Abstract
In camelids the ventral parts of compartments 1 and 2 (C1/C2) and the total surface of compartment 3 of the forestomach are lined with tubular glands, whereas in ruminants the surface of the forestomach is composed entirely of stratified, squamous epithelium. Thus, differences in absorption rates between these foregut fermenters can be expected. In five camels C1/C2 was temporarily isolated, washed and filled with buffer solutions. Absorption of short-chain fatty acids (SCFA) and net absorption of sodium and water were estimated relative to Cr-ethylenediaminetetraacetic acid as a fluid marker. SCFA were extensively absorbed in the forestomach; clearance rates of SCFA with different chain lengths were equal. After lowering the pH of solutions SCFA absorption rates increased, but much less than the increase of the non-ionized fraction. Absorption of propionate was lower when acetate had been added. Findings suggest that most of the SCFA in camels are transported in the ionized form, most likely via an anion exchange mechanism. Net water absorption is closely related to net sodium absorption. Apparently water absorption results from an iso-osmotic process. Differences between absorption mechanisms of SCFA from the forestomach of camelids and ruminants are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The two suborders within the order Artiodactyla, Tylopoda (with only the one family, Camelidae, old- and new-world camelids) and Ruminantia both have large forestomachs and both ruminate. Ruminantia developed more recently in the evolutionary process than Camelidae (Thenius 1979). Some basic differences exist in anatomy and in physiology of these two suborders. Concerning the forestomachs, substantial varieties are evident in macroscopic anatomy and in histology and ultra structure of the forestomach mucosa.
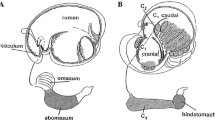
In Fig. 1 forestomach compartments of ruminants and of camelids are compared schematically. The forestomach of both these suborders consists of three different sections. In ruminants the rumen, reticulum and omasum and the HCl-secreting abomasums can be differentiated. In camelids, the forestomach consists of a large compartment 1 (C1) that is divided by a strong transversal muscular ridge into a cranial and a caudal portion. The relatively small compartment 2 (C2) is not efficiently separated from C1. Compartment 3 (C3), which originates from C2, is situated at the right side of C1. C3 is a long tube-like and intestine-like organ. HCl is produced only in the comparatively small hind stomach at the end of C3. The ventral regions of C1 and C2 are lined mainly by a glandular sac area, which are particularly prominent between the strong rib-like muscular ridges in the caudal C1.
Comparison of forestomach compartments in ruminants and in camelids (adapted from Lechner-Doll et al. 1995), compartment 1 (C1), compartment 2 (C2) and compartment 3 (C3)
Whereas in ruminants the surface epithelium of all sections of the forestomach are made up totally of a stratified, squamous, keratinized epithelium, in camelids only the dorsal part of C1 and C2, the strong rib-like muscular ridge and the small ridges between the glandular sacs are covered with a stratified epithelium (Fig. 1). The ventral part of C1, C2 and the whole surface of C3 are lined by a columnar surface epithelium and deep tubular glands (Fig. 1). This mucigenous glandular epithelium appears in light and in electron microscopic studies similar to cardiac glands of other mammals (Cummings et al. 1972; Luciano et al. 1979; Lechner-Doll et al. 1995). Large regions of the stomach in most macropod marsupials, sloths, pigs, colobus monkeys are lined with such a cardiac mucosa (Höller 1970; Gemmel and von Engelhardt 1977; Stevens and Hume 1996). In ruminants only a narrow band of cardiac glands is present at the cardia of the abomasum. Near the gastro-oesophageal junction of many mammals a rather small band of such a gland region also exists. Concerning the function of this cardiac mucosa little is known so far. Bicarbonate secretion was shown in the stomach of pigs (Höller 1970) and in the forestomach of llamas (Eckerlein and Stevens 1973; Rübsamen and von Engelhardt 1978). From C3 (von Engelhardt et al. 1979) and from the Pavlov pouch of C1 of the forestomach (Rübsamen and von Engelhardt 1978) of llamas an extensive absorption of short-chain fatty acids (SCFA), of sodium and of water was measured. From the temporarily isolated C1/C2 of two guanacos a rapid absorption of SCFA, sodium and water was reported, and it was found that absorption was two to three times faster than absorption from the rumen of sheep (von Engelhardt and Sallmann 1972).
We assumed that absorption rates of SCFA and absorption of sodium and water from the forestomach of camels are dissimilar to those of ruminants due to the decisive peculiarity of the large area of cardiac glands lining the forestomach of camelids. We therefore studied the absorption of the three major SCFA, the absorption of sodium and water from compartments 1 and 2 (C1/C2) in camels. Emphasis was placed on factors affecting absorption rates, and on potential transport mechanisms of SCFA across the forestomach epithelium of camels.
Materials and methods
Experimental animals
A total of five camels were used throughout the experiments (Table 1). At least 6 months prior to the studies all animals were fitted with a fistula in the caudal C1 (for details Dycker 2001). Camels were fed about 3 kg carrots, 2 kg beet pulp and medium-quality hay ad libitum daily. Water and mineralized salt licks were easily accessible.
