Abstract
The purpose of this study was to observe the regulatory effects of GABAA (γ-aminobutyric acid A) receptor on the N-methyl-d-aspartate (NMDA) receptor during excitotoxicity in spiral ganglion neurons in the rat cochlea induced by sodium salicylate (SS). Western blot illustrated SS decreased the expression of NMDA receptor 2B subunit (NR2B) surface protein through affecting GABAA receptor, but the total protein content did not significantly change. Y1472 and S1480 are important phosphorylation sites in NR2B, SS downregulated the Fyn-dependent phosphorylation of Y1472 in a manner not related to the CK2 (Casein Kinase 2) dependent phosphorylation of S1480, thus regulating the surface distribution and internalization of NMDA receptor through GABAA receptor. These results suggest that the modified pattern of dynamic balance between excitation and inhibition by coactivation of the GABAA receptor can attenuate the excitatory NMDA receptor under the action of SS, via inhibiting the Fyn-dependent phosphorylation of Y1472.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There are many causes of deafness, but ototoxic drugs are the most common cause of acquired deafness. Sodium salicylate, namely, the active ingredient in aspirin, is one of the most widely used anti-inflammatory analgesic drugs; however, the use of salicylic acid drugs is often accompanied by ototoxicity (Wei et al. 2010; Chen et al. 2013), which has serious effects on the quality of patients’ life who use these drugs long term and limits the further use of such drugs in clinical applications. Therefore, it is an increasingly urgent need to reduce the side effects of deafness associated with the use of salicylic acid drugs in clinical practice.
Studies have confirmed that salicylic acid drugs have direct effects on all parts of the auditory nervous system, from the outer hair cells to the auditory cortex (Wang et al. 2006; Caperton and Thompson 2011), and that these effects are mostly reversible (de Almeida-Silva et al. 2011). Both NMDAR and GABAAR are directly involved in the neurotoxic effects of sodium salicylate on the auditory system (Xu et al. 2005; Wang et al. 2006; Zou and Shang 2012; Sahley et al. 2013; Yao et al. 2015). Recently, the topic of receptor interactions has attracted increasing attention from researchers. A direct crosstalk between postsynaptic NMDAR and GABAAR contributes to the excitatory/inhibitory balance in the nervous system, which restricts each other and jointly regulate neuron activity. For example, in inferior colliculus (Cong et al. 2011), hippocampus (Chen and Wong 1995) and cerebellum (Robello et al. 1997), it is found that the activation of NMDAR could inhibit the current of GABAAR and that this inhibitory effect was regulated by Ca2+ in relation to CaMKII dependence. Other studies have shown that Systemic application of GABAergic agents reverses or prevents the hypermetabolic state (Kurumaji and McCulloch 1989), the excessive release of neurotransmitter (Giovannini et al. 1994), and the neurotoxic action of NMDAR (Ohkuma et al. 1994; Zhang et al. 2007; Oliveira-Pinto et al. 2015). These studies suggest that GABAAR’s regulation of NMDAR acts to compensate for and buffer the excessive excitation of neurons. However, studies on the regulatory effect of GABAAR on NMDAR in the peripheral auditory nervous system, such as in SGNs, or on the ototoxicity induced by sodium salicylate have rarely been reported so far.
The SGNs are the first targets for the ototoxicity of salicylic acid drugs (Chen et al. 2010), which has been widely described. Our previous study confirmed that sodium salicylate could affect the expression of NMDAR and GABAAR in SGNs (Tang et al. 2014; Yao et al. 2015). Therefore, we speculate that GABAAR plays an important role in the regulation of NMDAR in sodium salicylate-induced ototoxicity. To test this hypothesis, this study used western blot to explore the underlying mechanism of ototoxicity.
Materials and methods
Primary culture of spiral ganglion neurons (SGNs)
The experimental animals were provided by the Experimental Animal Center of Guangxi Medical University. The animal experimental program was approved by the Animal Ethics Committee of Guangxi Medical University. The primary culture method for SGNs was the same as that previously reported by this study group (Chen et al. 2016). Briefly, this experiment used 3-day-old Sprague–Dawley rats. The cochlea were removed under an upright microscope (Olympus, Japan). The helical ligament was separated with filament forceps and a microdissection needle, the basement membrane was removed, and only the cochlear axial spiral tube containing the spiral ganglion was retained. The tissue containing the SGNs was shredded and incubated in a solution containing 0.25% trypsin (Gibco, USA) at 37 °C for 10 min, and then the digestion was terminated. Following centrifugation at 1000 r/min for 5 min and the removal of supernatant, the pellet was resuspended and gently triturated. Dissociated SGNs was plated onto poly-d-lysine-coated 35-mm culture dishes with DMEM/F12 containing 10% fetal bovine serum (Gibco, USA), and all cultures were maintained in a humidified CO2 (5%) incubator (Thermo, USA) for 48 h at 37 °C.
