Abstract
For many mammalian species short-term fasting is associated with intestinal atrophy and decreased digestive capacity. Under natural conditions, strictly carnivorous animals often experience prey scarcity during winter, and they may therefore be particularly well adapted to short-term food deprivation. To examine how the carnivorous gastrointestinal tract is affected by fasting, small-intestinal structure, brush-border enzyme activities and hepatic structure and function were examined in fed mink (controls) and mink that had been fasted for 1–10 days. During the first 1–2 days of fasting, intestinal mass decreased more rapidly than total body mass and villus heights were reduced 25–40%. In contrast, tissue-specific activity of the brush-border enzymes sucrase, maltase, lactase, aminopeptidase A and dipeptidylpeptidase IV increased 0.5- to1.5-fold at this time, but returned to prefasting levels after 6 days of fasting. After 6–10 days of fasting there was a marked increase in the activity of hepatic enzymes and accumulation of intra-hepatic lipid vacuoles. Thus, mink may be a useful model for studying fasting-induced intestinal atrophy and adaptation as well as mechanisms involved in accumulation of intra-hepatic lipids following food deprivation in strictly carnivorous domestic mammals, such as cats and ferrets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For strictly carnivorous mammals such as the cat and mink, little is known regarding the structural and functional adaptation of the gastrointestinal tract (GIT) to fasting. However, profound alterations of the GIT have been recorded for omnivorous mammals (rodents and pigs) deprived of enteral feeding for several days. Such studies have found that fasting causes a reduction in mucosal mass, changes in brush-border enzyme activity (Ferraris and Carey 2000), impaired intestinal mucosal barrier (Altmann 1972; Alverdy et al. 1985; Johnson and Kudsk 1999), and increased bacterial translocation (Deitch et al. 1995; Spaeth et al. 1990). For hibernating ground squirrels, there is a marked reduction in intestinal mucosal mass and villus height, compared with summer-active ground squirrels. The overall architecture of the epithelium is well preserved, and though intestinal absorptive capacity is decreased at low environmental temperatures (7 °C), the ability of the intestine to transport ions and nutrients at normal body temperatures (37 °C) remains intact in the hibernating GIT (Carey 1990; Carey and Sills 1996). An example of carnivores that have been studied with regard to the effects of fasting on the GIT are snakes that consume huge meals at infrequent intervals (Secor et al. 1994; Secor and Diamond 2000). For these reptiles, there is a dramatic up-regulation of intestinal mass and brush-border transporter activity 1–3 days after feeding, followed by a subsequent rapid decrease in intestinal mass and function once digestion is completed.
For rats, mucosal mass reduction in response to fasting has been found to be accompanied by an increase in intestinal lactase activity coupled with decreases in sucrase, maltase, and aminopeptidase N (ApN) activities (McNeill and Hamilton 1971; Altmann 1972; Levine et al. 1974; Miura et al. 1992). Deprivation of the GIT of luminal nutrients by total parenteral nutrition (TPN) is characterised by atrophy of the small intestinal mucosa that appears similar to changes brought on by fasting (Levine et al. 1974; Ihara et al. 2000). For piglets maintained on TPN for 7 days, intestinal mucosal mass is reduced by 50% compared with enterally fed piglets, concurrent with significant reductions in villus height and crypt depth (Park et al. 1998). Lactase activity increases markedly and sucrase activity remains unaltered, whereas aminopeptidase A (ApA) and dipeptidylpeptidase IV (DPPIV) activities decrease in response to TPN in piglets (Park et al. 1998; Remillard et al. 1998; Sangild et al. 2002).
In their natural habitat, strictly carnivorous mammals may experience longer periods of fasting than herbivorous or omnivorous mammals (Dunstone 2004). This raises the possibility that their GIT is better adapted to short-term fasting and thereby less severely affected during periods of food scarcity. The first objective of this study was to investigate the intestinal structural and functional response to short-term fasting using the mink as a model for a strictly carnivorous mammal. The second objective was to explore whether the mink would be a useful model for studying fasting-induced metabolic syndromes of domestic carnivores. Fasting and metabolic stress can cause life-threatening intra-hepatic lipid accumulation and hepatic malfunction in cats and pregnant ferrets (Pazak et al. 1998; Batchelder et al. 1999), and a similar syndrome has been described in lactating mink (Wamberg et al. 1992). Hence, we determined the impact of fasting on different markers of hepatic function, lipid metabolism and hepatocyte morphology.
