which was then sectioned at 4 μm using a Bromma 2218 Historange microtome (LKB, Uppsala, Sweden). Sections were floated on to a poly-lysine coated glass microscope slide, and slides were dried overnight at 44°C.
Sections were blocked with PBS containing 1% bovine serum albumin (PBA) for 30 min, followed by 60 min of incubation in PBA containing the various primary antibodies. We used antibodies to measure the abundances of NKA, VHA, NKCC, HSP 60, 70, and 90, and ubiquitin. Antibodies to NKA (α5) and NKCC (T4) were purchased from the University of Iowa Developmental Studies Hybridoma Bank and were diluted with PBA by 1:100 and 1:200, respectively. VHA, HSP, and ubiquitin antibodies were purchased from Sigma. Anti-VHA antibody was diluted 1:1,000 with PBA while all HSP antibodies as well as the anti-ubiquitin antibodies were diluted 1:5,000 with PBA. α5-stained sections were incubated with a secondary goat anti-mouse antibody covalently bound to Pacific Blue (Molecular Probes, Eugene, OR, USA) that was diluted 1:100 with PBA. The remaining slides were stained with a secondary goat antibody covalently bound to Alexa 688 (Molecular Probes, Eugene, OR, USA) that were diluted 1:100 with PBA. After 60 min of incubation in secondary antibody with 5 μl RNase, slides were rinsed with PBS and counterstained for 10 min with propidium iodide, which was diluted 1:500 with distilled water.
Tissue microarray analysis
A laser scanning cytometer was used to quantify protein concentrations per unit area within individual branchial MRCs and within randomly assigned areas of tissue on TMAs (Phantoms) as described by Sardella et al. (2008). Immunohistochemical analysis of TMAs was conducted using a 40× objective (UPlanFL 40×/0.75/∞/0.17, Olympus, Melville, NY, USA) in combination with a UV laser (400 nm). WinCyte CompuColor was used to generate images by which the cells stained with Alexa 688 appeared green and those stained with Pacific Blue appeared blue. Contouring and event detection for TMAs was optimized as described in Lima and Kültz (2004) and Sardella et al. (2008), and protein concentrations are presented as relative fluorescent units (RFU).
Statistical analysis
Data was analyzed using a one-way analysis of variance (ANOVA), followed by a post hoc Tukey’s HSD test when treatment means were significantly different (α = 0.05; n = 6). Statistical tests were performed using SigmaStat version 3.0 and plots were made using SigmaPlot version 9.0.
Results
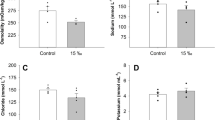
Green sturgeon survived all salinity transfers and acclimations throughout the experiment. There was a significant difference in plasma osmolality with acclimation salinity, where EST- and BAY-acclimated sturgeon showed an approximate 10% increase relative to FW-acclimated sturgeon (P < 0.01; Fig. 1a), but there was no difference in muscle water content (Fig. 1b). NKA was detected by fluorescence of Pacific Blue and was abundant within distinct large cells (MRCs) along the filamental and lamellar regions. Both VHA and NKCC were detected by Alexa 688 fluorescence and NKCC was localized similar to NKA within throughout the branchium within easily distinguished MRCs. NKA, NKCC, and VHA were present in all gill sections regardless of acclimation salinity (Fig. 2).
The effect of acclimation salinity on a plasma osmolality and b muscle water content of green sturgeon in freshwater (FW), estuary water (EST), and SF Bay water (BAY). Data are presented as means ± SE. Letters denote significant differences between salinities as determined by one-way ANOVA (P < 0.05; n = 6)
There was no significant difference in NKA activity between the acclimation salinities (Fig. 3a), but the NKA abundance within individual MRCs increased by 11% in BAY-acclimated sturgeon (P < 0.01; Fig. 3b). FW-acclimated sturgeon had the greatest NKA abundance when assessed at the level of the entire tissue; abundance was nearly 16% lower in EST- and BAY-acclimated groups (P < 0.01; Fig. 3c). There were no significant differences detected in VHA activity or abundance with acclimation salinity (Fig. 4). NKCC was present in FW-acclimated sturgeon (Fig. 5a), but the overall abundance was reduced by approximately 22% relative to sturgeon in EST or Bay water (P < 0.01; Fig. 5b). Caspase 3/7 activity was significantly affected by acclimation salinity (P < 0.05; Fig. 6), but differences were not detected by the post-hoc Tukey’s HSD test. The overall trend was for activity to increase with salinity.
