Abstract
The Eastern Grey Kangaroo (Macropus giganteus) occurs mostly in the wetter regions of eastern Australia. However, in the past 30–40 years it has moved into more arid regions (rainfall<250 mm), thus increasing its overlap zone with the xeric adapted Red Kangaroo (Macropus rufus). An increased access to water (supplied for domestic stock) may explain this range extension, but changes in the availability of preferred feed could also be involved. The water use, drinking patterns and thermoregulatory behaviour of these two species of kangaroo have been examined in a semi-free range study, during summer at an arid rangeland site. Foraging was largely nocturnal in both species and during the day they behaved to reduce heat loads. This was especially so for M. giganteus, which showed greater shade seeking. However, it still used more water (72±2.6 mL kg−1 day−1, mean ± SE) than M. rufus (56±7.6 mL kg−1 day−1) and drank twice as frequently. Although M. giganteus produced a less concentrated urine (1422±36 mosmol kg−1) than M. rufus (1843±28 mosmol kg−1), kidney physiology did not explain all of the differences in water metabolism between the species. Water from the feed and faecal water retention also appear to be involved. Broadly, a better access to reliable water and the utilisation of mesic microhabitats has enabled M. giganteus to make inroads into the changing rangelands of eastern Australia. However, changes in the vegetation, due to stock grazing, have also favoured M. giganteus, which is a grass eating specialist.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
What is it that limits animal population density in good and bad habitats? This was a crucial question posed by Krebs (2002) in his critical review of studies in population dynamics, in which he highlighted the complexity of population processes. Such questions are pertinent to kangaroos in the arid rangelands of eastern Australia because of recent range changes, most notably by the Eastern Grey Kangaroo, Macropus giganteus. These rangelands, on which sheep are extensively grazed, also support four species of grazing kangaroo. Like sheep, kangaroos also utilise foregut fermentation to facilitate plant fibre digestion (Hume 1999). Large populations of Red Kangaroos (M. rufus) occur in the hotter arid regions, while densities of the two species of Grey Kangaroo, the Eastern Grey Kangaroo (M. giganteus) and the Western Grey Kangaroo (M. fuliginosus) are highest in areas of more reliable rainfall (Caughley et al. 1987; Dawson 1995; McCullough and McCullough 2000). The Euro or Inland Wallaroo (M. robustus erubescens) occurs associated with hill country (Dawson and Denny 1969; Dawson 1995).
Of the kangaroos, M. giganteus appears the most mesic; its primary range is in wetter areas adjacent to the eastern coast of Australia (annual rainfall>500 mm) (Caughley et al. 1987; Dawson 1995). Yet, in the past 30–40 years it has expanded westward into the hot, arid rangelands, the prime habitat of M. rufus (rainfall<250 mm) (Denny 1975; Caughley et al. 1984), an increased provision of reliable watering sites for domestic stock (Caughley 1964, Caughley et al. 1984) being the usually accepted reason. In studies in small yards and the laboratory, a higher use of water by M. giganteus, relative to M. rufus, has been found, as well as differences in renal concentrating abilities (Blaney et al. 2000).
Doubt, however, has emerged concerning the primary role of water in facilitating the range extension of M. giganteus. Despite an initial focus on climatic factors and water (Caughley et al. 1984, 1987), Caughley et al. (1988) suggested that a renewable resource, possibly food, was a factor determining the inland boundary of M. giganteus, which falls where M. rufus has high densities. The diets of M. giganteus and M. rufus differ in less arid areas where M. giganteus predominate, with M. giganteus eating more grass (Griffiths and Barker 1966). Recently, Dawson et al. (2004) examined their diets and foraging behaviour at the inland edge of the range of M. giganteus and diets were found to differ, although there was much overlap. Daily foraging patterns and preference indices for feed types were also determined and Dawson et al. (2004) suggested that livestock induced changes in the rangeland vegetation, with annual grasses becoming more dominant, favoured M. giganteus, a grass eating specialist. The availability of green, annual grasses largely determines kangaroo survivorship and reproductive success in the arid rangelands (Moss and Croft 1999).