Materials and methods
Animals and experimental protocol
Twenty-eight female mink (7 months of age, average body mass 1,339±73 g) were randomly selected and divided into five groups. Mink were housed in individual cages (Research Station Rørrendegård, Copenhagen, Denmark) with free access to water. Prior to the start of the study all animals were fed commercial mink food with the following distribution of metabolisable energy (ME): 31% protein, 50% fat, 19% carbohydrate (Sjaellands Pelsdyrfoder, Højby, Denmark). Mink were fasted for 0 (control), 1, 2, 4, 6 (all n=5) or 10 days (n=3). After the experimental protocol was completed the mink were anaesthetised with a mixture of tiletamin, zolazepam, xylasin and butorphanol (Pharmacy Services, KVL, Copenhagen, Denmark, 0.1 mg/kg i.m.). Animals were weighed, a cardial blood sample (8 ml) was collected and a lethal dose of sodium pentobarbital (Pharmacy Services, KVL, Copenhagen, Denmark, 200 mg/ml) was injected intracardially. Blood collected was stabilised in EDTA-containing tubes kept on ice and centrifuged (15 min, 4,000 rpm, 4 °C), and the plasma was stored at −20 °C. All experiments were carried out according to the National Guidelines for Animal Experimentation (National Committee on Animal Experimentation, Denmark).
Following sacrifice, the small intestine from the pyloric sphincter to the colon was rapidly excised by cutting along the mesenteric border, emptied of its contents and rinsed in ice-cold physiological saline. The small intestine was measured and divided into three segments of equal length designated as proximal, middle, and distal small intestine. Because the small intestine of mink is very short it was decided to analyse only the proximal and distal segments for regional responses. The proximal and distal segments were weighed, and from the middle of each segment, a 2-cm tissue sample was snap-frozen in liquid nitrogen and stored at −80 °C for later determination of enzyme activity. A second 2-cm tissue sample was fixed in 4% paraformaldehyde at 4 °C for later histological preparation. Finally the liver was inspected for macroscopic signs of lipid accumulation, weighed, and a sample collected from the right lateral lobe was fixed in 4% paraformaldehyde for later histological preparation.
Morphometry
Paraformaldehyde-fixed samples were embedded in paraffin, sectioned (3 µm), mounted on slides (Menzel-Gläser), and stained with haematoxylin and eosin. From each intestinal sample, we measured villus height (μm), villus width (μm) and crypt depth (μm) from 15 representative villus-crypt columns using a light microscope (Orthoplane, Leitz, Germany) and NIH Image J software (version 1.22c, National Institutes of Health, Bethesda, MD). Liver sections were evaluated for intrahepatic lipid accumulation by light microscopy and graded from 0–5 in accordance with Biourge et al. (1994). In this scaling system, specimens graded 0–2 are considered to have normal liver histological features, but with gradual increases in the number of lipid vacuoles. Grades 3–5 indicate a further progressive increase in the number and distribution of cytoplasmic lipid vacuoles within the hepatocytes and the hepatic acini.