The effect of acclimation salinity on green sturgeon a NKA activity, b MRC NKA abundance, and c total NKA abundance. Microarray data is presented as relative fluorescent units (RFU), see Fig. 1 for statistical details
The effect of acclimation salinity on green sturgeon a VHA activity, b MRC VHA abundance, and c total VHA abundance. Microarray data is presented as relative fluorescent units (RFU), see Fig. 1 for statistical details
a Immunohistochemical plate of a FW-acclimated green sturgeon gill stained with anti-NKCC antibody, counterstained with the nuclear stain propidium iodide, and b the effect of acclimation salinity of total gill NKCC abundance. Microarray data is presented as relative fluorescent units (RFU), see Fig. 1 for statistical details
The effect of acclimation salinity on green sturgeon gill caspase 3/7 activity. See Fig. 1 for statistical details
Finally, there were no significant differences in the abundance of HSP 60, 70, or 90 with salinity acclimation. HSPs were detected on TMAs using Alexa 688 and no differences were seen within MRCs or when assessed along the epithelium as a whole. There was also no difference in the abundance of ubiquitin, which similar to HSPs was present in all salinities to some degree and detected by Alexa 688 fluorescence (data not shown).
Discussion
As has been observed previously by Allen and Cech (2007), green sturgeon of this age/size class were able to acclimate to salinities greater than FW. Despite 100% survival, plasma osmolality was significantly increased in sturgeon acclimated to EST or BAY water, relative to those in FW (Fig. 1a). This difference in osmolality was similar to what was observed by Allen and Cech (2007) for individuals of this size, and fits within a pattern where the differences between salinities tend to decrease with size. Based on our data and those of Allen and Cech (2007), we speculate that the differences in plasma osmolality would become more subtle as the fish continued to grow and would ultimately not be observed. An increased plasma osmolality in sturgeon of this size class may be typical for this species and not necessarily a detrimental effect; it also remains a possibility that it may be crucial in driving the upregulation of mechanisms for SW acclimation. The elevated osmolality did not appear to be stressful to the fish, as there were no increases in HSP or ubiquitin abundances in the EST or BAY group. Moreover, HSP 70 expression, which is commonly associated with osmotic stress (Werner et al. 2007), remained unchanged despite the higher plasma osmolality. The increased plasma osmolality within the EST and BAY sturgeon also did not correspond with a decrease in muscle water content, which remained consistent between the salinity treatments (Fig. 1b).
We measured the abundances of NKA, VHA, and NKCC in green sturgeon gills and all were present regardless of acclimation salinity (Fig. 2). NKA was present in abundance along the branchial epithelium (blue fluorescence) and can be used to clearly distinguish MRCs. These cells were located along the filamental epithelium as has been described for many species (Carmona et al. 2004; Katoh and Kaneko 2003; Lima and Kültz 2004; Wendelaar Bonga and van der Meij 1989), as well as along the secondary lamellae, which has been seen in this and other sturgeon species previously (Allen and Cech 2007; Carmona et al. 2004). NKA is known to drive both ion absorption and excretion by dramatically different mechanisms in FW- and SW-acclimated fishes, respectively (Marshall 2002; Perry 1997). Our results indicate that this enzyme was present in greater abundance within the MRCs of BAY-acclimated sturgeon relative to those in FW (Fig. 3a). This has also been observed using TMAs from Mozambique tilapia, which is a commonly used teleost model for osmoregulatory studies (Sardella et al. 2008). While the MRCs of the BAY-acclimated sturgeon had more NKA per unit area, the FW-acclimated sturgeon had a greater abundance of NKA within the epithelium as a whole. A greater abundance in FW-acclimated sturgeon may result from the presence of more than one MRC type. The presence of at least two distinct MRC types has been described for FW-acclimated teleosts (Goss et al. 2001), both of which are NKA abundant; this is likely the case for green sturgeon as well. We did not conduct counts of MRCs, but it has been shown in tilapia that FW individuals had more MRCs, while SW had fewer but larger MRCs (Sardella et al. 2008). Despite the different patterns of abundance, we did not detect any difference in the activity of this enzyme with salinity (Fig. 3c), which was also observed between FW and SW-acclimated shortnosed sturgeon (A. brevirostrum) (Jarvis and Ballantyne 2003; Rodriguez et al. 2003), however SW-acclimated Siberian sturgeon (A. baerii) have been shown to have a greater activity relative to those in FW. Regression analysis between abundance pattern and activity pattern of individual sturgeon revealed no significant relationship (not presented). Direct correlation between abundance and in vitro activity may be unlikely, however, as these measurements of activity represent V max of NKA and do not necessarily reflect in vivo activity at physiological temperature and osmolality. Furthermore, abundance as determined by TMA does not take into account if the enzyme is in active form, as dissociated α sub-units and covalently modified enzymes (phosphorylated) are also labeled by the α5 antibody. Lastly, it is also reasonable to conclude that NKA activity patterns vary with species and most likely individual size.
