Abstract
Seasonal adjustments in body mass and thermogenesis are important for the survival of small mammals during acclimatization in the temperate zone. To determine the contributions of short photoperiod and cold temperatures to seasonal changes in thermogenesis and body mass in Mongolian gerbils (Meriones unguiculatus), body mass, basal metabolic rate (BMR), nonshivering thermogenesis (NST), energy intake and energy digestibility were determined in seasonally acclimatized and laboratory acclimated animals. Body mass showed significant seasonal changes and decreased to a minimum in winter. Both BMR and NST increased in winter, and these changes were mimicked by exposing animals to short photoperiod or cold temperatures in the animal house. Digestible energy intake also increased significantly in winter, and also during exposure of housed animals to both short photoperiod and cold. These results suggest that Mongolian gerbils overcome winter thermoregulatory challenges by increasing energy intake and thermogenesis, and decreasing body mass to reduce total energy requirements. Short photoperiod and cold can serve as effective environmental cues during seasonal acclimatization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-hibernating small mammals in the temperate zone face the shortage of available food and increased energy demand in winter (Merritt 1995; Wang and Wang 1996; Jackson et al. 2001). Potential imbalances in food supply and energy demand pose strong selective pressure for the evolution of physiological and behavioral adaptations that enhance their probability of survival over winter (Nagy 1993a; Jackson et al. 2001).
An important physiological strategy for small mammals to cope with cold winter is to increase the capacity for heat production, particularly nonshivering thermogenesis (NST) in brown adipose tissue (Heldmaier et al. 1982; Klaus et al. 1988; Merritt and Zegers 1991; Merritt 1995; Wang and Wang 1996; Kronfeld-Schor et al. 2000). Seasonal cycles of NST have been studied in several small mammals including Djungarian hamsters (Heldmaier et al. 1982), desert spiny mice (Acomys russatus) (Kronfeld-Schor et al. 2000), Masked shrews (Sorex cinereus) (Merritt 1995), Gapper’s red-backed voles (Clethrionomys gappei) (Merritt and Zegers 1991), plateau pikas (Ochotona curzoniae), and root voles (Microtus oeconomus) (Wang and Wang 1996). Most studies showed that increased thermogenic capacity during cold periods was due to the increased capacity of NST (Merritt et al. 2001).
Changes in body mass are the result of the balance between energy intake and expenditure and decrease in body mass is one of the means to save energy (Merritt et al. 2001). Most small mammals, such as Djungarian hamsters (Phodopus sungorus), South American field mice (Abrothrix andinus), prairie voles (M. ochrogaster), and meadow voles (M. pennsylvanicus) decrease their body mass in cold seasons (Iverson and Turner 1974; Steinlechner et al. 1983; Bartness and Wade 1985; Bozinovic et al. 1990; Voltura 1996). However, some species such as collared lemmings (Dicrostonyx groenlandicus), Syrian hamsters (Mesocricetus auratus), and Pampas mice (Akodon azarze) increase their body mass by accumulating energy reserves for the coming winter (Bartness and Wade 1984; Nagy et al. 1995; Del Valle and Busch 2003). The maintenance of a constant body temperature is expensive for winter-active small mammals. However, there are relatively few data on energy budgets for seasonal acclimatized small mammals.
Photoperiod and temperature are two important environmental factors that are involved in seasonal control of body mass and thermogenesis in small mammals (Heldmaier et al. 1981, 1982; Jansky et al. 1986; Wang et al. 1999; Knopper and Boily 2000; Powell et al. 2002; Peacock et al. 2004). Generally, short photoperiod and/or cold can significantly reduce body mass and enhance thermogenic capacity in some small mammals (Dark et al. 1983; Voltura and Wunder 1998; Wang et al. 1999; Klingenspor et al. 2000; Knopper and Boily 2000; Peacock et al. 2004). Whereas with seasonal variations, short photoperiod and/or cold can significantly increase body mass in some small rodent species such as collared lemmings (Powell et al. 2002) and golden hamsters (Jansky et al. 1986).