What is then the role of water in the changing kangaroo distributions in the dry country of eastern Australia? Although laboratory studies indicate a lesser ability in renal water conservation by M. giganteus, relative to M. rufus, there is much uncertainty regarding their relative water needs in the wild, arid rangelands. Although Nagy and Bradshaw (2000) have produced allometric relationships indicating marked differences in field water use between arid zone and mesic marsupials, the base data for M. giganteus (Nagy et al. 1990) was obtained in conditions very different from those of the rangeland. Often overlooked in these discussions is the impact of behaviour in reducing water needs. M. robustus erubescens uses microhabitats to moderate environmental extremes and water use in the rangelands (Dawson and Denny 1969; Dawson et al. 1975). M. giganteus seeks dense shade on hot days (McCarron 1990). The current study hypothesised that behavioural responses and microhabitat selection may alleviate the water requirements of M. giganteus. Consequently, time budgets, microhabitat selection and environmental factors were examined in conjunction with a study of the water relations of M. giganteus and M. rufus in summer. It was considered essential to undertake the study in semi-free range conditions that would allow for a sufficient number of comparable animals and appropriate experimental control. The study was made in conjunction with the diet study of Dawson et al. (2004), which is fundamental to an understanding of the role of ecophysiology in determining distributions of kangaroo species in arid Australia.
Materials and methods
Study site
This study was carried out at ‘Fowlers Gap’, the Arid Zone Research Station of the University of New South Wales. It lies in the far north-west of New South Wales (31°S, 142°E) and is 112 km north of the city of Broken Hill. The field work occurred in late summer, February–March 1999. An experimental enclosure of approximately 8 ha with kangaroo-proof fencing was used. Kangaroos had not grazed in it for several years and sheep and feral herbivores had been excluded for 20 years. It was well vegetated with small shrubs (mainly bladder saltbush, Atriplex vesicaria), grasses and a few clumps of shade trees (see Dawson et al. 2004 for details of the vegetation). The enclosure contained a water trough. A centrally located 7 m high observation tower enabled behaviour assessment. A 6-channel weather station (Monitor Sensors, Caboolture, Qld., Australia) continuously monitored the air (T a), and black globe (T bg) temperatures, together with solar radiation, relative humidity, wind speed and rainfall. Black globe temperature is influenced by the T a, solar radiation influx, long wave radiation exchange, and wind speed, and provides a good integrated measure of the ‘effective’ environmental temperature.
Climatic conditions
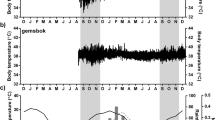
The conditions were fine and varied from warm to hot. During the measurement of water turnovers and behavioural observations (12–20th March), the climatic conditions were stable. The days were sunny with little or no clouds and light winds; the wind rarely exceeded 12 km−1. The pattern in T a is shown in Fig. 1, together with T bg and the solar radiation influx. The mean daily maximum T a was 31°C (range 29–34°C), while the mean daily minimum T a was 18°C (range 14–21°C); these mean values were the same as the mean summer values for Broken Hill (Cunningham et al. 1981). The maximum values for T bg, near 50°C, occurred on cloudless afternoons with little wind and T bg generally exceeded 40°C between 12:00 and 15:00 h. The minimum values for T bg occurred just before sunrise and were 2–3°C below T a. Relative humidity showed a cyclical pattern that somewhat mirrored T a; it reached a maximum value near 50% before sunrise and declined below 30% by early afternoon.
Animals
Adult females are the most abundant age/sex class in kangaroo populations (Dawson 1995). Seven adult females (without pouch young) of each species were examined; in part of the study data on only six M. rufus were gathered since one animal died accidentally toward the end of the study. The kangaroos had been in captivity for extended periods and were somewhat habituated to researchers; they were placed in the enclosure for approximately a month prior to the study. The kangaroos were fitted with identifying polyvinyl collars (2.5 cm wide), which were marked with individual patterns by coloured reflective tape. When required, the animals were caught using a carbon dioxide powered dart gun (Black Wolf, DEEB Monash University, Victoria); the darts contained 0.5 mL of Zoletil 100 (50 mg mL−1).