The VHA data was more difficult to interpret, and it remains unclear what role this transporter has in sturgeon gills. VHA is one potential mechanism for Na+ absorption in FW-acclimated fishes, whereby the transport of protons at the expense of ATP, provides an electromotive force to drive Na+ movement through an apical Na+ channel (Marshall 2002; Perry 1997). Furthermore, it has been shown previously that the activity of this enzyme decreased with SW-exposure in rainbow trout (Oncorhynchus mykiss) (Lin and Randall 1993). Our data does not indicate that this mechanism was crucial to green sturgeon Na+ absorption in this study, as the abundance and activity of VHA were unchanged among the salinity groups (Fig. 4). The role of VHA in acid–base regulation, and the possibility that an apical Na+/H+-exchanger (NHE) may be present in sturgeon gills, further complicated drawing conclusions from these data. More experiments are needed to elucidate the role of VHA in sturgeon, in addition to characterizing their mechanism of Na+ absorption.
The role of NKCC has been much better defined and when present on the basolateral membrane of MRCs has been characterized as part of the mechanism for ion excretion in SW-acclimated fishes (Marshall 2002). In this model, the Na+ gradient produced by NKA is used to drive two Cl− into the MRC where they diffuse though an apical Cl− channel and into the environment (Marshall 2002). Much like the NKA-labeled slides, NKCC could be used to define MRCs along both the filament and lamellae. NKCC was present within the MRCs of sturgeon regardless of acclimation salinity, although the abundance was greatest in EST- and BAY-acclimated fish (Fig. 5b). It is not surprising that the EST- and BAY-acclimated sturgeon had a greater abundance of NKCC as the function of this transporter is crucial to hypoosmoregulation. The presence of this transporter in sturgeon that had been reared and held in FW may indicate that at this age/size, sturgeon are pre-acclimating to SW, or at least for entry into water of variable salinity such as an estuary. Similar results have been observed in the anadromous Atlantic salmon (Salmo salar) where there was essentially no difference between FW and SW individuals with respect to NKCC abundance (Hiroi and McCormick 2007). Furthermore, injection of cortisol and growth hormone, which have been associated with salmonid smoltification, resulted in an upregulation of NKCC in FW-acclimated Atlantic salmon (Pelis and McCormick 2001a, b). There have been previous discussions of how age/size affects SW-readiness in sturgeon (Allen and Cech 2007; McEnroe and Cech 1985; McKenzie et al. 2001; Rodriguez et al. 2003), and the age of initial expression of NKCC may provide some insight into the precise age at which sturgeon become SW-ready.
Based on caspase 3/7 activity and similar to what has been observed in teleosts transferred from FW to SW, it appears that the rate of apoptosis is greater in green sturgeon acclimated to BAY water. Apoptosis is typically associated with the turnover rate of the branchial epithelium (Sardella et al. 2004b; Wendelaar Bonga and van der Meij 1989), and it has been shown that cellular turnover is accelerated in SW versus FW-acclimated individuals (Wendelaar Bonga and van der Meij 1989). There is considerable work to be done on elucidating the cellular restructuring of the epithelium of green sturgeon, but we have presented some basal evidence that mechanisms similar to those of euryhaline teleosts may be in place. Anadromy is observed across a very wide range of species, including very primitive groups such as lamprey (Agnatha). It has been previously suggested that the mechanistic similarity between groups that evolved in SW (lamprey) and those that are thought to have evolved in FW (Osteichthyes) indicates a convergent evolution with respect to these mechanisms (Wright 2007).