Mongolian gerbils (Meriones unguiculatus) mainly live in the Inner Mongolian grasslands of China, Mongolia, and the region of Beigaer in Russia (Zhang and Wang 1998). In these regions, winter lasts for more than 6 months. It has been reported that free-living Mongolian gerbils show seasonal changes in NST but a relatively stable BMR (Wang et al. 2003). Mongolian gerbils have a wide thermal neutral zone (TNZ) and can maintain maximal energy intake over a wide range of ambient temperatures, in contrast to other cold desert mammals (Wang et al. 2000; Liu et al. 2002). Short photoperiod or cold can induce an increase in BMR, NST, and energy intake, but cannot cause the change in body mass alone (Li et al. 2001, 2003, 2004). No data are available on the energy intake of seasonally acclimatized and laboratory acclimated Mongolian gerbils so far. We hypothesized that Mongolian gerbils can enhance their winter survival by adjusting body mass, energy intake, and thermogenesis seasonally. We predicted that they can increase thermogenic capacity and energy intake, and decrease body mass in short photoperiod or cold conditions. In the present study, we traced seasonal changes in body mass, energy intake, basal metabolic rate (BMR), and nonshivering thermogenesis (NST) in Mongolian gerbils in an outdoor enclosure and determined the effects of photoperiod and temperature in the laboratory for 4-week acclimated animals.
Material and methods
Animals and experimental designs
The animals were the offspring of adult Mongolian gerbils captured in Inner Mongolian Grasslands in May 1999 and transported to the Institute of Zoology, Chinese Academy of Sciences in Beijing, China. Gerbils were housed in groups (3–5) in plastic cages (30×15×20 cm3 high) with sawdust bedding. All the animals were maintained under 16L:8D photoperiod with light on at 0400 h, and room temperature was kept at 23±1°C. Subjects were fed ad libitum with standard rat chow and water.
Experiment I
To test for seasonal changes in body mass, energy intake, and thermogenesis, we moved the gerbils (70–90 days of age) from the animal house to an outdoor enclosure, and held them individually in plastic cages (30×15×20 cm3 high). After 1 month stabilization in the outdoor enclosure, body mass was monitored at 15-day intervals, and environmental temperature, energy intake, BMR, and NST were measured in August, October, and December of 2001, and February, May and June of 2002.
Experiment II
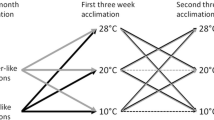
To test for photoperiod and temperature effects on seasonal changes in the physiological parameters measured in Experiment I, gerbils (70–90 days of age) were randomly assigned to the following four experimental regimes in the animal house: long photoperiod (LD, 16L:8D) and warm (23°C); long photoperiod (LD, 16L:8D) and cold (5°C); short photoperiod (SD, 8L:16D) and warm (23°C); short photoperiod (SD, 8L:16D) and cold (5°C). The animals were acclimated for 4 weeks. Body mass was monitored every 3 days and energy intake, BMR, and NST were measured at the start and end of the experiments as described previously. This experiment was conducted from March to May in 2001.
Metabolic trials
Basal metabolic rate was measured by using an established closed-circuit respirometer (Gorecki 1975; Song and Wang 2003a; Liu et al. 2004) at 29°C within their thermal neutral zone (Li et al. 2001; Wang et al. 2003). Briefly, the metabolic chamber volume was 3.6L and the temperature inside the chamber was maintained by a water bath (±0.5°C). KOH and silica gel were used to absorb carbon dioxide and water respectively in the metabolic chamber. Gerbils were fasted for 3 h before being moved into the metabolic chambers. After 60 min in the chambers, oxygen consumption was recorded for a further 60 min at 5 min intervals. The two stable consecutive lowest readings were taken to calculate BMR and corrected to standard temperature and pressure (STP) (Song and Wang 2003a; Liu et al. 2004). Body temperature was measured before and after each test. All metabolic measurements were taken between 10:00 and 17:00 h to minimize any effects of circadian rhythms.
Nonshivering thermogenesis was measured on the next day and induced with subcutaneous injections of norepinephrine (NE) (Shanghai Harvest Pharmaceutical Co. LTD) at 25±1°C which is near the lower critical temperature (Wang et al. 2003; Li et al. 2004). The dosage of NE was calculated according to the equation described by Heldmaier (1971): NE dosage (mg/kg) =6.6 M −0.458b (g), where M b is body mass in gram. Oxygen consumption was recorded for 60 min with 5 min intervals. The two consecutive highest recordings of oxygen consumption were taken to calculate the maximum NST (Wang and Wang 1996; Wang et al. 1999; Li et al. 2001), and corrected to the STP conditions.