Methods
The water turnover rate (WTR) and total body water (TBW) content were measured using tritiated water (Denny and Dawson 1975; Nagy and Costa 1980). The kangaroos were weighed up to 0.05 kg, using a Salter 50 kg hanging balance. Subsequent to a background blood sample (5 mL) being taken from the lateral tail vein, the kangaroos were injected intraperitoneally with 37 MBq of tritiated water in 1 mL of isotonic saline. An equilibrium blood sample was taken after 6–7 h (Denny and Dawson 1975), the animals having been held in a small shaded pen. The kangaroos were then released into the experimental enclosure and left with minimal disturbance for a week. The animals were then sacrificed, reweighed and a final blood sample (10 mL) taken from the heart. The urine (5–10 mL) was collected and the kidneys removed. Forestomach samples were also taken for diet analysis (see Dawson et al. 2004).
The blood was immediately cooled and centrifuged within 2 h at 3,000g for 30 min. Plasma was obtained and stored frozen until analysis. Water was extracted from the plasma samples by vacuum sublimation (Vaughan and Boling 1961). Three 25 μL aliquots of the extracted water were each mixed with 4 mL of Packard Ultima Gold High-flash point LSC-cocktail and counted in a WinSpectral 1414 liquid scintillation counter. The TBW was estimated from the dilution of 3H in the equilibrium sample. The WTR was determined from the change in the specific activity of the samples over the experimental period (Nagy and Costa 1980).
The blood and urine samples were analysed for osmolality and the concentrations of sodium, potassium, chloride, magnesium, calcium and protein (plasma only). Osmolality was determined using a freezing point depression osmometer (Knaver Osmometer). Concentrations of Na+, K+, Ca2+ and Mg2+ were determined using a GBC 906AA atomic absorption spectrophotometer. The samples analysed for Na+ and K+ were diluted in a caesium chloride solution (2.5 g L−1), while the samples analysed for Mg2+ and Ca2+ were diluted in lanthanum chloride (7.2 mmol L−1). A Radiometer CMT 10 chloride titrator was used to measure Cl− concentrations. Plasma protein concentrations were measured by refractometry, using an AO T/C Refractometer (American Optical Instrument Company). The samples for plasma and urine urea concentrations were unfortunately lost in a refrigeration failure.
The length, width and depth (mm) of the right kidneys were measured with vernier callipers. The kidneys were then cut coronally in the frontal plane to produce mirror images. Indices to assess the proportions of cortical and medullary tissue were determined. Two thickness indices were determined. These were the relative thickness of the medulla (RMT) (Sperber 1944) and the percent medullary thickness (PMT) (Heisinger and Breitenbach 1969).
Both medullary thickness and the total thickness were measured transverse to the long axis of the kidney and the kidney size was determined as (length × width × depth)1/3. Two area indices were determined following Brownfield and Wunder (1976): the percent medullary area (PMA) and relative medullary area (RMA). The area of the cortical and medullary segments were determined by tracing areas from the cut sections onto transparent mm2 graph paper.
Behavioural observations
Four days were dedicated to 24 h behavioural observations. A point-sampling technique (Dunbar 1976) was used for the quantitative recording of kangaroo behaviour from the 7 m high tower. Scans were made every 10 min during the day. The positioning of kangaroos in the shade or in the sun was noted. At night, when the observations were more difficult, scans were made every 20 min. Night observations were made using a weak spotlight and Nikon ×12 marine binoculars. The behaviour of each species was initially categorised into three types, with sub-categories; however, the following report is largely based on the main categories:
Foraging: where the animal was consuming or searching for food, which included eating, slow searching (i.e. the movement while feeding within a patch, requiring one or two steps) and fast searching (usually walking fast between food patches).
Resting: all non-active behaviour where the animal appeared relaxed, which included lying, crouching and standing.
Active non-foraging (locomotion): where the kangaroo was moving or alert, sometimes in response to a disturbance. Miscellaneous behaviours that were uncommon, such as active grooming and drinking, were also included in this category.