Experimental design
Camels had been trained to tolerate the experimental procedure. Animals were tethered; they easily could stand up and lie down. C1 and C2 were emptied through the fistula, forestomach contents was stored at 40°C and was returned back into the forestomach at the end of the experiments. C1 and C2 were rinsed about ten times with circa 20 l warm (40°C) 0.9% NaCl-solution. Then, a flexible 2 m long nose-oesophagus tube (external diameter 6 mm) was placed through the nose into the oesophagus up to the forestomach. The saliva collection device (von Engelhardt and Sallmann 1972), adapted to the size of camels, was fixed at the end of the tube, and the collection device was pulled into the oesophagus about 40 cm proximal of the cardia. A foam rubber ring was placed around the tube at the nostril; thereby, the collection device was kept in position inside the oesophagus. The inflated cuff of the collection device prevented saliva passing into the forestomach. In preliminary tests a satisfactory position of the collecting device during longer experimental periods and a complete collection of saliva had been confirmed. The secreted saliva was sucked off and was collected (approximately 5 l/h). An inflatable balloon catheter (20 cm long, external diameter 15 mm) was placed in the canal between C2 and C3; thereby, an outflow of fluid from C1/C2 into C3 was prevented (Fig. 2) (for details Dycker 2001). The accuracy of all calculations depends on the perfect retention of the water soluble marker Cr-ethylenediaminetetraacetic acid (CrEDTA) in C1/C2. It therefore was essential that a complete closure of the canal between C2 and C3 was achieved with the balloon catheter. For checking, at the end of the experiments the entire solutions were sucked off from the temporarily isolated forestomach, which was weighed, and therein the present CrEDTA was then calculated.
View of the temporarily isolated forestomach in camels. In camels with a permanent forestomach fistula contents from the forestomach is removed, a saliva collection device is placed in the oesophagus, and a balloon catheter is positioned in the canal between compartment 2 (C2) and compartment 3 (C3). Thereby inflow and outflow of fluid into and from the forestomach is blocked. The forestomach is washed and filled with buffer solution. Buffer solution is continuously pumped from and back into the forestomach, and samples can be taken from this circular flow; pH was measured and adjusted by adding acid (adapted from Dycker 2001)
After C1/C2 had been washed and the washing solutions had been sucked off, 30 l of a buffer solution (Table 2) was poured into C1/C2. For estimating fluid volume and water absorption 40 ml of the water soluble, non-absorbable fluid marker CrEDTA had been added into the buffer solutions (9 mg/ml Cr as CrEDTA; Binnert et al. 1968). Test solutions were warmed up to 37°C and were continuously gassed with a mixture of 95% O2 and 5% CO2. The fistula was closed, and with a tube pump (Mulifix Record, MR 25, Schwinherr, Schwäbisch Gemünd) test solution was pumped out from the cranial C1 and back into the caudal C1 of the forestomach continuously at a rate of 2 l/min.
When control buffer solution 1 (solute concentrations similar to forestomach contents, Table 2) was added to C1/C2 the pH of the solution in the forestomach increased within 2 h from pH 6.76 (±0.05 to pH 7.22 (±0.11 (Fig. 3). In order to control pH effects on absorption rates, in all further experiments the pH of buffer solutions in C1/C2 were adjusted to the pH specified for the respective experiment by titration with H2SO4 (Fig. 2).
Changes of pH after adding buffer solution 1 (solute concentrations similar to forestomach contents) into the temporarily isolated compartments 1 and 2 when the pH was not continuously adjusted; n = 13; three experiments each with camels Sei, Em, Su and four experiments with camel Ro; means and standard error of mean are given
Samples at 15 ml were taken from the forestomach at 15 min intervals throughout the 2-h experiments. Samples were withdrawn with a syringe without contact to ambient air, and were stored in icy water up to the end of the 2-h experiments. Osmolarity and HCO −3 -concentrations were measured immediately after each experiment. Then, samples were centrifuged for 20 min. at 4,000×g, and the supernatant was stored at −18°C up to the time of analysis.
In order to accomplish sufficient mixing the first sample was taken 5 min after addition of the buffer solutions, and this time was defined t 0. The starting fluid volume at t 0 (V 0) was the sum of the added fluid volume and the fluid that had still remained in the forestomach after sucking off the washing solutions.
At the end of the experiments the buffer solutions were sucked off, and the stored forestomach contents as well as the collected saliva were returned into C1/C2. To achieve a normal microbial milieu in forestomach contents between experiments at least a 2-week interval was scheduled for each camel between successive experiments.
The experimental periods lasted 2 h. Changes in osmolarity, sodium- and SCFA-concentrations in buffer solution 1 (solute concentrations similar to forestomach contents) during these 2 h were measured in 13 experiments with four camels (Ro, Em, Su, Sa). As well as in the other experiments samples were taken and analysed at 15 min. intervals. Osmolarity decreased already during the warm-up before the experiments from 300 mosm/kg to 286 ± 1.69 mosm/kg. During the 2-h experimental period osmolarity (y) decreased linearly from approximately 285 to 265 mosm/l (y = 0.15x + 284; r = 0.98; x time in minutes); sodium concentrations (y) (y = 0.08x + 117.7; r = 0.98) and SCFA-concentrations (y) (y = 0.14x + 91.2; r = 0.99) declined almost in parallel.