Energy intake and digestibility
Energy intake was measured for 3-day intervals as described previously (Song and Wang 2001, 2002; Liu et al. 2002). During each test, gerbils were housed individually in stainless steel mesh metabolic cage (0.24×0.24×0.24 m3 high), in which food and water were provided ad libitum. Uneaten food and feces were collected after the 3-day test, and separated manually and oven-dried at 70°C for at least 72 h. The caloric values of food and feces were determined by Parr1281 oxygen bomb calorimeter (Parr Instrument, USA). Dry matter intake, gross energy intake, digestible energy, and digestibility were calculated by the following equations (Grodzinski and Wunder 1975; Song and Wang 2001; Liu et al. 2002, 2003):
It should be noted that all digestibilities are apparent digestibilities.
Data analysis
Data analysis was carried out using SPSS package (SPSS 1998). Distributions of all variables were tested for normality using the Kolmogorov-Smirnov test. Abnormally distributed data were transformed to natural logarithms for normalization. Seasonal data such as body mass, BMR, NST, and energy intake were obtained from the same animals and were analyzed by general linear model (GLM) repeated measures ANOVA, followed by an LSD post-hoc test for individual comparisons. To remove the effect of body mass on these parameters, BMR, NST, and energy intake were scaled to the 0.67 power of body mass (M 0.67b ) as proposed for rodents (Hayssen and Lacy 1985; Pei et al. 2001). Two-way ANCOVA was used to detect the effects of photoperiod and temperature on body mass, BMR, NST, and energy intake, using body mass as the covariate. Differences among groups were detected by LSD post-hoc tests. All values in the text are expressed as mean ± SEM, and P<0.05 was considered to be statistically significant.
Results
Mean, maximum and minimum ambient temperatures during seasonal acclimatization are shown in Table 1.
Experiment I
Body mass
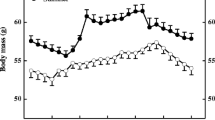
Body mass of Mongolian gerbils showed significant seasonal changes (F (20, 100)=18.907, P<0.01, Fig. 1). Body mass remained stable from July to September (LSD, P>0.05) and then decreased from September to a minimum in November (LSD, P<0.05, Fig. 1). After that, body mass began to increase and reached a maximum in June. Compared with November, body mass of gerbils in June increased by 47% (LSD, P<0.05, Fig. 1).
BMR and NST
Both BMR and NST showed significant seasonal changes (BMR, F (5, 25)=8.854, P<0.01; NST, F (5, 25)=17.879, P<0.01, Fig. 2). Scaling the data to M −0.67b resulted in the same statistical outcome (not shown in Fig. 2). From August through December, BMR and NST increased significantly, reached a peak in December and February (BMR: 118.3±3.7 mlO2/h; NST: 361.0±32.9 O2/h) respectively, then declined to a minimum in June(BMR: 98.2±8.0 mlO2/h; NST: 228.9±50.0 O2/h). Compared with December, BMR and NST decreased by 17 and 31% in June, respectively (LSD, P<0.05, Fig. 2).
Energy intake and digestibility
Gross energy intake varied significantly over the seasons (F (5, 25)=2.977, P<0.05, Table 2). Energy intake in February increased by 51% compared with that in June (LSD, P<0.05). Similar patterns were seen in digestible energy intake (F (5, 25)=2.638, P<0.05), which was the highest in February and lowest in June (LSD, P<0.05, Table 2). Digestibility showed no significant seasonal variation.
Experiment II
Body mass
Prior to acclimation, no differences were found in the body mass of Mongolian gerbils between groups. During acclimation, no significant changes were observed among the other three groups, even though animals in warm and short photoperiod conditions increased their body mass significantly (t= −3.560, P<0.01, Table 3). At the end of the experiment, no effect of photoperiod and temperature on final body mass was detected (photoperiod, F=0.057, P>0.05; temperature, F=0.446, P>0.05; interaction, F=0.507, P>0.05, Table 3).