Enzyme assays
Each frozen sample from the proximal and distal region of the small intestine was extracted in 1% Triton X-100 (10 ml g−1 tissue) and homogenised (0 °C, 4×20 s). Homogenates were assayed for dissacharidase (lactase, maltase and sucrase) and peptidase (aminopeptidase N, dipeptidylpeptidase IV and aminopeptidase A) activities, as previously described (Sangild and Elnif 1996). Briefly, sucrose (0.01 M; no. 194018, ICN, Aurora, OH, USA) and lactose (0.12 M; L-3625, Sigma Chemical) dissolved in sodium maleate buffer (50 mM, pH 6.0) were used as substrates for sucrase-isomaltase (SI, EC 3.2.1.48-10) and lactase-phloridzin hydrolase (EC 3.2.1.23-62) respectively. Maltose (0.0112 M; L-5885, Sigma Chemical) was used to measure maltase activity, which represents the combined activity of sucrase-isomaltase and maltase-glucoamylase (EC 3.2.1.20). Aminopeptidase N (ApN, EC 3.4.11.2), dipeptidyl peptidase IV (DPP IV, EC 3.4.14.5) and aminopeptidase A (ApA, EC 3.4.11.7) activities were measured using three peptidase-specific substrate solutions: 10 mM l-alanin-4-nitroanilide (Merck, Darmstadt, Germany) in 50 mM Tris-HCl, pH 7.3; 15 mM glycyl-l-proline-4-nitro-anilide (Bachem, Bubendorf, Switzerland) in 50 mM Tris-HCl, pH 8.0; and 10 mM α-l- glutamic acid 4-nitroanilide (Institute of Protein Chemistry, Hørsholm, Denmark) in 50 mM Tris-HCl, pH 8.0 respectively. For all enzymes, activity is expressed per gram intestine and one unit (U) of activity represents 1 μmol of substrate hydrolysed per minute at 37 °C.
Haematology
Alanine aminotransferase (ALT), aspartate aminotransferase (AST) and serum urea nitrogen (BUN) in plasma were measured spectrophotometrically at 340 nm, cholesterol and triglyceride at 505 nm, and total bilirubin (TBIL) at 545 nm using the ADVIA 1650 Chemistry System (Bayer, Munich, Germany). Plasma concentrations of nonesterified fatty acids (NEFA) were quantified enzymatically using a Wako Nefa C test kit (Wako Chemichals GmbH, Neuss, Germany) and then evaluated colorimetrically at 550 nm.
Statistical analysis
Body, small-intestinal and liver mass data were analysed by analysis of co-variance (ANCOVA, SAS/STAT version 8.1, SAS Institute, Cary, NC), with initial body mass as the covariate, fasting as the fixed variable and animal as the random variable. Remaining data were analysed by a two-way ANOVA using the MIXED procedure of SAS. Fasting and intestinal region were considered as fixed variables, while animal and year were included as random variables. The results are expressed as the adjusted least-squares means (LSmeans) ±SEM, except where noted. When a significant treatment effect was found, differences between means were tested by the least significant difference test or Student’s t-test. P=0.05 was used as the critical level of significance for all statistical evaluations.
Results
Clinical observations
The mink remained physically active after fasting, with the exception of one mink that was less active but still remained alert after 10 days of fasting. Initial body mass tended to be higher for the mink fasted for 1 and 10 days (1,483±147 g) compared with the other groups (1,276±79 g). With fasting, mink experienced a significant biphasic pattern of mass loss, with a rapid loss during the first 4 days of fasting (16% reduction), no reduction from day 4 to 6, followed by a second phase of mass loss between day 6 and 10 (additional 16% reduction) (Fig. 1).
Small intestinal mass and morphometry
Small intestinal mass decreased (P<0.05) after 1–2 days of fasting (22%), remained stable the following days of fasting and further decreased (by 13%) at 10 days of fasting (Fig. 1). This decrease in mass was observed in both the proximal and distal small intestinal regions (data not shown). For fed control mink, villi of the proximal small intestine were twice as high as those of the distal small intestine (1,179±29 vs. 579±38 μm, P<0.01) (Fig. 2). For the proximal small intestine villus height decreased by 30% (P<0.001) after 1 day of fasting and by 36% after 2 days with no further decrease during the subsequent 8 days of fasting (Fig. 2). Fasting had no consistent effects on either villus width (average 101±5 μm, data not shown) or crypt depth (495±30 μm) for the proximal small intestine (Fig. 2). Similarly for the distal small intestine, villus heights decreased by 21% after 2 days of fasting with no further reduction thereafter (Fig. 2). Villus width (125±9 μm, data not shown) of the distal small intestine did not change with fasting, but crypt depth decreased by 40% after 10 days (557±30 vs. 332±38 μm, P<0.0001) (Fig. 2).