Treatment of the cultures
After 48 h of cell culture, drug intervention was carried out with normal culture medium or culture medium (all contained 10 μM GABA and 10 μM NMDA) with one of the following treatments: SS (0.5, 5 mM and 50 mM SS), GABAAR agonist Mus (10, 100 and 1000 μM) and GABA (0.1, 1 and 10 mM), GABAAR antagonist Bic (20, 100 and 500 μM) and Gabazine (10, 100 and 1000 μM), 10 μM PP2, 0.5 mM SS+10 μM Mus, 0.5 mM SS+20 μM Bic, 5 mM SS+10 μM Mus, or 5 mM SS+20 μM Bic. The above drugs were purchased from Sigma, USA. The treatments were terminated 30 min later.
Western blot
Total protein was obtained after 30 min of incubation with a lysate buffer including a protease inhibitor, a phosphatase inhibitor and radio immunoprecipitation assay. Extraction of surface protein was performed according to the instructions of the Cell Fractionation Kit (Cell Signaling Technology, USA). The brief steps are as follows. 400 µl of cell suspension was added into a 1.5 ml tube, centrifuged for 5 min at 500 (g) at 4°C, aspirated the supernatant and resuspended pellet in 500 μl of Cytoplasm Isolation Buffer. The mixture was vortexed for 5 s, incubated on ice for 5 min, centrifuged for 5 min at 500(g) and resuspended pellet in 500 μl of Membrane Isolation Buffer. Then the mixture was vortexed for 15 s, incubated on ice for 5 min and centrifuged for 5 min at 8000(g). Save the supernatant that is the surface protein. The protein concentration was determined with the Protein Assay Kit (Thermo, USA). The buffer supernatant containing 40 μg of protein was added to an SDS (sodium dodecyl sulfate)-polyacrylamide gel for electrophoretic separation. The protein was transferred to a polyvinylidene fluoride membrane with a stable 100 mA current, the membrane was cut according to the molecular weight markers (Thermo, USA), and then the membrane containing the target protein was blocked with 5% fat-free milk at room temperature for 1.5 h. The following antibodies were used: anti-NMDAR2B antibody (rabbit, 1:1000, Abcam, UK), anti-Fyn antibody (rabbit, 1:1000, Abcam, UK), anti-NMDAR2B (phospho-Y1472) antibody (rabbit, 1:1000, Millipore, USA), anti-Casein Kinase 2 beta antibody (rabbit, 1:1000, Abcam, UK), and anti-NMDAR2B (phospho-S1480) antibody (rabbit, 1:1000, Abcam, UK). The polyvinylidene fluoride membrane was incubated at 4 °C overnight and was then incubated with goat anti-rabbit fluorescence secondary antibody (1:500, Earthox, USA) for 1.5 h at room temperature. After washing with tris buffered saline tween, the membrane was scanned using an infrared scanning device (Odyssey, USA). The band intensities (gray values) were measured with ImageJ software, and the β-3 tubulin protein was used as the internal reference. Protein relative expression level = (target band grayscale/reference band grayscale)/(control group grayscale/reference band grayscale) × 100%.
Statistical analysis
Statistical analysis involved the use of SPSS version 20.0 (SPSS Inc., USA). All experimental data are shown as the mean ± standard error of the mean. The significant differences among three or more groups were examined using one-way analysis of variance (ANOVA) with LSD or SNK tests if the ANOVA indicated a significant difference. A P value < 0.05 was considered to indicate a statistically significance.
Results
Sodium salicylate (SS) and activated GABAAR down-regulated the expression of the NMDA receptor 2B subunit (NR2B) surface protein
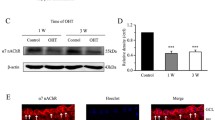
To verify the effect of SS on NMDAR, SGNs were treated with SS (0.5, 5 and 50 mM) for 30 min, which significantly reduced the expression of NR2B surface protein by 13.49, 36.04 and 37.58% (F = 20.214, P = 0.000 < 0.05), as compared to the control group. The effects of SS was in a concentration-dependent manner. But there is no significance between 5 mM SS group and 50 mM SS group (P = 0.795 > 0.05) (Fig. 1), that is to say the saturated concentration of SS is 5 mM. In addition, SGNs were treated with Mus (GABAAR agonist) (10, 100 and 1000 μM) to activate GABAAR, which also decreased the NR2B surface protein expression levels by 8.44, 35.90 and 39.87% (F = 423.826, P = 0.000 < 0.05), as compared to the control group. After treatment of GABA (0.1, 1 and 10 mM), the expression of NR2B surface protein was reduced by 41.62, 67.56 and 74.64% (F = 155.733, P = 0.000 < 0.05), as compared to the control group. There is also no significance between 100 μM Mus group and 1000 μM Mus group (P = 0.829 > 0.05) or between 1 mM GABA group and 10 mM GABA group (P = 0.102 > 0.05) (Fig. 2), and the saturated concentration of Mus and GABA were 0.1, 1 mM, respectively. However, all the treatments had no significant change in total protein (SS group: F = 1.006, P = 0.439 > 0.05; Mus group: F = 0.706, P = 0.575 > 0.05; GABA group: F = 0.231, P = 0.872 > 0.05). The results suggested that SS or GABAAR activation promoted NMDAR internalization.