BMR and NST
Initial BMR and NST showed no differences among groups. During acclimation, NST increased by 41% under short photoperiod and cold conditions (t= −8.661, P<0.01, Table 3). At the end of acclimation, temperature had a significant effect on BMR, with cold inducing a 16% higher BMR than that after warm acclimation conditions (F=8.153, P<0.01), but there was no effect of photoperiod, and no significant interaction between photoperiod and temperature (photoperiod, F=0.732, P>0.05; interaction, F=0.246, P>0.05). Both short photoperiod and cold caused NST to increase by 11 and 21% respectively (photoperiod, F=4.978, P<0.05; temperature, F=18.570, P<0.01, Table 3). There was no significant interaction between photoperiod and temperature on NST (F=3.015, P>0.05).
Energy intake and digestibility
There were no significant effects of photoperiod and temperature on dry matter intake, gross energy intake, digestible energy intake or digestibility before acclimation (P>0.05). However, dry matter intake (Photoperiod, F=4.400, P<0.05, temperature, F=46.725, P<0.01), gross energy intake (Photoperiod, F=4.400, P<0.05, temperature, F=46.725, P<0.01), digestible energy intake (Photoperiod, F=1.872, P>0.05, temperature, F=44.692, P<0.01), and digestibility (Photoperiod, F=18.844, P<0.01, temperature, F=7.771, P<0.01) under short photoperiod and/or cold increased more than under long photoperiod and warm conditions after 3 days of acclimation (Table 4), and there was a significant interaction between photoperiod and temperature (P<0.01). The effect of temperature persisted to the end of acclimation (dry matter intake, F=324.297, P<0.01; gross energy intake, F=324.297, P<0.01; digestible energy intake, F=3245.300, P<0.01; digestibility, F=2.441, P>0.05, Table 4). At the end of acclimation, dry matter intake, gross energy intake, and digestible energy intake in the two cold groups increased by 70 and 71%, 70 and 71%, 73 and 71% respectively compared with that of the initial. There were no significant differences in digestibility.
Discussion
In the present study, it was clear that Mongolian gerbils showed seasonal changes in body mass, which was lowest in winter and highest in summer. This pattern is similar to a sympatric species—Brandt’s voles (M. brandtii) (Li and Wang 2005), and other small mammals in temperate zones (Iverson and Turner 1974; Steinlechner et al. 1983; Klaus et al. 1988; Bozinovic et al. 1990; Merritt 1995; Wang and Wang 1996). In the Djungarian hamster, body mass in winter decreased by 30% compared with summer (Steinlechner et al. 1983). Iverson and Turner (1974) reported that mean body mass of M. pennsylvanicus also decreased by 30–40% from August (summer) to February (winter). Winter-active small mammals have great energy demands in cold periods and it has been thought that a decrease in body mass helps them to cope with winter stress by reducing their total energy requirements (Merritt and Zegers 1991; Merritt 1995; Merritt et al. 2001). The decrease in body mass could result from changing energy reserves and/or thermoregulatory heat production (Klaus et al. 1988; Voltura and Wunder 1998; Merritt et al. 2001; Bartness et al. 2002). However, a decrease in body mass will increase the ratio of surface-to-volume, which can cause greater heat loss, and thus increase living costs. Generally, small mammals possess less cooling resistance than large mammals and, therefore, imposed much more cost for endothermy, especially for winter-active small mammals (Merritt 1995; Merritt et al. 2001).
Short photoperiod and/or cold can cause a decrease in body mass of some small mammals (Iverson and Turner 1974; Heldmaier et al. 1982; Steinlechner et al. 1983; Bartness and Wade 1985; Klingenspor et al. 2000; Knopper and Boily 2000; Zhao and Wang 2005). For Djungarian hamsters, the decrease in body mass in winter was mainly caused by short photoperiod (Knopper and Boily 2000), while in species of Microtus, such as M. pennsylvanicus, it resulted mainly from lack of food (Iverson and Turner 1974). In the present study, both short photoperiod and cold had no significant effects on body mass in 4-week acclimated Mongolian gerbils. We recently reported that under constant long photoperiod, cold can increase BMR, NST, and energy intake in this species, but not body mass (Li et al. 2004). We have also found that short photoperiod alone can increase BMR and energy intake in Brandt’s voles, but not body mass and NST (Zhao and Wang 2005). There is also evidence that acclimation time and photoperiod history can influence responses to environmental factors (Nagy 1993; Veloso and Bozinovic 2000).