Enzyme activities
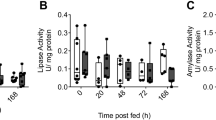
The data were statistically analysed across the values obtained from both the proximal and distal small intestine. Intestinal lactase activity increased by 35% after 2 days of fasting (0.23±0.04 to 0.31±0.04 U g−1, P<0.05) and then subsequently decreased to control levels (Fig. 3A). Maltase activity increased by 100% during the first 4 days of fasting (from 12.0±2.7 to 24.2±2.7 U g−1, P<0.0001), then decreased to control levels during the subsequent days (13.1±3.2 U g−1 at day 10) (Fig. 3A). Sucrase activity increased by 50% during the first two days of fasting (1.1±0.2 to 1.7±0.2 U g−1 on day 2 of fasting, P<0.01), then similarly decreased to control levels during the following days (Fig. 3A). After 1 day of fasting, the activity of ApA increased by 53% (2.0±0.2 to 3.1±0.2 U g−1, P<0.01) and then decreased to control levels (1.61±0.31 U g−1 on day 10) (Fig. 3B). DPPIV activity increased by 46% after 4 days of fasting (3.6±0.6 to 5.2±0.6 U g−1, P<0.05) and then returned to fed control levels by day 6 (Fig. 3B). There was no significant effect of fasting on ApN activity analysed across proximal and distal small intestine (average 6.0±0.6 U g−1). Although an increase of 38% was observed after 1–2 days of fasting in the proximal small intestine (6.3±0.8 to 8.6±0.8 U g−1, P<0.05), it was followed by a subsequent decrease to control levels (data not shown).
Disaccharidase-specific and B peptidase-specific activities across the small intestine in mink fasted for 0 (control), 1, 2, 4, 6 or 10 days (LSmeans±SEM, n=5). ApA Aminopeptidase A, ApN aminopeptidase N, DPPIV dipeptidyl peptidase IV. Means marked with the same letter do not differ significantly (P<0.05)
Liver mass and morphology
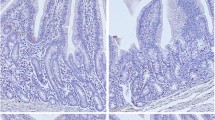
Liver mass decreased more rapidly than body mass during the first day of fasting (18% reduction). From 1 to 6 days of fasting, liver mass remained stable, then increased from 6 to 10 days of fasting, reaching a mass similar to that of fed control mink (Fig. 1). Based on the scaling system of Biourge et al. (1994), liver specimens from mink either fed or fasted for 1, 2 or 4 days were considered normal (grades 0–2), whereas the specimens from animals fasted for 6–10 days clearly had accumulated more lipid vacuoles (grades 3–5) (Table 1, Fig. 4).
Representative light micrographs of hematoxylin- and eosin-stained tissue sections of the livers from fasted mink. Hepatic structure was normal in A fed mink (biopsy score 0); B at 2 days of fasting, small intracellular lipid vacuoles were visible (biopsy score 2) and at C 6 (biopsy score 4) to D 10 (biopsy score 5) days of fasting lipid vacuoles increased in size and distribution. All images were obtained at 100× magnification
Haematology
After 10 days of fasting, plasma concentration of hepatic-associated enzymes increased compared to fed animals (Table 1), ALT increased 15-fold (P<0.0001) and AST increased 7-fold (P<0.0001). Fasting had no effect on BUN, total bilirubin or triglyceride levels. Cholesterol levels decreased after 1–2 days of fasting (P<0.05) and thereafter increased to prefasting levels, whereas NEFA increased after 1 day of fasting (P<0.05), remained elevated for the next 6 days, followed by a decrease to prefasting levels (Table 1).
Discussion
This study in mink demonstrates that small-intestinal mass and villus height are significantly reduced after only 1 day of fasting. Nevertheless, the reduction in digestive capacity may be temporarily compensated for by an initial up-regulation of enterocyte function as reflected in an increased intestinal brush-border enzyme activity. This may allow the mink intestine to preserve brush-border function, thereby providing full capacity for substrate hydrolysis if feeding is resumed after a short period of fasting.