Estimation of transmural PDs
Transmural electrical potential differences (PDs) were measured between the solution in C1/C2 and blood in the jugular vein using two KCl-agar-bridges. One electrode was dipped into solution 1 in the forestomach, and the other electrode was connected by a T-piece with an infusion tube that was connected to an in-dwelling catheter (Vasculon, Plus, 18G, 13 mm, BOC Ohmed AB, Sweden) in the jugular vein. Continuously a sterile electrolyte solution (Sterofundin) was dropped into the tube (one drop/5 s). Agar–KCl-bridges were connected via 3 M KCl-solution with calomel-electrodes (Ingold, Steinbach, Germany), and PD was measured with a millivoltmeter (Digital pH meter 646, Knick, Berlin). In each of three camels (Em, Su and Er) mean PD was recorded three times for 2 h when the control buffer solution 1 had been in C1/C2.
Buffer solutions
In order to establish adequate compositions of the buffer solutions first of all on three camels at five successive days solute concentrations were analysed in their forestomach contents (Table 2). Depending on the protocols eight different buffer solutions were made up (Table 2). Solute concentrations in the control buffer solution 1 were similar to those in the forestomach contents. All buffer solutions were titrated to the respective pH. The final osmolarity was adjusted to 300 mosm/l with mannitol.
Analytical methods
Short-chain fatty acids were analysed by gas chromatography (gas chromatograph 588 A with injector 7672 A with integrator 3396 B; Hewlett-Packard, Böblingen, Germany). Samples were deproteinized with 96% formic acid and subsequently centrifuged at 3,600×g for 10 min. Separation was carried out at 130°C isothermally on chromosorb WAW 80/100 mesh with 20% neopentyl glycosuccinate and 2% orthophosphoric acid. The detection temperature was 250°C.
Chloride was determined by coulometric titration (CMT 10 Chlorid Titrator, Radiometer, Willich, Germany), sodium and potassium by flame photometry (FLM3, Radiometer, Willich, Germany). Osmolarity was measured by freezing point depression (Roebing Osmometer, Berlin). Chromium concentration of the fluid marker CrEDTA was measured with an atom absorption spectrophotometer (Perkin Elmer 2100, Überlingen, Germany). Bicarbonate was analysed with a blood gas analysis instrument (278 Blood Gas System, Ciba Corning Diagnostics, Fernwald, Germany).
Calculations
Fluid volume
Fluid volume in C1 and C2 at the beginning of the experimental period (V 0) is the buffer solution volume added (30 l) plus the remaining solutions in the forestomach after sucking off the washing solution (estimated from changes of CrEDTA concentrations in the respective buffer solution added). V 0 is the amount of CrEDTA added with the 30 l buffer solution (Mr0) (mg) divided by the marker concentration 5 min. after adding the buffer solution with CrEDTA (Cr0) (mg/l), V 0 = Mr0/Cr0. Time zero (t 0) is the start of the measuring period, i.e. 5 min. after adding the buffer solution into the forestomach. Throughout the 2-h experiments Cr-concentrations were fitted to a linear regression, and the concentrations at t 0 (Cr0) and at t 120 (Cr120) were deduced from this regression line; t 120 is the time and V 120 is the fluid volume at the end of the 2-h experimental period, V 120 = Cr0/Cr120 V 0.
Net water absorption or secretion rates
The net water absorption or secretion is deduced from changes of fluid volumes in the forestomach throughout the experimental period, ΔV = V 0 − V 120; where ΔV is the net water absorption during 120 min.
Net absorption rates of sodium, absorption rates of SCFA and gain of bicarbonate
Net absorption or secretion from or into the forestomach is the quantity of the respective solute in the forestomach fluid at the start of the experimental period − the quantity at the end, X sol = (C sol-0 V 0 − C sol-120 V 120)/Δt; where X sol is net absorption or secretion of the respective solute; C sol-0 and C sol-120 concentrations of the respective solute at t 0 and t 120; Δt time between t 0 and t 120 in minutes.
Clearance rates for SCFA
Clearance rates are an assessment of the permeability of the forestomach mucosa for SCFA. Clearance rates are estimated from the net absorption divided by the mean concentration of the SCFA in the forestomach fluid at the beginning and at the end of the experimental period, ClSCFA = X SCFA/[(C SCFA-0 + C SCFA-120)/2]; where ClSCFA is the clearance rate of the respective SCFA; X SCFA absorption rates of SCFA; C SCFA-0 and C SCFA-120 concentrations of SCFA at t 0 and t 120.
Statistics
Results were expressed as means ± standard error of mean; n designates the number of measuring periods or samples. Effects of animals on absorption and secretion rates were tested by a one-way analysis of variance. Differences between mean values were checked using the paired or unpaired two-side t-test, as appropriate. In excess of two variables, multiple regression analysis was applied. Regression analysis was done by the method of least squares fit, and for significance correlations were tested with the t-test. Correlation coefficients are given as estimates for dependences. Curve fittings for Michaels–Menten-Kinetics were done using the “graphpad-inplot” computer programme.