Small mammals cope with cold mainly by increasing their capacity for thermogenesis (Heldmaier 1982; Klaus et al. 1988; Merritt and Zegers 1991; Merritt 1995; Wang and Wang 1996; Kronfeld-Schor et al. 2000; Li and Wang 2005). In the present study, Mongolian gerbils increased BMR and NST in winter, consistent with previous findings in the laboratory and field (Li et al. 2001; Wang et al. 2003) and similar to other rodent species living in cold regions (Heldmaier et al. 1982; Wang and Wang 1996; Li and Wang 2005). Spiny mice living in a hot rocky desert also increased NST capacity by 112–170% higher in winter than in summer (Kronfeld-Schor et al. 2000). Heldmaier et al. (1982) showed that the NST capacity of Djungarian hamsters increased by 71% in winter compared with summer. Plateau pikas and root voles living in Qinghai-Tibet alpine meadow (Wang and Wang 1996) and Brandt’s voles in Inner Mongolian grasslands (Li and Wang 2005) also showed similar patterns. Enhancement of thermogenesis in winter is common for temperate and arctic small mammals (Rosenmann et al. 1975; Heldmaier et al. 1982; Merritt and Zegers 1991; Merritt 1995; Merritt et al. 2001).
During seasonal acclimatization many factors such as temperature, photoperiod, and food quality and quantity can all affect NST capacity in small mammals (Heldmaier et al. 1982; Wunder and Gettinger 1996; Nespolo et al. 1999; Wang et al. 1999). Cold appears to be the dominant factor in the inducion of increased NST capacity, while short photoperiod can also stimulate the development of NST (Heldmaier and Steinlechner 1981; Heldmaier et al. 1982). In the present study, both short photoperiod and cold induced a significant enhancement in BMR and NST in Mongolian gerbils, as has been shown in some other small mammals (Heldmaier et al. 1982; Wang et al. 1999; Zhao and Wang 2005). The high BMR has been found to be due to the increased development of body organs, especially the gastrointestinal tract, associated with the processing of high food intakes (Speakman et al. 2000; Song and Wang 2002, 2003b). The high NST is due to the increased thermogenic properties of brown adipose tissue mitochondria, such as increased cytochrome c oxidase activity and increased contents of uncoupling protein (Heldmaier et al. 1981, 1982; Klaus et al. 1988; Klingenspor et al. 1989; Zhao and Wang 2005; Li and Wang 2005). Speakman (1996) suggested that small mammals entering winter have a choice of thermoregulatory strategies. They can choose low BMR/low NST, which results in low total energy demands and therefore high survival in mild winters. Alternatively, they can maintain high BMR/high NST in severe cold winters, as suggested by the results of this study with Mongolian gerbils, which enable them to survive long cold periods in extreme climates.
The balance between energy acquisition and expenditure is critical to an animal’s survival and reproductive success (Bozinovic et al. 2004; Nagy and Negus 1993). This balance depends on the interplay among energy intake, digestion processing, and the energy allocation to alternative functions such as thermoregulation, growth, reproduction, and others (Nagy and Negus 1993; Wang and Wang 1996; Bacigalupe and Bozinovic 2002). In Mongolian gerbils, energy intake increased during winter and cold conditions, partly in response to increases in BMR and NST in winter. Liu et al. (2002) also found that Mongolian gerbils increase food intake in cold conditions. Collared lemmings housed at 5°C had a 37% higher food intake than those housed at 18°C (Nagy and Negus 1993). We found that in Brandt’s voles, the winter decrease in body mass was accompanied by increased energy intake and enhanced NST as well as by decreased body fat mass and reduced levels of circulating leptin: we suggest that leptin may serve as a starvation signal in the regulation of energy balance (Li and Wang 2005).
In summary, Mongolian gerbils decreased body mass and increased BMR, NST, and energy intake in winter. Short photoperiod and cold seem to be the environmental cues involved. The increase in energy intake and thermogenesis under cold conditions can enhance winter survival. Decreased body mass also helps by lowering total energy requirements. Another possible interpretation for the seasonal variations in body mass is that Mongolian gerbils are hyperphagic in late winter and early spring to fatten before breeding ensues. This possibility needs to be further investigated.