The pattern of mass loss in response to fasting in mink was similar to that described for other mammalian species (Hoffer 1994). An initial rapid mass reduction is observed from days 1 to 4 of fasting due to the mobilisation of glycogen deposits and labile proteins (gluconeogenic phase). Subsequently, there is a switch to ketone-body utilisation and fat catabolism, resulting in a slower mass reduction from days 4 to 6 of fasting (protein conservation phase). By day 10 of fasting, most lipid reserves are depleted and protein mobilisation increases, causing a return to a more rapid mass reduction (Hoffer 1994).
The intestinal tract is a metabolically expensive organ to maintain and an initial rapid decrease in intestinal mass is an effective way to minimise energy expenditure during food scarcity (Ferraris and Carey 2000; Secor 2001). In the present study, during the first 2 days of fasting, the reductions in intestinal mass (22%) and villus height (33%) were more pronounced than the loss in total body mass (8%). There were no further decreases in villus height for the rest of the period and intestinal mass remained stable for the following 4 days. From days 6 to 10 of fasting, the intestinal mass reduction (14%) was similar to the reduction in body mass (16%). This strikingly rapid decrease in intestinal mass has also been observed in rats, where 1–3 days of fasting resulted in significant reductions in intestinal mass and villus height (Steiner et al. 1968; McManus and Isselbacher 1970). In addition, for rats maintained on TPN for 15 days, a rapid and significant reduction in mucosal mass occurs after 3 days of TPN, with no further reduction thereafter (Hughes and Dowling 1980). TPN administration avoids the effects of a catabolic body condition, but it is generally accepted that the deprivation of enteral nutrients during administration of TPN induces intestinal atrophy similar to fasting (Levine et al. 1974; Miura et al. 1992; Ihara et al. 2000). The reduction in intestinal mass observed in the present study from 6–10 days of fasting was probably a result of the catabolic effects of fasting, while in rats maintained on TPN for 15 days no further reduction was observed after the initial reduction (Hughes and Dowling 1980).
For mink, villus height was twice as high in the proximal as in the distal small intestine. Nevertheless, fasting resulted in significant villus atrophy in both regions. This observation differs from studies in rats, where 3 days of fasting caused significant villus atrophy in the jejunum only, with no atrophy in the ileum (Hughes and Dowling 1980; Holt et al. 1986). Villus atrophy in response to fasting may be related to decreased enterocyte proliferation, increased apoptosis, and changes in enterocyte dimensions (Ferraris and Carey 2000; Dahly et al. 2002). These parameters may, however, differ among species. Hence, for hibernating ground squirrels, the intestinal epithelium turnover time is 3 weeks compared with 3–5 days for non-hibernating squirrels (Carey 1990). For rats fasted for 3 days, the number of mitoses per crypt and the cell migration ratio decreases significantly in the jejunum (Brown et al. 1963), whereas for the ileum, no change occurs (Holt et al. 1986). Furthermore, increased apoptosis for apical enterocytes is observed in both jejunal and ileal segments after 24 h of fasting in rats (Iwakiri et al. 2001; Martins et al. 2001), and a reduced enterocyte height after 3 days of fasting has been noted in mice and rats (Brown et al. 1963).
In studies on digestive enzyme activities along the small intestine in blue fox, mink, ferret, and rat, Oleinik (1995) found that most brush-border enzyme activities were highest in rats. In addition, rats exhibited a significant gradient in brush-border enzyme activity, decreasing from the proximal to the distal small-intestinal regions, whereas the carnivores had a smaller proximal to distal gradient. In rats, the most distal part of the small intestine may serve to provide reserve intestinal digestive capacity when digestive demands are particularly high. In carnivores, such a “reserve zone” may not exist, possibly because they have a shorter intestine (Oleinik 1995). The length of the mink small intestine is only four times its body length, whereas for rats the intestine is 10 times its body length (Stevens and Hume 1995). Our findings, that fasting affected morphometric parameters and brush-border enzyme activities in both the proximal and distal regions of the small intestine in mink, support the view that carnivore species may have less intestinal reserve capacity.