Data analysis
The data was analysed using Statistica/Mac software. For most analyses a single factor ANOVA was used to compare the means from the species. Behavioural observations were analysed by species and time. The observations were transformed into proportions, which were arcsine transformed prior to analysis. For an initial analysis the behavioural observations were collapsed into 3 h blocks, starting at midnight. A Student–Newman–Keuls (SNK) multiple range test was applied when significant differences were indicated by the ANOVA. If significant differences were indicated the specific blocks were further examined as 1 h blocks. Data on the proportion of animals in the shade was compared for 2 h blocks between 6:00 and 18:00 h. Drinking frequencies were compared using a Chi-squared test. The tables show mean ± SE.
Results
Water metabolism and kidney structure
Mature females (without young) were used to provide the basic data, thus eliminating the variability associated with sexual dimorphism and the reproductive status. Body masses did not differ between M. giganteus and M. rufus and were maintained during the experiment (Table 1). There was no significant difference between the two species in the TBW (Table 1). However, the WTR in M. giganteus was significantly higher than that of M. rufus, with M. giganteus using 480 mL more water per day than M. rufus. The kidney size did not differ between the species (Table 2). However, differences occurred in most of the indices that were used to assess kidney function (Table 2). M. giganteus had significantly lower values for the medullary thickness index, PMT, and also for the area indices, RMA and PMA, all of which indicate a lesser concentrating ability in this species. The RMT of M. giganteus was not significantly lower than that of M. rufus in this study. The data for kidney indices suggested differences in electrolyte handling between the two kangaroo species. The characteristics of their plasma and urine are shown in Table 3. In the plasma, significant differences between the species were not seen but differences in the patterns of osmolality and electrolyte concentrations in the urine were noted. Urine osmolality in M. giganteus was lower than in M. rufus (Table 3) and this was reflected in the higher urinary concentrations of Na+ and K+ in M. rufus. Concentrations of Cl− in the urine of both species were high but variable; the urea concentrations could not be determined.
Behaviour
Behavioural patterns were similar for both species of kangaroo (Fig. 2). At night and during the cooler daylight hours, the kangaroos mostly foraged; although, at these times, resting and other activities (e.g. moving to water and drinking) also occurred (Fig. 2). As the T a and solar radiation increased in the morning (Fig. 1) the kangaroos generally moved into the shade and rested, mostly by lying down (Fig. 2). M. giganteus had a significantly greater focus on shade throughout the day (P<0.05) (Fig. 3). The difference in the use of shade was obvious in the morning since M. rufus foraged longer (Fig. 2), but it also occurred throughout the day (Fig. 3). Notably, M. giganteus largely used the dense shade of trees, whilst M. rufus also utilised the sparser shade offered by shrubs. The majority of both species were foraging again by 17:00 h.
The daily time use of kangaroos in late summer at Fowlers Gap Station. The behaviour was grouped into the broad categories of foraging (dark grey), resting (light grey) and locomotion (white), the latter additionally including drinking and other minor activities. a The behaviour patterns of M. rufus; b The behaviour patterns of M. giganteus. Sunrise was ∼6:00 h and sunset was ∼18:30 h
When resting in the middle of the day the kangaroos were mostly lying, but crouching, a posture that reduces the radiation heat inflow, was more common, overall, in M. giganteus (P<0.5) (Fig. 3). Individuals of both species were crouching more between 12:00 and 14:00 h than at other resting times (P<0.001). The crouching increased from below 20%, through the morning periods, to peak near 40%, in both species, between 12:00 and 13:00 h; it then declined, more quickly in M. rufus. Between 14:00 and 16:00 h the crouching in M. rufus was half (16%, P<0.01) that in M. giganteus.