References
Bacigalupe LD and Bozinovic F (2002) Design, limitations and sustained metabolic rate: lessons from small mammals. J Exp Biol 205:2963–2970
Bartness TJ and Wade GN (1984) Photoperiodic control of body weight and energy metabolism in Syrian hamsters (Mesocricetus auratus): role of pineal gland, melatonin, gonads, and diet. Endocrinology 114:492–498
Bartness TJ and Wade GN (1985) Photoperiodic control of seasonal body weight cycles in hamsters. Neurosci Biobehav Rev 9:599–612
Bartness TJ, Demas GE, Song CK (2002) Seasonal changes in adiposity: the roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Exp Biol Med 227:363–376
Bozinovic F, Novoa FF, Veloso C (1990) Seasonal changes in energy expenditure and digestive tract of Abrothrix andinus (Cricetidae) in the Andes Range. Physiol Zool 63:1216–1231
Bozinovic F, Bacigalupe LD, Vasquez RA, Visser GH, Veloso C, Kenagy GJ (2004) Cost of living in free-ranging degus (Octodon degus): seasonal dynamics of energy expenditure. Comp Biochem Physiol A 137:597–604
Dark J, Zucker I, Wade GN (1983) Photoperiodic regulation of body mass, food intake, and reproduction in meadow voles. Am J Physiol 245:R334–R338
Del Valle JC and Busch C (2003) Body composition and gut length of Akodon azarae (Muridae: Sigmodontinae): relationship with energetic requirements. Acta Theriol 48(3):347–357
Gorecki A (1975) Kalabukhov-Skvortsov respirometer and resting metabolic rate measurement. In: Grodzinski W, Klekowski R, Duncan ZA (eds) Methods for ecological energetics. Blackwell, Oxford, pp 309–313
Grodzinski W, Wunder BA (1975) Ecological energetics of small mammals. In: Golley FB, Petrusewicz K, Ryszkowski L (eds) Small mammls: their productivity and population dynamics. Cambridge University Press, Cambridge, pp 173–204
Hayssen V, Lacy RC (1985) Basal metabolic rate in mammals: taxonomic differences in the allometry of BMR and body mass. Comp Biochem Physiol 81A:741–754
Heldmaier D (1971) Zitterfreie warmebidung und körpergröbe säugetieren. Z Vergl Physiol 73:222–248
Heldmaier G and Steinlechner S (1981) Seasonal control of energy requirements for thermoregulation in the Djungarian hamster (Phodopus sungorus), living in natural photoperiod. J Comp Physiol B 142:429–437
Heldmaier G, Steinlechner S, Rafael J, and Vsiansky P (1981) Photoperiodic control and effects of melatonin on nonshivering thermogenesis and brown adipose tissue. Science 212:917–919
Heldmaier G, Steinlechner S, Rafael J, Latteier B (1982) Photoperiod and ambient temperature as environmental cues for seasonal thermogenic adaptation in the Djungarian hamster, Phodopus sungorus. Int J Biometeorol 26:339–345
Heldmaier G, Steinlechner S, Rafael J (1982) Nonshivering thermogenesis and cold resistance during seasonal acclimation in the Djungarian hamster. J Comp Physiol B 149:1–9
Iverson SL, Turner BN (1974) Winter weight dynamics in Microtus pennsylvanicus. Ecology 55:1030–1041
Jackson DM, Hambly C, Trayhurn P, Speakman JR (2001) Associations between energetics and over-winter survival in the short-tailed field vole Microtus agrestis. J Anim Ecol 70:633–640
Jansky L, Haddad G, Pospisilova D, Dvorak P (1986) Effect of external factors on gonadal activity and body mass of male golden hamsters (Mesocricetus auratus). J Comp Physiol B 156:717–725
Klaus S, Heldmaier G, Ricquier D (1988) Seasonal acclimation of bank voles and wood mice: nonshivering thermogenesis and thermogenic properties of brown adipose tissue mitochondria. J Comp Physiol B 158:157–164
Klingenspor M, Klaus S, Wiesinger H, Heldmaier G (1989) Short photoperiod and cold activate brown fat lipoprotein lipase in the Djungarian hamster. Am J Physiol 257:R1123–R1127