Results
Transmural PDs
Mean PD between forestomach control buffer solution 1 and blood in the three camels Em, Su and Er was –30 ± 0.78 mV; PD in the forestomach was always negative to blood. PD was also measured in these three camels when the forestomach was filled with normal forestomach contents; ten measurements were done in each camel. Marked regional differences were seen. PD measured in forestomach contents in the cranial C1 was −32.4 ± 1.01 mV, in the caudal C1 −32.6 ± 1.36 mV and in C2 −52.0 ± 1.03 mV, respectively.
Absorption rates and clearance rates of SCFA
Initially, absorption of SCFA from the temporarily isolated C1/C2 was studied with the control buffer solution 1 with a composition similar to forestomach contents of camels (Table 2). Absorption of SCFA was extensive, and absorption rates of the three SCFA depended on their concentrations in the buffer solutions (acetate 70, propionate 15, butyrate 10 mmol/l) (Fig. 4). The high clearance rates of SCFA from buffer solution 1 in the forestomach (Fig. 5) documented a high permeability of the forestomach mucosa for the three SCFA. Although chain length and lipid solubility of the three SCFA are markedly different, clearance rates of Ac−/HAc, of Pr−/HPr and of Bu−/HBu were similar (no significant differences between respective findings in Fig. 5).
Absorption of acetate/acetic acid (Ac − /HAc), propionate/propionic acid (Pr − /HPr), butyrate/butyric acid (Bu − /HBu), net absorption of sodium and of water from the temporarily isolated compartments 1 and 2. Camels Ro, Sei, Em and Su; n = 13; buffer solution 1 (solute concentrations similar to forestomach contents); means and standard error of mean are given
Clearance rates of acetate/acetic acid (Ac − /HAc), propionate/propionic acid (Pr − /HPr), butyrate/butyric acid (Bu − /HBu) from the temporarily isolated compartments 1 and 2 in camels Ro, Sei, Em and Su; n = 13; buffer solution 1 (solute concentrations similar to forestomach contents); means and standard error of mean are given; estimations from data in Fig. 4; n.s. not significant
Clearance rates in Fig. 5 indicated that SCFA are mainly not absorbed from the forestomach of camels in the lipid-soluble, non-ionized form. This was partly confirmed in the later experiments when the pH of solutions in the forestomach was decreased from 6.7 to 6.0 (Fig. 6). For Ac−/HAc the difference between mean values at pH 6.7 and at pH 6.0 is not significant. At the lower pH absorption of Pr−/HPr was about 50% (P < 0.05) and of Bu−/HBu 25% (P < 0.01) higher. In the buffer solution at pH 6.0 concentrations of the undissociated SCFA fraction is about five times higher than at pH 6.7. Compared to this elevation the increase of SCFA absorption rates are exceedingly small. Thus, most likely only a smaller amount of SCFA had been absorbed by diffusion of SCFA in the undissociated form. The majority may be transported across the epithelium by a saturable carrier-mediated anion exchanger.
Absorption of acetate/acetic acid (Ac − /HAc), propionate/propionic acid (Pr − /HPr), butyrate/butyric acid (Bu − /HBu) from the temporarily isolated compartments 1 and 2 at pH 6.7 with buffer solution 1 in six experiments with five camels, and at pH 6.0 in nine experiments with buffer solution 2 with camels Ro, Sei and Em; n.s. not significant, *P < 0.05, **P < 0.01
In order to test this hypothesis, concentrations of Pr−/HPr were increased stepwise from 25 to 100 mmol/l. The respective absorption rates dependent on Pr−/HPr concentrations, and the indication of saturation kinetic are shown in Fig. 7. V max is 473 mmol/h, and K m is 40 mmol/l.
Effects of stepwise increased propionate/propionic acid (Pr − /HPr) concentrations on Pr − /HPr absorption from the temporarily isolated compartments 1 and 2 at pH 6.7. Buffer solutions 7, 6, 5 and 4, with 25, 50, 75 and 100 mmol/l Pr − /HPr and without Ac−/HAc and Bu−/HBu; camels Ro, Sei and Em; n = 9; V max 473 mmol/h; K m 40.1 mmol/l; means and standard error of mean are given
For a carrier-mediated transport we may expect a competitive inhibition. In two test series buffer solutions 5 and 8 were used. Both contained 50 mmol/l Pr−/HPr. But, in buffer solution 8 an additional 50 mmol/l Ac−/HAc was present. In the presence of Ac−/HAc mean absorption of Pr−/HPr was significantly lowered by 26% (Fig. 8).
Absorption of propionate/propionic acid (Pr − /HPr) from the temporarily isolated compartments 1 and 2 with buffer solution 6 (n = 10) with 50 mmol/l Pr − /HPr (no Ac − /HAc and Bu−/HBu) and with buffer solution 8 (n = 6) with 50 mmol/l Pr − /HPr and 50 mmol/l Ac − /HAc (no Bu−/HBu); camels Ro, Sei and Em; means and standard error of mean are given; **P < 0.01
Bicarbonate gain and SCFA absorption
The anion exchanger could be a SCFA−-HCO −3 exchange. As a result, a gain of HCO −3 in the forestomach fluid had to be expected. In the studies presented in Fig. 4 (experiments with control buffer solution 1) also HCO −3 -concentrations were measured throughout the 2-h experiments. From the increases of HCO -3 -concentrations the gain of HCO -3 was calculated. Mean gain of HCO -3 was 82.3 ± 12.6 mmol/h. That was only slightly higher than 30% of the simultaneously observed SCFA absorption.