Other differences in behaviour occurred between the species that could have an impact on the use of water. The total daily time active (foraging plus other activities) for M. rufus was 0.48±0.007 days (11.5 h), while for M. giganteus it was 0.52±0.002 days (12.5 h). In a broad analysis of daily activity in 3 h blocks, this difference between the species was not significant (P=0.19); however, the time of the day had significant impacts on kangaroo behaviour (P<0.0001) and significant interactions between the time and species were indicated (P<0.0001). To further examine activity patterns, the activity was partitioned into foraging and non-foraging activities (mainly locomotion and drinking). The foraging time differed significantly between species (P<0.05). The mean total daily foraging time for M. rufus was 0.414±0.0023 days (9.9 h), while for M. giganteus it was 0. 473±0.0032 days (11.4 h). A more detailed examination of the blocks where differences were indicated, showed that M. rufus foraged significantly more between 6:00 and 9:00 h (P<0.05). This was notably the case between 7:00 and 8:00 h (P<0.01), by which time the solar radiation and T bg had risen noticeably and M. giganteus was seeking shade (Figs. 1, 3). Conversely, in the evening between 21:00 and 24:00 h, M. giganteus foraged more (P<0.05). The amount of other non-foraging activities (mainly locomotion and drinking) also differed, with M. giganteus moving more than M. rufus (P<0.05); the difference was notable in the period 18:00–21:00 h (P<0.01) when M. giganteus moved regularly to drink. M. giganteus drank more than twice as often as M. rufus (P<0.001). The M. giganteus individuals drank on 13 instances, i.e. about each second day, but only three cases were noted for M. rufus; drinking usually occurred between 18:00 and 23:00 h.
Discussion
It is apparent from our direct comparisons and from previous investigations that M. giganteus has, relative to M. rufus, a lower capability for dealing with the problems of water relations in the hot arid rangelands. This low capability occurs despite their behavioural adjustments. In our study, which was conducted during conditions that approximated mean summer temperatures, the use of water by M. giganteus was 71.8±2.6 mL kg−1 day−1, as compared with 56.1±7.6 mL kg−1 day−1 for M. rufus (Table 1), i.e. M. giganteus used about half a litre (480 mL) more water per day than M. rufus. On an average, M. giganteus drank at 2 days intervals, which was more than twice as frequently as M. rufus, confirming the initial report of Caughley (1964). In similar weather, tagged wild M. rufus drank at a modal frequency of 5 days but many individuals returned to water much less frequently (Dawson et al. 1975). Sheep drink daily or even twice a day in these conditions (Dawson et al. 1975).
Structural differences occured in the kidneys of the two species of kangaroo (Table 2) and part of the difference in their WTR and drinking frequency appears linked to kidney structure and function. Species differences in plasma osmolality and electrolyte plasma concentrations did not occur (Table 3); they were comparable with previous data (Dawson et al. 1975; Denny and Dawson 1977; Blaney et al. 2000). However, the findings for urine concur with those of Blaney et al. (2000), who showed in laboratory studies that M. giganteus had renal water conserving capacities inferior to those of M. rufus; the mean maximal osmolalities during dehydration were 2,444 and 3,135 mosmol kg−1 for M. giganteus and M. rufus, respectively. The imbalance among the electrolytes (Table 3) is likely to be associated with urea (Denny and Dawson 1977). Although any significant difference in the RMT between the species was not found, a greater RMT in M. rufus has been previously reported in more extensive studies (Denny and Dawson 1977; Blaney et al. 2000) and our data shows similar patterns for the other renal structural indices (Table 2). Brownfield and Wunder (1976) considered the area indices, PMA and RMA, to be better indicators of kidney capabilities than the PMT and RMT, which relate the thickness of the renal medulla (i.e. the lengths of the loops of Henle) to the size of the kidney. Beuchat (1990) and Greenwald (1989) confirm that, overall, these indices are good predictors of renal water conserving abilities.
Are aspects of ecophysiology, other than kidney function, important to the water needs of M. giganteus relative to M. rufus? Urine collections from normally hydrated M. giganteus and M. rufus in metabolism cages (Denny and Dawson 1977; Blaney et al. 2000) indicate that differential urine production accounts for less than half of the 0.5 L difference in water turnover that was noted. In addition to the kidneys, thermoregulatory and digestive systems are also involved in water relations.