In this study, 2–4 days of fasting caused a significant increase in the specific activity of lactase, maltase, sucrase, ApA and DPPIV across regions, and an increase of ApN activity specifically in the proximal region. After 10 days of fasting, the specific activity of all enzymes had returned to prefasting levels. In a study on intestinal adaptation in rats fasted 1–5 days, the activity of lactase in the jejunum and of lactase and ApA in the ileum increased initially and then decreased. However, jejunal ApA and sucrase activity only decreased, and sucrase activity in the ileum remained unchanged (Raul et al. 1982). In rats fasted or maintained on TPN for 5 days, ApN and DPPIV activities initially increased for the fasted rats, whereas both groups experienced significant decreases in jejunal mucosal mass, cell proliferation and sucrase and maltase activities (Ihara et al. 2000).
The increase in brush-border specific activity in fasted mink may result from decreased enterocyte turnover or changes in enterocyte size (Ferraris and Carey 2000; Dahly et al. 2002). Brush-border enzyme activities are highest in mature enterocytes; with decreasing enterocyte migration, the ratio of mature to immature cells increases (Ferraris and Carey 2000). It appears that the carnivorous mink is well adapted to preserve its digestive capacity, despite loss of mucosal mass following short-term fasting. The compensatory increase in brush-border enzyme-specific activities lasted 4–6 days, whereafter the activities decreased to basal levels, resulting in a net reduction in hydrolytic capacity at 10 days of fasting. Although similar effects of fasting on selected enzymes have been reported for omnivorous mammals (Altmann 1972; Levine et al. 1974; Park et al. 1998), it is noteworthy that the tissue-specific activities of all six enzymes measured in this study remained at or above the fed control levels for the whole length of the small intestine even after 10 days of fasting.
During the initial fasting period, liver mass decreased rapidly, possibly due to utilisation of intrahepatic glycogen stores. However, by 10 days of fasting, liver mass had increased to prefasting levels, despite the concurrent loss in body mass. We found increased accumulation of lipid vacuoles within the liver by 6–10 days of fasting and at the same time plasma levels of hepatic-associated enzymes (ALT, AST) increased, indicative of hepatic malfunction (Center 1998; Brown et al. 2000). Nevertheless, after 10 days of fasting, the mink showed minimal behavioural change and there were no changes in plasma triglyceride, TBIL or BUN levels. Concurrent with the increased hepatic lipid accumulation there was an increase in circulating NEFA from 1–6 days of fasting followed by a slight decrease at 10 days. Feline hepatic lipidosis (FHL) is a relatively common disease of domestic cats, and it is initiated by a prolonged period of anorexia and metabolic stress (Brown et al. 2000). FHL is biochemically associated with elevated serum levels of ALT, AST, triglycerides, TBIL and NEFA because liver function is compromised as a result of profound intra-hepatic lipid accumulation and sometimes there is a decrease in BUN (Center 1998; Pazak et al. 1998). For ferrets, pregnancy toxaemia is characterised by an extreme catabolic state and accumulation of intra-hepatic lipid vacuoles that appears similar in aetiology to FHL (Batchelder et al. 1999). Pregnancy toxaemia and FHL are both potentially fatal conditions if not treated. Hepatic lipidosis is not commonly diagnosed in mink, although the syndrome “nursing sickness” seems to have a similar metabolic aetiology (Wamberg et al. 1992). Our results suggest that hepatic malfunction and mucosal atrophy with only moderate enterocyte dysfunction may be associated with conditions of anorexia and metabolic stress in strict carnivorous mammals. Thus mink could serve as a useful model for studying fasting-induced intestinal atrophy as well as mechanisms causing accumulation of intra-hepatic lipid vacuoles in other strictly carnivorous mammals.