Net absorption of sodium and water
Mean net absorption of sodium and of water are presented in Fig. 4. The variability of these net absorption rates between the four camels was large. For mean net water absorption great individual differences were found; in two camels a mean net water absorption was present (Em 788 ± 194 ml/h; Ro 529 ± 21 ml/h), whereas in the other two camels a net water secretion was found (Su −247 ± 13 ml/h; Sei −201 ± 224 ml/h). The two camels with the mean net water absorption had the higher and the two camels with the mean net water secretion had the lower mean net sodium absorption rates (mean net sodium absorption rates: Em 486 ± 50 mmol/h; Ro 171 ± 29 mmol/h; Su 102 ± 12 mmol/h; Sei 84 ± 6 mmol/h).
A close correlation between net water absorption (y) and net sodium absorption (x) was found. For the experiments presented in Fig. 4 the interdependence was y = 3.39x − 386; r = 0.81; P < 0.001; n = 13. The correlation was even closer for a larger number (n = 34) of other experiments (Fig. 9). From the equation in Fig. 9 it is apparent that water was secreted into the forestomach of the three camels at a rate of 853 ml/h when the net sodium absorption rate was zero.
Discussion
Forestomach epithelial cells in camelids
It is most likely that the greater part of absorption and secretion in the forestomach of camelids is carried out by the glandular epithelium. The epithelium lining the surface areas of the glandular region is composed of about 40 μm high tall columnar cells. The fine structure in camels (Lechner-Doll et al. 1995) is comparable to that in llamas (Cummings et al. 1972; Luciano et al. 1979). The main characteristics of these cells are numerous apical tiny microvilli, many mitochondria, a well developed Golgi apparatus and numerous mucous granules. The basolateral membrane of the epithelium and the intercellular space are enlarged similar to other epithelia with a high absorptive capacity. The ultra structure of these cells at the epithelial surface in the glandular region resembles cells in the gall bladder and small intestine. Glands are defined as cardiac glandular mucosa. Many endocrine cells are present in this area (Luciano et al. 1980).
Concerning functions of the glandular mucosa in camelids little is known. During the washing procedure of C1/C2 mucous substances accumulate in the washing solutions in camels and also in llamas (personal observations; von Engelhardt and Sallmann 1972; Hinderer 1978). Similarly in Pavlov pouches prepared in the glandular region of the cranial portion of C1 of llamas (Rübsamen and von Engelhardt 1978) and in the cardiac gland region of the stomach in pigs (Höller 1970) secretion of mucus was observed. In both these studies HCO −3 secretion was noted.
Solute concentration in forestomach contents of camelids
Concentration of sodium in the contents in C1/C2 of llamas (von Engelhardt et al. 1979), of two guanacos (von Engelhardt and Sallmann 1972) and of three camels (Table 2) was on average 70% higher than that in rumen contents of sheep (von Engelhardt and Hauffe 1975). Concentrations of potassium, chloride and SCFA as well as the osmolarity were similar in sheep and in camelids, but mean pH was slightly higher in camelids than in sheep. Also, mean PD between forestomach contents and blood was similar in camels and in sheep (Gäbel 1988).
Absorption of SCFA
The SCFA are absorbed rapidly in the forestomach in camels (Fig. 4) and in ruminants (Gäbel 1995). Absorption rates depend on surface area of the mucosa of the respective compartments, permeability of the forestomach mucosa for SCFA and transport characteristics for SCFA. These parameters can be rather different in various species and under different experimental conditions.
The clearance rates of SCFA in the temporarily isolated forestomach are a parameter for the permeability of the mucosa for SCFA. Clearance rates for SCFA from the isolated reticulo-rumen of cattle fed hay (Thorlacius and Lodge 1973) were similar to camels (Fig. 5) (3,200–3,700 ml/h). Rather high clearance rates of about 2,000 ml/h had been estimated from the temporarily isolated C1/C2 of two guanacos (von Engelhardt and Sallmann 1972) and from Pavlov pouches prepared in the cranial C1 of llamas (Rübsamen 1976). Mean clearance rates of SCFA from the isolated reticulo-rumen in sheep fed hay had been 1,260 and 630 ml/h (Marek 1991; Gäbel et al. 1991, respectively).