Due to their greater use of shade, exposure to a higher T bg may be eliminated as a cause of a higher use of water by M. giganteus. Laboratory studies inform us that both species are excellent homeotherms, with little difference in evaporative water use (Dawson et al. 2000a, b). However, responses in natural settings can be more complex. Although no differences in T b were seen at high T a in the laboratory (Dawson et al. 2000a, b), McCarron et al. (2001), earlier at this site, found differences in T b between M. giganteus and M. rufus during hot days. They found that in the wild M. rufus maintained a T b 2°C higher than M. giganteus during the hottest part of the day, but the implications of this, with regard to water needs, are uncertain. Actually, the greater shade seeking by M. giganteus (Fig. 3) should have reduced its relative thermoregulatory water needs. Solar radiation in the open peaked near 900 W m−2 in the middle of the day (Fig. 1). In the open, T bg approached 50°C; whereas, in full shade, it would differ little from T a. A further behavioural response to high heat loads is crouching in kangaroos, which reduces the surface area for heat inflow (Russell and Harrop 1976); this also occurred more significantly in resting M. giganteus (Fig. 3). The reduced morning foraging by M. giganteus relative to M. rufus (Fig. 2), was compensated for by extra foraging at night. Similar broad foraging patterns have been seen in semi-arid woodlands during hot and dry conditions (McCullough and McCullough 2000).
The water intake in feed is important to the total water turnover and drinking needs (Green 1989; Blaney et al. 2000). When M. giganteus and M. rufus were feeding in small yards on lush, green grass in winter, the WTRs were much elevated and did not differ. Neither species drank (Blaney et al. 2000). Even in the arid zone much water can come from feed. The grass eaten by the kangaroos studied contained 40–60% water, with other plants containing 55–75% water (Dawson et al. 2004). Feed intakes in the wild are not available and though the two species foraged for slightly different times (Fig. 2) studies in pens give similar maintenance intakes of near 600 g dry matter per day (adjusted to the size of the kangaroos) on feeds of comparable digestibility (Hume 1974; Dellow and Hume 1982). The field metabolic rates in summer seem to be not much higher than the maintenance requirements (McCarron et al. 2001), so the forage-water intakes would be about 600–800 mL day−1. When this intake is subtracted from the total daily water needs (i.e. WTR), M. giganteus would be left with an additional water requirement around twice that of M. rufus, which it appears to meet by more frequent drinking. At the other end of the gut, M. rufus possibly looses less water via faeces than M. giganteus. The large intestine of M. giganteus is significantly shorter than that of M. rufus (Osawa and Woodall 1992), and in other kangaroos the colon length correlates with faecal water retention (Freudenberger and Hume 1993).
Over much of the arid rangelands of eastern Australia reliable water sources were extremely scarce prior to the advent of domestic grazing. The water needs of sheep in the region mean that water must be provided at regular distances; sheep usually forage within 5 km of water. The expansion of stock watering sites has increased markedly over the past 30–40 years, in order to facilitate a more effective use of the rangeland (Dawson et al. 2004). The original kangaroos of this region, M. rufus and M. robutus erubescens, are more mobile and have a much less reliance on water than stock (Dawson et al. 1975).
Is range expansion, by the more mesic M. giganteus, only due to the provision of more water or are other factors involved? Expansion should only be facilitated if adequate and appropriate feed is available (Caughley et al. 1988; Dawson et al. 2004); evidence that M. giganteus is simply replacing M. rufus is lacking. An increase in water sources has also spread grazing pressure by sheep and cattle and, as a result, annual grasses and herbaceous forbs have increased (James et al. 1999), with shrubs of the family Chenopodiacae (saltbushes and bluebushes) being much reduced (Leigh and Mulham 1971). This will have benefited both M. giganteus and M. rufus, which both have a marked preferences for grass, with that of M. giganteus being the greater. M. giganteus has a significantly larger foregut than M. rufus and may handle more fibrous grasses (Dawson et al. 2004). However, the once dominant halophytic saltbushes and bluebushes are usually avoided as feed by both kangaroos (Dawson et al. 2004). Elsewhere in arid Australia, kangaroo populations have increased in association with changes in vegetation due to domestic stock (Newsome 1971). The interaction of feed and water is highlighted by the observation of Caughley et al. (1985) that, in drought, kangaroos of both species died near water, due to starvation.