In summary, green sturgeon were capable of surviving and acclimating to EST and BAY water at this age/size range, and defended both the plasma and intracellular compartments. We have shown the extent to which osmo- and ionoregulatory mechanisms are developed in 6 month old sturgeon following salinity acclimations, and it appears that sturgeon mechanisms are quite similar to that of teleosts with respect to the typical transporters involved, although the role of VHA versus NHE remains to be determined. Finally, of the bioindicators of possible SW-readiness that we have characterized, expression of NKCC on the basolateral membrane of MRCs was the most intriguing, and it would be interesting to determine when this protein is first synthesized in order to characterize the precise age/size at which sturgeon are capable of tolerating SW transfers.
Abbreviations
- DPS:
-
Distinct population segment
- TMA:
-
Tissue microarray
- LSC:
-
Laser scanning cytometry
- NKA:
-
Na+, K+-ATPase
- NKCC:
-
Na+, K+, 2Cl− co-transporter
- VHA:
-
Vacuolar-type H+-ATPase
- MRC:
-
Mitochondrion-rich cell
- g/l:
-
Grams per liter
- °C:
-
Degrees celsius
- FW:
-
Freshwater
- SW:
-
Seawater
- EST:
-
Estuary water
- BAY:
-
SF Bay water
- NEM:
-
N-Ethylmaleimide
References
Allen PJ, Cech JJ (2007) Age/size effects on juvenile green sturgeon (Acipenser medirostris), oxygen consumption, growth, and osmoregulation in saline environments. Environ Biol Fish 79:211–229
Altinok I, Galli SM, Chapman FA (1998) Ionic and osmotic regulation capabilities of juvenile Gulf of Mexico sturgeon (Acipenser oxyrinchis de sotoi). Comp Biochem Physiol 120A:609–616
Carmona R, Garcia-Gallego M, Sanz A, Domezain A, Ostos-Garrido MV (2004) Chloride cells and pavement cells in gill epithelia of Acipenser naccarii: ultra structural modifications in seawater-acclimated specimens. J Fish Biol 64:553–566
Ernst SA, Riddle CV, Karnaky KJ (1980) Relationship between localization of Na+, K+-ATPase, cellular fine-structure, and reabsorptive and secretory electrolyte transport. Curr Top Memb Trans 13:355–385
Goss GG, Adamia S, Galvez F (2001) Peanut lectin binds to a subpopulation of mitochondria-rich cells in the rainbow trout epithelium. Amer J Physiol 281:R1718–R1725
Hiroi J, McCormick SD (2007) Variation in salinity tolerance, gill Na+/K+-ATPase, Na+/K+/2Cl− co-transporter and mitochondria-rich cell distribution in three salmonids Salvelinus namaycush, Salvelinus fontinalis and Salmo salar. J Exp Biol 210:1015–1024
Jarvis PL, Ballantyne JS (2003) Metabolic responses to salinity acclimation in juvenile short-nosed sturgeon Acipenser brevirostrum. Aquaculture 219:891–909
Katoh F, Kaneko T (2003) Short-term and long-term replacement of branchial chloride cells in killifish transferred from seawater to freshwater, revealed by morphofunctional observations and a newly established ‘time-differential double fluorescent staining’ technique. J Exp Biol 206:4113–4123
Kelly JT, Klimley AP, Crocker CE (2007) Movements of green sturgeon, Acipenser medirostris, in the San Francisco Bay estuary, California. Environ Biol Fish 79:281–295
Lima R, Kültz D (2004) Laser scanning cytometry and tissue microarray analysis of salinity effects on killifish chloride cells. J Exp Biol 207:1729–1739
Lin H, Randall DJ (1993) H+-ATPase activity in crude homogenates of fish gill tissue: Inhibitor sensitivity and environmental and hormonal regulation. J Exp Biol 180:163–174
Lindley TE, Moser ML, Erickson DI, Belchik M, Welch DW, Rechisky EL (2008) Marine migration of North American green sturgeon. Trans Am Fish Soc 137:182–194
Marshall WS (2002) Na+, Cl−, Ca2+, and Zn2+ transport by fish gills: a retrospective review and prospective synthesis. J Exp Zool 293:264–283
Martinez-Alvarez RM, Sanz A, Garcia-Gallego M, Domezain A, Domezain J, Carmona R, Ostos-Garrido MV, Morales AE (2005) Adaptive branchial mechanisms in the sturgeon Acipenser naccarii during acclimation to seawater. Comp Biochem Physiol A 141:183–190