Clearance of the three SCFA with increasing chain length and thus increasing lipid solubilities could provide an indication of transport mechanism involved in absorption. In camels (Fig. 5) and also in the temporarily isolated forestomach of guanacos (von Engelhardt and Sallmann 1972) clearance rates for Ac−/HAc, Pr−/HPr and Bu−/HBu were similar, indicating that diffusion of SCFA in the non-ionized, lipid-soluble form across the epithelia is of minor importance. In Pavlov pouches in C1 of llamas, on the other hand, clearance rates of Pr−/HPr were 50% higher than those of Ac−/HAc, and of Bu−/HBu were 50% higher than those Pr−/HPr (Rübsamen and von Engelhardt 1978). We have no proof whether functions of this epithelium of these Pavlov pouches several weeks after surgery were still normal; the transmural PD of −35 mV in these pouches indicated that at least net sodium transport was similar to that in the isolated forestomach. In the washed reticulo-rumen of cattle (Thorlacius and Lodge 1973), in vitro isolated sheets of cattle rumen mucosa (Stevens and Stettler 1966) and in the washed reticulo-rumen of sheep (Südermann 1986) clearance rates increased with chain length of SCFA, although these increases were considerably less than the increments in lipid solubility.
An increase in SCFA absorption when the pH in the forestomach fluid is lowered would be a further indication of passive diffusion of undissociated SCFA. A stimulatory effect of low pH on SCFA absorption has been reported repeatedly in studies with sheep and cattle (Stevens and Stettler 1966; Weigand et al. 1975; Thorlacius and Lodge 1973; Dijkstra et al. 1993; Kramer et al. 1996; Pitt et al. 1996; Sehested et al. 1999). However, in all these studies the stimulatory effect of a lowered pH did not reach the extent predicted by the Hendersson–Hasselbalch equilibrium of HSCFA and SCFA−. In camels, SCFA absorption was somewhat stimulated when the pH was lowered, too (Fig. 6). Nonetheless, these elevations were mostly less than those in sheep and cattle. Such low effects of pH on SCFA absorption were also reported for the guinea pig caecum and colon (Oltmer and von Engelhardt 1994). The latter could be explained by the neutral pH microclimate at the epithelial surface as shown for the colon of guinea pig (Genz et al. 1999), colonic crypts of mice (Chu and Montrose 1996), rat jejunum (Shiau et al. 1985) and gastric surface (Chu et al. 1999). In the glandular region of the forestomach in camelids such a pH microclimate at the luminal surface could be responsible for the minor pH effects on SCFA absorption (Fig. 6). The prominent mucus secretion and resulting mucus layer at the epithelial surface could support such a pH microclimate. Furthermore, a mucus layer could provide preconditions for an effective unstirred layer.
The missing effects of chain lengths of SCFA, the small pH effects of buffer solutions, as well as the similarities of clearance rates of the three SCFA indicate that in camels SCFA absorption is not the result of passive diffusion of the undissociated fatty acids. Findings point at a transepithelial SCFA transport primarily in the ionized form. The concept of a carrier-mediated transport is supported by saturation effect of Pr−/HPr absorption (Fig. 7) and by competitive inhibition (Fig. 8). The calculated apparent K m (40 mmol/l) for Pr−/HPr is much lower in camels than that estimated for sheep (178 and 112 mmol/l; Michelberger 1994; Dijkstra et al. 1993, respectively). The low K m indicates that the SCFA transporters in the mucosa of the camel forestomach are more specific than that in ruminants.
In the ruminant forestomach and also in the large intestine convincing evidence of the presence of an anion exchanger has been presented (Gäbel 1995; Gäbel et al. 2002; Vidyasagar et al. 2005). In the aforementioned experiments the estimated levels of HCO −3 gain were not precise. The temporarily isolated forestomach is a partially open system for CO2, the carbonic anhydrase activity in forestomach epithelium is most likely high, and the equilibrium between HCO −3 and CO2 is instantaneously adjusted. Thus, CO2 disappears rapidly from the buffer solutions in the forestomach. Nevertheless, a substantial gain of HCO − 3 in forestomach fluid was seen, but the measured HCO −3 - increase cannot be a reliable estimate for the quantitative relations of a potential anion exchanger. We assume that SCFA−/HCO −3 exchange is a major mechanism involved in SCFA absorption in the forestomach of camelids. In contrast, in ruminants, besides a SCFA−/HCO −3 exchange, a distinct portion of SCFA in the reticulo-rumen is transported via simple diffusion in the protonated form (Gäbel 1995; Gäbel and Sehested 1997; Gäbel and Aschenbach 2006). However, in the omasum of sheep Ac−/HAc is obviously transported predominately by diffusion of the unionized HAc (Ali et al. 2006).
Net absorption of sodium and water
A close relationship between sodium and water absorption is a fundamental and generally accepted process for water absorption for most epithelia in the gastrointestinal tract and in the kidney. This also had been demonstrated for the reticulo-rumen of sheep (Südermann 1986). Likewise, in camels a close relationship exists (Fig. 9). It is interesting to note that in camels when net sodium absorption was zero a net water secretion of 853 ml/h was calculated. This may demonstrate the basic secretory functions of the cardiac glands; this is different from processes in the forestomach of ruminants. In camels, for the net absorption of one litre of water, net sodium absorption was 162 mmol/l which is approximates isotonic water absorption. In sheep twice as much sodium disappeared when 1 l water was absorbed (Südermann 1986). In camelids as well as in ruminants sodium absorption from the forestomach takes place against an electrochemical gradient as a result of an active transport process. Whereas in ruminants transepithelial sodium transport has been studied in detail (Leonhard-Marek 2002; Leonhard-Marek et al. 2005; Abdoun et al. 2005) we have so far no detailed respective information on transport mechanisms in camelids.