Klingenspor M, Niggemann H, Heldmaier G (2000) Modulation of leptin sensitivity by short photoperiod acclimation in the Djungarian hamster, Phodopus sungorus. J Comp Physiol B 170:37–43
Knopper LD and Boily P (2000) The energy budget of captive Siberian hamster, Phodopus sungorus, exposed to photoperiod changes: mass loss is caused by a voluntary decrease in food intake. Physiol Biochem Zool 73:517–522
Kronfeld-Schor N, Haim A, Dayan T, Zisapel N, Klingenspor M, Heldmaier G (2000) Seasonal thermogenic acclimation of diurnally and nocturnally active desert spiny mice. Physiol Biochem Zool 73:37–44
Li QF, Sun RY, Huang CX, Wang ZK, Liu XT, Hou JJ, Liu JS, Cai LQ, Li N, Zhang SZ, Wang Y (2001) Cold adaptive thermogenesis in small mammals from different geographical zones of China. Comp Biochem Physiol A 129:949–961
Li XS, Wang DH (2005) Regulation of body weight and thermogenesis in seasonally acclimatized Brandt’s voles (Microtus brandti). Horm Behav (in press)
Li XS, Wand DH, Yang JC (2003) Effect of photoperiod on body weight and energy metabolism in Brandt’s voles (Microtus brandti) and Mongolian gebils (Meriones unguiculatus) (in Chinese with English summary). Acta Theriol Sin 23:304–311
Li XS, Wang DH, Yang M (2004) Effects of cold acclimation on body weight, serum leptin level, energy metabolism and thermogenesis in the Mongolian gerbil (Meriones unguiculatus) (in Chinese with English summary). Acta Zool Sin 50:334–340
Liu H, Wang DH, Wang ZW (2002) Maximum metabolizable energy intake in the Mongolian gerbil (Meriones unguiculatus). J Arid Environ 52:405–411
Liu H, Wang DH, Wang ZW (2003) Energy requirement during reproduction in female Brandt’s voles (Microtus brandti). J Mammal 84(4):1410–1416
Liu JS, Wang DH, Sun RY (2004) Metabolism and thermoregulation in three species of rodent from Northeastern China. J Therm Biol 29(3):177–183
Merritt JF (1995) Seasonal thermogenesis and body changes in body mass of masked shrews, Sorex cinereus. J Mammal 76:1020–1035
Merritt JF and Zegers DA (1991) Seasonal thermogenesis and body mass dynamics of Clethrionomys gapperi. Can J Zool 69:2771–2777
Merritt JF, Zegers DA, Rose LR (2001) Seasonal thermogenesis of southern flying squirrels (Glaucomys volans). J Mammal 82:51–64
Nagy TR (1993) Effects of photoperiod history and temperature on male collared lemmings, Dicrostonyx groenlandicus. J Mammal 74:990–998
Nagy TR and Negus NC (1993) Energy acquisition and allocation in male collared lemmings (Dicrostonyx groenlandicus): effects of photoperiod, temperature, and diet quality. Physiol Zool 66:737–560
Nagy TR, Gower BA, Stetson MH (1995) Endocrine correlates of seasonal body weight dynamics in the collared lemming (Dicrostonyx groenlandicus). Am Zool 35:246–258
Nespolo RF, Opazo JC, Rosenmann M, Bozinovic F (1999) Thermal acclimation, maximum metabolic rate and nonshivering thermogenesis in Phyllotis xanthopygus (Rodentia) inhabiting the Andean range. J Mammal 80:742–748
Peacock WL, Krol E, Moar KM, McLaren JS, Mercer JG, Speakman JR (2004) Photoperiodic effects on body mass, energy balance and hypothalamic gene expression in the bank vole. J Exp Biol 207:165–177
Pei YX, Wang DH, Hume ID (2001) Effects of dietary fibre on digesta passage, nutrient digestibility, and gastrointestinal tract morphology in the granivorous Mongolian gerbils (Meriones unguiculatus). Physiol Biochem Zool 74:742–749
Powell CS, Blaylock ML, Wang R, Hunter HL, Johanning GL, Nagy TR (2002) Effects of energy expenditure and Ucp1 on photoperiod-induced weight gain in collard lemmings. Obes Res 10:541–550
Rosenmann M, Morrison P, Feist D (1975) Seasonal changes in the metabolic capacity of red-backed voles. Physiol Zool 48:303–310
Song ZG, Wang DH (2001) Maximum energy assimilation rate in Brandt’s vole (Microtus brandti) from Inner Mongolian grassland (in Chinese with English summary) . Acta Theriol Sin 21:271–278