Abbreviations
- ALT :
-
alanine aminotransferase
- ApA :
-
aminopeptidase A
- ApN :
-
aminopeptidase N
- AST :
-
aspartate aminotransferase
- BUN :
-
blood urea nitrogen
- BM :
-
body mass
- DPPIV :
-
dipeptidyl peptidase IV
- GIT :
-
gastrointestinal tract
- FHL :
-
feline hepatic lipidosis
- NEFA :
-
non-esterified fatty acids
- TBIL :
-
total bilirubin
- TPN :
-
total parenteral nutrition
References
Altmann GG (1972) Influence of starvation and refeeding on mucosal size and epithelial renewal in the rat small intestine. Am J Anat 133:391–400
Alverdy JC, Chi HS, Selivanov V, Morris J, Sheldon GF (1985) The effect of route of nutrient administration on the secretory immune system. Curr Surg 42:10–13
Batchelder MA, Bell JA, Erdman SE, Marini RP, Murphy JC, Fox JG (1999) Pregnancy toxemia in the European ferret (Mustela putorius furo). Lab Anim Sci 49:372–379
Biourge VC, Groff JM, Munn RJ, Kirk CA, Nyland TG, Madeiros VA, Morris JG, Rogers QR (1994) Experimental induction of hepatic lipidosis in cats. Am J Vet Res 55:1291–1302
Brown B, Mauldin GE, Armstrong J, Moroff SD, Mauldin GN (2000) Metabolic and hormonal alterations in cats with hepatic lipidosis. J Vet Intern Med 14:20–26
Brown HO, Levine ML, Lipkin M (1963) Inhibition of intestinal epithelial cell renewal and migration induced by starvation. Am J Physiol 205:868–872
Carey HV (1990) Seasonal changes in mucosal structure and function in ground squirrel intestine. Am J Physiol 259:R385–R392
Carey HV, Sills NS (1996) Hibernation enhances d-glucose uptake by intestinal brush border membrane vesicles in ground squirrels. J Comp Physiol B 166:254–261
Center SA (1998) Nutritional support for dogs and cats with hepatobiliary disease. J Nutr 128:2733S–2746S
Dahly EM, Guo Z, Ney DM (2002) Alterations in enterocyte proliferation and apoptosis accompany TPN-induced mucosal hypoplasia and IGF-I-induced hyperplasia in rats. J Nutr 132:2010–2014
Deitch EA, Xu D, Naruhn MB, Deitch DC, Lu Q, Marino AA (1995) Elemental diet and IV-TPN-induced bacterial translocation is associated with loss of intestinal mucosal barrier function against bacteria. Ann Surg 221:299–307
Dunstone N (2004) Food and foraging. The mink. T & AD Poyser, London, pp 62–100
Ferraris RP, Carey HV (2000) Intestinal transport during fasting and malnutrition. Annu Rev Nutr 20:195–219
Hoffer LJ (1994) Adaptation to starvation. In: Shills ME (ed) Modern nutrition in health and disease. Lea and Fibiger, Philadelphia, pp 927–949
Holt PR, Wu S, Yeh KY (1986) Ileal hyperplastic response to starvation in the rat. Am J Physiol 251:G124–G131
Hughes CA, Dowling RH (1980) Speed of onset of adaptive mucosal hypoplasia and hypofunction in the intestine of parenterally fed rats. Clin Sci (Lond) 59:317–327
Ihara T, Tsujikawa T, Fujiyama Y, Ueyama H, Ohkubo I, Bamba T (2000) Enhancement of brush border membrane peptidase activity in rat jejunum induced by starvation. Pflugers Arch 440:75–83
Iwakiri R, Gotoh Y, Noda T, Sugihara H, Fujimoto K, Fuseler J, Aw TY (2001) Programmed cell death in rat intestine: effect of feeding and fasting. Scand J Gastroenterol 36:39–47
Johnson CD, Kudsk KA (1999) Nutrition and intestinal mucosal immunity. Clin Nutr 18:337–344
Levine GM, Deren JJ, Steiger E, Zinno R (1974) Role of oral intake in maintenance of gut mass and disaccharide activity. Gastroenterology 67:975–982
Martins MJ, Hipolito-Reis C, Azevedo I (2001) Effect of fasting on rat duodenal and jejunal microvilli. Clin Nutr 20:325–331
McManus JP, Isselbacher KJ (1970) Effect of fasting versus feeding on the rat small intestine. Morphological, biochemical, and functional differences. Gastroenterology 59:214–221