We conclude that SCFA are absorbed from C1/C2 of camels extensively and mainly in the ionized from, most likely by a saturable SCFA−/HCO −3 exchange mechanism. A non-ionic diffusion of SCFA across the forestomach mucosa in C1/C2 is of minor impact compared with ruminants. A distinctive unstirred layer at the surface of the forestomach mucosa of camelids may be considerably more distinct than in ruminants. The close relationship between net absorption of sodium and of water is similar to processes in other intestinal epithelia.
Abbreviations
- Ac−/Hac:
-
Acetate/acetic acid
- Bu−/Hbu:
-
Butyrate/butyric acid
- C1/C2:
-
Compartments 1 and 2
- CrEDTA:
-
Cr-ethylenediaminetetraacetic acid
- Pr−/HPr:
-
Propionate/propionic acid
- SCFA:
-
Short-chain fatty acids
References
Abdoun K, Stumpff F, Wolf K, Martens H (2005) Modulation of electroneutral Na transport in sheep rumen epithelium by luminal ammonia. Am J Physiol 289:G508–G520
Ali O, Shen Z, Tietjen U, Martens H (2006) Transport of acetate and sodium in sheep omasum: mutual, but asymmetric interactions. J Comp Physiol B 176:477–487
Binnert WTA, van´t Kloster Th, Frens AM (1968) Soluble chromium indicator measured by atomic absorption in digestion experiments. Vet Rec 82:470
Chu S, Montrose MH (1996) Non-ionic diffusion and carrier-mediated transport drive extracellular pH regulation of mouse colonic crypts. J Physiol 494:783–793
Chu S, Tanaka S, Kaunitz JD, Montrose MH (1999) Dynamic regulation of gastric surface pH by luminal pH. J Clin Invest 103:605–612
Cummings JF, Munnell JL, Vallenas A (1972) The mucigenous glandular mucosa in the complex stomach of two new-world camelids, the llama and guanaco. J Morphol 137:71–110
Dijkstra J, Boer H, van Bruchem J, Bruining M, Taminga S (1993) Absorption of volatile fatty acids from the rumen of lactating dairy cows as influenced by volatile fatty acid concentration, pH, and rumen liquid volume. Br J Nutr 69:385–396
Dycker Ch (2001) Resorption und Sekretion von kurzkettigen Fettsäuren und Elektrolyten im Vormagen des Kameles. Vet Med Thesis, Hannover
Eckerlein RH, Stevens CE (1973) Bicarbonate secretion by the glandular saccules of the llama stomach. Cornell Vet 63:436–445
Gäbel G (1988) Natrium- und Chloridtransport im Pansen von Schafen: Mechanismen und ihre Beeinflussung durch intraruminale Fermentationsprodukte. Habilitationsschrift, School of Vetenary Medicine, Hannover
Gäbel G (1995) Transport of short-chain fatty acids in the ruminant forestomach. In: Cummings JH, Rombeau JL, Sakata T (eds) Physiological and clinical aspects of short-chain fatty acids. Cambridge University Press, Cambridge, pp 133–148
Gäbel G, Aschenbach JR (2006) Ruminal SCFA absorption: channelling acids without harm. In: Sejrsen K, Hevelplund T, Nielsen MO (eds) Ruminant physiology—digestion, metabolism and impact of nutrition on gene expression, immunology and stress. Academic Publications, Wageningen, The Netherlands, pp 173–195
Gäbel G, Sehested J (1997) SCFA transport in the forestomach of ruminants. Comp Biochem Physiol A 118:367–374
Gäbel G, Bestmann M, Martens H (1991) Influence of diet, short-chain fatty acids, lactate and chloride on bicarbonate movement across the reticulo-rumen wall of sheep. J Vet Med A 38:523–529
Gäbel G, Aschenbach JR, Müller F (2002) Transfer of energy substrates across the ruminal epithelium: implications and limitations. Anim Health Res Rev 31:15–30
Gemmel RT, von Engelhardt W (1977) The structure of the cells lining the stomach of the tammar Wallaby (Macropus eugenii). J Anat 123:723–733
Genz AK, von Engelhardt W, Busche R (1999) Maintenance and regulation of the pH microclimate at the luminal surface of the distal colon of guinea-pig. J Physiol 517:507–519
Hinderer S (1978) Kinetik des Harnstoffwechsels beim Lama bei proteinarmen Diäten. Thesis, Stuttgart-Hohenheim
Höller H (1970) Untersuchungen über Sekret und Sekretion der Cardiadrüsenzone im Magen des Schweines. Zbl Vet Med A 17:685–711; 857–873
Kramer T, Michelberger T, Gürtler H, Gäbel G (1996) Absorption of short-chain fatty acids across ruminal epithelium of sheep. J Comp Physiol B 166:262–269
Lechner-Doll M, von Engelhardt W, Abbas HM, Mousa L, Luciano L, Reale E (1995) Particularities in forestomach anatomy, physiology and biochemistry of camelids compared to ruminants. In: Tisserand JL (ed) Elevage et alimentation du dromadaire–Camel production and nutrition. Options méditerranéennes, Serie B:Etudes et Recherches Nr 13, CIHEAM, Paris, pp 19–32