Song, ZG, Wang DH (2002) Relationships between metabolic rates and body composition in the Mongolian gerbil (Meriones unguiculatus) (in Chinese with English summary). Acta Zoologica Sinica 48:445–451
Song ZG, Wang DH (2003a) Metabolism and thermoregulation in the striped hamster Cricetulus barabensis. J Therm Biol 28:509–514
Song, ZG, Wang DH (2003b) Relationships between metabolic rate and body composition in Brandt’s voles (Microtus brandti) (in Chinese with English summary). Acta Theriol Sin 23:230–234
Speakman (1996) Energetics and the evolution of body size in small terrestrial mammals. Symp Zool Soc Lond 69:63–81
Speakman JR, Johnson MS (2000) Relationships between resting metabolic rate and morphology in lactating mice: what tissues are the major contributors to resting metabolism?. In: Heldmaier G, Klingenspor M (eds) Life in the cold. Springer, Berlin Heidelberg New York, pp 497–486
Steinlechner S, Heldmaier G, Becker H (1983) The seasonal cycle of body weight in the Djungarian hamster: photoperiod control and the influence of starvation and melatonin. Oecologia 60:401–405
Veloso C and Bozinovic F (2000) Interplay between acclimation time and diet quality on basal metabolic rate in females of degus Octodon degus (Rodentia: octodontidae). J Zool 252:531–533
Voltura MB (1996) Seasonal variation in body composition and gut capacity of the prairie vole (Microtus ochrogaster). Can J Zool 75:1714–1719
Voltura MB and Wunder BA (1998) Effects of ambient temperature, diet quality, and food restriction on body composition dynamics of the prairie vole, Microtus ochrogaster. Physiol Zool 71:321–328
Wang DH, Wang ZW (1996) Seasonal variations on thermogenesis and energy requirements of plateau pikas Ochotona curzoniae and root voles Microtus oeconomus. Acta Theriol 41:225–236
Wang DH, Sun RY, Wang ZW, Liu JS (1999) Effects of temperature and photoperiod on thermogenesis in plateau pikas (Ochotona curzoniae) and root voles (Microtus oeconomus). J Comp Physiol B 169:77–83
Wang DH, Wang YS, Wang ZW (2000) Metabolism and thermoregulation in the Mongolian gerbils Meriones unguiculatus. Acta Theriol 45(2):183–192
Wang DH, Wang ZW, Wang YS, Yang JC (2003) Seasonal changes of thermogenesis in Mongolian gerbils (Meriones unguiculatus) and Brandt’s voles (Microtus brandti) (Abstract). Comp Biochem and Physiol A 134:S96
Wunder BA, Gettinger RD (1996) Effects of body mass and temperature acclimation on the nonshivering thermogenic response of small mammals. In: Geiser F, Hulbert AJ, Nicol SC (eds) Adaptions to the cold: tenth international hibernation symposium. University of New England Press, Armidale, pp 131–139
Zhang ZB, Wang ZW (1998) Ecology and management of rodent pests in agriculture (In Chinese with English summary). Ocean Press, Beijing
Zhao ZJ,Wang DH (2005) Short photoperiod enhances thermogenic capacity in Brandt's voles. Physiol Behav 85(2):143-149
Acknowledgements
We are grateful to Professor Ian Hume, University of Sydney, Australia, for his helpful comments and correcting language errors. This study was supported by grants from the National Natural Science Foundation of China (No. 30170151 and 30430140) and the Chinese Academy of Sciences (No. KSCX2-SW-103) to DHW, and the State Key Laboratory of Integrated Management of Pest Insects and Rodents (IMP0302 and IMP0405).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume
Rights and permissions
About this article
Cite this article
Li, XS., Wang, DH. Seasonal adjustments in body mass and thermogenesis in Mongolian gerbils (Meriones unguiculatus): the roles of short photoperiod and cold. J Comp Physiol B 175, 593–600 (2005). https://doi.org/10.1007/s00360-005-0022-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-005-0022-2