McCormick SD (1993) Methods for non-lethal gill biopsy and measurement of Na+, K+-ATPase activity. Can J Fish Aquat Sci 50:656–658
McEnroe M, Cech JJ (1985) Osmoregulation in the juvenile and adult white sturgeon, Acipenser transmontanus. Environ Biol Fish 14:23–30
McKenzie DJ, Cataldi E, Romano P, Owen SF, Taylor EW, Bronzi P (2001) Effects of acclimation to brackish water in the growth, respiratory metabolism, and swimming performance of young-of-the-year Adriatic sturgeon (Acipenser naccarii). Can J Fish Aquat Sci 58:1104–1112
Moyle PB (2002) Inland fishes of California. University of California Press, Berkely
Pelis RM, McCormick SS (2001a) Effects of growth hormone and cortisol on Na+, K+, 2Cl− co-transporter localization and abundance in the gills of Atlantic salmon. Gen Comp Endoc 124:134–143
Pelis RM, McCormick SS (2001b) Effects of growth hormone and cortisol on Na+ -K+ -2Cl− co-transporter localization and abundance in the gills of Atlantic salmon. Gen Comp Endo 124:134–143
Perry SF (1997) The chloride cell: structure and function in the gills of freshwater fishes. Ann Rev Physiol 59:325–347
Rodriguez A, Gisbert E, Gallardo MA, Santilari S, Ibarz A, Sanchez JJ, Castello-Orvay F (2003) Osmoregulation in juvenile Siberian sturgeon (Acipenser baerii). In: proceedings of the IX National Congress of Agriculture, Spain
Sardella B, Cooper J, Gonzalez R, Brauner CJ (2004a) The effect of temperature on juvenile Mozambique tilapia hybrids (Oreochromis mossambicus × O. urolepis hornorum) exposed to full-strength and hypersaline seawater. Comp Biochem Physiol A 137:621–629
Sardella B, Matey V, Cooper J, Gonzalez RJ, Brauner CJ (2004b) Physiological, biochemical, and morphological indicators of osmoregulatory stress in California Mozambique tilapia (Oreochromis mossambicus × O. urolepis hornorum) exposed to hypersaline water. J Exp Biol 207:1399–1413
Sardella BA, Kültz D, Cech JJ, Brauner CJ (2008) Salinity-dependent changes in Na+/K+-ATPase content of mitochondria-rich cells contribute to the differences in thermal tolerance of Mozambique tilapia. J Comp Physiol 178:249–256
van der Heijden AJH, van der Meij CJM, Flik G, Wendelaar-Bonga S (1999) Ultrastructure and distribution dynamics of chloride cells in tilapia larvae in fresh water and seawater. Cell Tissue Res 297:119–130
Wendelaar Bonga S, van der Meij CJM (1989) Degeneration and death, by apoptosis and necrosis, of the pavement and chloride cells in the gills of the teleost Oreochromis mossambicus. Cell Tissue Res 255:235–243
Werner RG, Linares-Casenave J, Van Eenennaam JP, Doroshov SI (2007) The effect of temperature stress on development and heat-shock protein expression in larval green sturegon (Acipenser medirostris). Environ Biol Fish 79:191–200
Wright PA (2007) Ionic, osmotic and nitrogenous waste excretion. In: McKenzie DJ, Farrell AP, Brauner CJ (eds) Fish physiology. Elsevier, New York, pp 283–319
Acknowledgments
This work was supported by a CALFED SeaGrant postdoctoral fellowship awarded to BAS, and a CALFED Science program grant (SP2006-1035) awarded to DK. We would like to sincerely acknowledge Serge Doroshov, Silas Hung, Robert Kaufmann, and Joel van Eenennaam for their successful effort at spawning green sturgeon in captivity. We also would like to thank Paul Lutes and Erik Hallen of the Center for Aquatic Biology, and Aquaculture, Enio Sanmarti, Amanda Schwabe, and Cesar Morales of the Department of Animal Science, as well as Christa Woodley and Joseph Cech Jr in the Department of Wildlife, Fish, and Conservation Biology at UC Davis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Sardella, B.A., Kültz, D. Osmo- and ionoregulatory responses of green sturgeon (Acipenser medirostris) to salinity acclimation. J Comp Physiol B 179, 383–390 (2009). https://doi.org/10.1007/s00360-008-0321-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-008-0321-5