Leonhard-Marek S (2002) Divalent cations reduce the electrogenic transport of monovalent cations across rumen epithelium. J Comp Physiol B 172:635–641
Leonhard-Marek S, Stumpff F, Brinkmann I, Breves G, Martens H (2005) Basolateral Mg2+/Na+ exchange regulates apical nonselective cation channel in sheep rumen epithelium via cytosolic Mg2+. Am J Physiol 288:G630–G645
Luciano L, Voss-Wermbter G, Behnke M, von Engelhardt W, Reale E (1979) Die Struktur der Vormagenschleimhaut beim Lama (Lama guanacoe und Lama lamae), I. Vormägen. Gegenbauers Morphol Jahr 125:519–549
Luciano L, Reale E, von Engelhardt W (1980) The fine structure of the stomach mucosa of the llama (Llama guanacoe). II. The fundic region of the hind stomach. Cell Tissue Res 208:207–228
Marek M (1991) Resorption von kurzkettigen Fettsäuren und Elektrolyten aus dem Retikulorumen von Schafen: Beeinflussung durch Nahrungsentzug und intraruminale Infusion von Natrium-Butyrat. Vet Med Thesis, Berlin
Michelberger T (1994) Propionsäure-Transport über das isolierte Pansenepithel des Schafes. Vet Med Thesis, Berlin
Oltmer S, von Engelhardt W (1994) Absorption of short-chain fatty acids from the in-situ-perfused caecum and colon of the guinea pig. Scand J Gastroenterol 29:1009–1016
Pitt RE, van Kessel JS, Fox DG, Pell AN, Barry MC, van Soest PJ (1996) Prediction of ruminal fatty acids and pH within the net carbohydrate and protein system. J Anim Sci 74:226–244
Rübsamen K (1976) Sekretion und Resorption in der Cardiadrüsenzone des Lamas. Thesis, Stuttgart-Hohenheim
Rübsamen K, von Engelhardt W (1978) Bicarbonate secretion and solute absorption in the glandular pouch in the forestomach of the llama. Am J Physiol 235:E1–E6
Sehested J, Diernaes L, Möller PD, Skadhauge E (1999) Ruminal transport and metabolism of short-chain fatty acids (SCFA) in vitro: effect of SCFA chain length and pH. Comp Biochem Physiol A 123:359–368
Shiau YF, Fernandez P, Jackson MH, McMonagle S (1985) Mechanisms maintaining a low pH microclimate in the intestine. Am J Physiol 248:G608–G617
Stevens CE, Hume ID (1996) Comparative physiology of the vertebrate digestive system. Cambridge University Press, Cambridge
Stevens CE, Stettler BK (1966) Factors affecting the transport of volatile fatty acids across rumen epithelium. Am J Physiol 210:365–372
Südermann M (1986) Untersuchungen über den Einfluß energiereicher Fütterung auf Transportvorgänge der Pansenwand. Vet Med Thesis, Hannover
Thenius E (1979) Die Evolution der Säugetiere. Gustav Fischer Verlag, Stuttgart
Thorlacius SO, Lodge GA (1973) Absorption of steam-volatile fatty acids from the cow as influenced by diet, buffer and pH. Can J Anim Sci 53:279–288
Vidyasagar S, Barmeyer Ch, Geibel J, Binder HJ, Rajendran VM (2005) Role of short-chain fatty acids in colonic HCO3 secretion. Am J Physiol 288:G1217–G1226
von Engelhardt W, Hauffe R (1975) Role of the omasum in absorption and secretion of water and electrolytes in sheep and goats. In: McDonald IW, Warner ACI (eds) Digestion and metabolism in the ruminants. The University of New England publishing Unit, Australia, pp 216–230
von Engelhardt W, Sallmann HP (1972) Resorption und Sekretion im Pansen des Guanakos (Lama guanacoe). Zbl Vet Med A 19:117–132
von Engelhardt W, Ali KE, Wipper E (1979) Absorption and secretion in the tubiform forestomach (compartment III) of the llama. J Comp Physiol 132:337–341
Weigand E, Young JW, McGilliard AD (1975) Volatile fatty acid metabolism by rumen mucosa from cattle fed hay or grain. J Dairy Sci 58:1294–1300
Acknowledgements
The skilled technical assistance of G. Becker, M. Burmester and K. Hansen is greatly appreciated. We thank F. Herkenrath and M. Rhode for careful training of the camels and for help throughout the experiments. We are grateful to Frances C. Sherwood-Brock for editing of the English manuscript and Dr Roger Busche for his assistance in preparing illustrations and the electronic version of the manuscript. Ch. Dycker was financially supported by the H. Wilhelm Schaumann Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
von Engelhardt, W., Dycker, C. & Lechner-Doll, M. Absorption of short-chain fatty acids, sodium and water from the forestomach of camels. J Comp Physiol B 177, 631–640 (2007). https://doi.org/10.1007/s00360-007-0161-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-007-0161-8