In conclusion, M. giganteus is expanding into arid rangelands because livestock water sources have increased. Relative to the common arid zone kangaroo M. rufus, M. giganteus has high water needs and the stock waters provide for these. As these waters have spread so have vegetation changes associated with heavy grazing pressures. Perhaps unexpectedly, stock grazing has promoted annual grasses, thus providing extra resources for kangaroos, especially M. giganteus which is a grass specialist. However, because of its behavioural responses to heat, it would be expected that the spread of M. giganteus would be limited to habitats where substantial tree and scrub cover is provided.
References
Blaney CE, Dawson TJ, McCarron HCK, Buffenstein R, Krockenberger AK (2000) Water metabolism and renal function and structure in eastern grey kangaroos (Macropus giganteus): responses to water deprivation. Aust J Zool 48:335–345
Beuchat CA (1990) Metabolism and scaling of urine concentrating ability in mammals: resolution of a paradox? J Theor Biol 143:113–122
Brownfield MS, Wunder BA (1976) Relative medullary area: a new structural index for estimating urinary concentrating capacity of mammals. Comp Biochem Physiol 55A:69–75
Caughley G (1964) Social organisation and daily activity of the red kangaroo and the grey kangaroo. J Mammal 45:429–436
Caughley G, Brown B, Dostine P, Grice D (1984) The grey kangaroo overlap zone. Aust Wildl Res 11:1–10
Caughley G, Grice D, Barker R, Brown B (1988) The edge of the range. J Appl Ecol 57:771–785
Caughley G, Grigg GC, Smith L (1985) The effect of drought on kangaroo populations. J Wildl Manage 49:679–685
Caughley G, Short J, Grigg GC, Nix H (1987) Kangaroos and climate: and analysis of distribution. J Appl Ecol 56:751–761
Cunningham GM, Mulham WE, Milthorpe PL, Leigh JH (1981) Introduction. In: Plants of western New South Wales. Soil Conservation Service of New South Wales, Sydney, pp 9–26
Dawson TJ (1995) Kangaroos: biology of the largest marsupials. University of New South Wales Press, Sydney
Dawson TJ, Blaney CE, Munn AJ, Krockenberger A, Maloney SK (2000a) Thermoregulation by kangaroos from mesic and arid habitats: influence of temperature on routes of heat loss in eastern grey kangaroos (Macropus giganteus) and red kangaroos (Macropus rufus). Physiol Biochem Zool 73:374–381
Dawson TJ, Denny MJS (1969) A bioclimatical comparison of the summer day microenvironments of two species of arid-zone kangaroo. Ecology 50:328–332
Dawson TJ, Denny MJS, Russell EM, Ellis BA (1975) Water use and diet preferences of free ranging kangaroos, sheep and feral goats in the Australian arid zone during summer. J Zool, Lond 177:1–23
Dawson TJ, McTavish KJ, Ellis BA (2004) Diets and foraging behaviour of red kangaroos and eastern grey kangaroos in the arid shrub land: is feeding behaviour involved in the range expansion of the eastern grey kangaroo in the arid zone? Aust Mammal 20:169–178
Dawson TJ, Munn AJ, Blaney CE, Krockenberger A, Maloney SK (2000b) Ventilatory accommodation of oxygen demand and respiratory water loss in kangaroos from mesic and arid environments, the eastern grey kangaroo (Macropus giganteus) and the red kangaroo (M. rufus). Physiol Biochem Zool 73:382–388
Denny MJS (1975) The occurrence of the eastern grey kangaroo (Macropus giganteus Shaw) west of the Darling River. Search (Sydney) 6:89–90
Denny MJS, Dawson TJ (1975) Comparative metabolism of tritiated water by macropodid marsupials. Am J Physiol 228:1794–1799
Denny MJS, Dawson TJ (1977) Kidney structure and function of desert kangaroos. J Appl Physiol 42:636–642
Dellow DW, Hume ID (1982) Studies on the nutrition of macropodine marsupials 1. Intake and digestion of lucerne hay and fresh grass, Phalaris aquatica. Aust J Zool 30:391–398
Dunbar RIM (1976) Some aspects of research design and their implication in the observational study of behaviour. Behaviour 58:78–98
Freudenberger DO, Hume ID (1993) Effects of water restriction on digestive function in two macropodid marsupials from divergent habitats and the feral goat. J Comp Physiol B 163:247–257