McNeill LK, Hamilton JR (1971) The effect of fasting on disaccharidase activity in the rat small intestine. Pediatrics 47:65–72
Miura S, Tanaka S, Yoshioka M, Serizawa H, Tashiro H, Shiozaki H, Imaeda H, Tsuchiya M (1992) Changes in intestinal absorption of nutrients and brush border glycoproteins after total parenteral nutrition in rats. Gut 33:484–489
Oleinik VM (1995) Distribution of digestive enzyme activities along intestine in blue fox, mink, ferret and rat. Comp Biochem Physiol A 112:55–58
Park YK, Monaco MM, Donovan SM (1998) Delivery of total parenteral nutrition (TPN) via umbilical catheterization: development of a piglet model to investigate therapies to improve gastrointestinal structure and enzyme activity during TPN. Biol Neonate 73:295–305
Pazak HE, Bartges JW, Cornelius LC, Scott MA, Gross K, Huber TL (1998) Characterization of serum lipoprotein profiles of healthy, adult cats and idiopathic feline hepatic lipidosis patients. J Nutr 128:2747S–2750S
Raul F, Noriega R, Doffoel M, Grenier JF, Haffen K (1982) Modifications of brush border enzyme activities during starvation in the jejunum and ileum of adult rats. Enzyme 28:328–335
Remillard RL, Guerino F, Dudgeon DL, Yardley JH (1998) Intravenous glutamine or limited enteral feedings in piglets: amelioration of small intestinal disuse atrophy. J Nutr 128:2723S–2726S
Sangild PT, Elnif J (1996) Intestinal hydrolytic activity in young mink (Mustela vison) develops slowly postnatally and exhibits late sensitivity to glucocorticoids. J Nutr 126:2061–2068
Sangild PT, Petersen YM, Schmidt M, Elnif J, Petersen TK, Buddington RK, Greisen G, Michaelsen KF, Burrin DG (2002) Preterm birth affects the intestinal response to parenteral and enteral nutrition in newborn pigs. J Nutr 132:2673–2681
Secor SM (2001) Regulation of digestive performance: a proposed adaptive response. Comp Biochem Physiol A 128:565–577
Secor SM, Diamond JM (2000) Evolution of regulatory responses to feeding in snakes. Physiol Biochem Zool 73:123–141
Secor SM, Stein ED, Diamond J (1994) Rapid upregulation of snake intestine in response to feeding: a new model of intestinal adaptation. Am J Physiol 266:G695–G705
Spaeth G, Berg RD, Specian RD, Deitch EA (1990) Food without fiber promotes bacterial translocation from the gut. Surgery 108:240–246
Steiner M, Bourges HR, Freedman LS, Gray SJ (1968) Effect of starvation on the tissue composition of the small intestine in the rat. Am J Physiol 215:75–77
Stevens CE, Hume ID (1995) Comparative physiology of the vertebrate digestive system. Cambridge University Press, Cambridge
Wamberg S, Clausen TN, Olesen CR, Hansen O (1992) Nursing sickness in lactating mink (Mustela vison). II. Pathophysiology and changes in body fluid composition. Can J Vet Res 56:95–101
Acknowledgements
Supported by the Danish Mink Breeders Association and the Danish Agricultural and Veterinary Research Council. The experiments were carried out according to the National Guidelines for Animal Experimentation (National Committee on Animal Experimentation, Denmark).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume
Rights and permissions
About this article
Cite this article
Bjornvad, C.R., Elnif, J. & Sangild, P.T. Short-term fasting induces intra-hepatic lipid accumulation and decreases intestinal mass without reduced brush-border enzyme activity in mink (Mustela vison) small intestine. J Comp Physiol B 174, 625–632 (2004). https://doi.org/10.1007/s00360-004-0452-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-004-0452-2