Griffiths M, Barker R (1966) The plants eaten by sheep and by kangaroos grazing together in a paddock in south-western Queensland. CSIRO Wildl Res 11:145–167
Green B (1989) Water turnover in free-living macropodoids. In: Grigg G, Jarman P, Hume I (eds) Kangaroos, wallabies and rat kangaroos. Surrey Beatty and Sons, Chipping Norton, pp 223–229
Greenwald L (1989) The significance of renal relative medullary thickness. Physiol Zool 62:1005–1014
Heisinger JF, Breitenbach RP (1969) Renal structural characteristics as indexes of renal adaptation in the genus Sylvilagus. Physiol Zool 42:160–172
Hume ID (1974) Nitrogen and sulphur retention and fibre digestion by euros, red kangaroos and sheep. Aust J Zool 22:13–23
Hume ID (1999) Marsupial nutrition. Cambridge University Press, Cambridge
James CD, Landsberg J, Morton SR (1999) Provision of watering points in the Australian arid zone: a review of effects on biota. J Arid Environ 44:87–121
Krebs CJ (2002) Beyond population regulation and limitation. Wildl Res 29:1–10
Leigh JH, Mulham WE (1971) The effect of defoliation on the persistence of Atriplex vesicaria. Aust J Agric Res 22:239–244
McCarron HCK (1990) Environmental physiology of the eastern grey kangaroo (Macropus giganteus Shaw). PhD Thesis, University of New South Wales, Sydney
McCarron HCK, Buffenstein R, Fanning FD, Dawson TJ (2001) Free-ranging heart rate, body temperature and energy metabolism in eastern grey kangaroos (Macropus giganteus) and red kangaroos (Macropus rufus) in the arid regions of south east Australia. J Comp Physiol B 171:401–411
McCullough DR, McCullough Y (2000) Kangaroos in outback Australia: comparative ecology and behaviour of three coexisting species. Columbia University Press, New York
Moss GL, Croft DB (1999) Body condition of the red kangaroo (Macropus rufus) in arid Australia: the effect of environmental condition, sex and reproduction. Aust J Ecol 24:97–109
Nagy KA, Bradshaw SD (2000) Scaling of energy and water fluxes in free-living arid-zone Australian marsupials. J Mammal 81:962–970
Nagy KA, Costa DP (1980) Water flux in animals: analysis of potential errors in the tritiated water method. Am J Physiol 238:R454–R465
Nagy KA, Sanson GD, Jacobsen NK (1990) Comparative field energetics of two macropod marsupials and a ruminant. Aust Wildl Res 17:591–599
Newsome AE (1971) The ecology of red kangaroos. Aust Zool 16:32–50
Osawa R, Woodall PF (1992) A comparative study of the macroscopic and microscopic dimensions of the intestine in five macropods (Marsupialia: Macropodidae). II. Relationship with feeding habits and fibre content of the diet. Aust J Zool 40:99–113
Russell EM, Harrop CJF (1976) The behaviour of red kangaroos (Megaleia rufa) on hot summer days. Z Tierpsychol 40:396–426
Sperber I (1944) Studies on the mammalian kidney. Zool Bidrag Fran Ups 22:249–432
Vaughan BE, Boling EA (1961) Rapid assay procedures for tritium-labelled water in body fluids. J Lab Clin Med 57:159–164
Acknowledgements
We thank the staff at the University of New South Wales Arid Zone Research Station, Fowlers Gap for their assistance. Dr. SK Maloney gave valuable advice on the statistical analysis. The kangaroos were kept under the provisions of a scientific fauna licence granted by the New South Wales, National Parks and Wildlife Service. Approval for the study (ACE 99/23) was given by the University of New South Wales Animal Care and Ethics Committee. This study was funded by Australian Research Council grants to TJD.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Dawson, T.J., McTavish, K.J., Munn, A.J. et al. Water use and the thermoregulatory behaviour of kangaroos in arid regions: insights into the colonisation of arid rangelands in Australia by the Eastern Grey Kangaroo (Macropus giganteus). J Comp Physiol B 176, 45–53 (2006). https://doi.org/10.1007/s00360-005-0030-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-005-0030-2