Different concentrations of Mus (10, 100 and 1000 μM) and GABA (0.1, 1 and 10 mM) were applied to cultured SGNs for 30 min before cell lysis; surface protein and total protein of NR2B were analyzed by Western blot. *P < 0.05, compared with control group (n = 3 for all groups, \(\mathop {\overline{x} }\limits^{{}}\) ± s)
GABAAR inhibition up-regulated the expression of the NMDA receptor 2B subunit (NR2B) surface protein
To further confirm the effect of GABAAR on NMDAR, we observed the effect of different concentrations of GABAAR antagonist (Bic and Gabazine) on the surface protein of NR2B, on the premise of adding a small dose of GABA (10 μM) to the medium. Obviously, after treatment of Bic (20, 100, 500 μM) for 30 min, the expression of NR2B surface protein was increased by 15.73, 59.00 and 61.90%, respectively (F = 33.530, P = 0.000 < 0.05), as compared to the control group. Similarly, after application of Gabazine (10, 100 and 1000 μM), the expression levels of NR2B surface protein was increased by 30.63, 79.54 and 81.26% (F = 42.147, P = 0.000 < 0.05), as compared to the control group. However, there is also no significance between 100 μM Bic group and 500 μM Bic group (P = 0.712 > 0.05) or between 100 μM Gabazine group and 1000 μM Gabazine group (P = 0.847 > 0.05) in NR2B surface protein expression. What’s more, there was no significant change in total protein after the treatment of GABAAR inhibitors for all groups (Bic group: F = 0.858, P = 0.501 > 0.05; Gabazine group: F = 0.549, P = 0.663 > 0.05) (Fig. 3). The results suggest that attenuating GABAAR inhibition may promote excitotoxic damage by increasing the function of NMDAR. Similar studies have also found that in the spinal dorsal horn nerve, anterior cingulate cortical neurons, application of Bic can cause hyperalgesia and also induce the delivery of NMDAR to the synapse (Baba et al. 2003; Buesa et al. 2006; Torsney and MacDermott 2006); electrophysiological records also show that attenuation of GABAergic inhibition can induce a new NMDAR-mediated synaptic current in the dorsal horn of the spinal cord (Wang et al. 2005).
Different concentrations of Bic (20, 100, 500 μM) and Gabazine (10, 100 and 1000 μM) were applied to cultured SGNs for 30 min before cell lysis; surface protein and total protein of NR2B were analyzed by Western blot. *P < 0.05, compared with control group (n = 3 for all groups, \(\mathop {\overline{x} }\limits^{{}}\) ± s)
Sodium salicylate (SS) decreased NMDA receptor 2B subunit (NR2B) surface protein expression through GABAAR, and the effects of SS and GABAAR modulation operated by the same mechanism
To verify the effect of the combination of SS and GABAAR on NMDAR, we added SS with Mus or Bic to observe the effect on the surface protein of NR2B. Compared with using Mus alone, the NR2B surface protein expression in the 0.5 mM SS+Mus group and 5 mM SS+Mus group was decreased by 30.82% (F = 30.160, P = 0.000 < 0.05) and 55.33% (P = 0.000 < 0.05), respectively. In contrast, compared with the Bic group, the expression of the NR2B surface protein in the 0.5 mM SS+Bic group and the 5 mM SS+Bic group was decreased by 14.23% (P = 0.035 < 0.05) and 29.72% (P = 0.000 < 0.05), respectively. However, there was no significant change in total protein expression between the treatment group and the control group (F = 1.111, P = 0.401 > 0.05) (Fig. 4).
Western blot analyzed surface protein and total protein of NR2B after 0.5 or 5 mM SS, 10 μM Mus and 20 μM Bic co-application for 30 min. *P < 0.05, compared with control group; #P < 0.05, compared with Mus group; &P < 0.05, compared with Bic group (n = 3 for all groups, \(\mathop {\overline{x} }\limits^{{}}\) ± s)
The above experiments proved that SS and GABAAR activation had a superposition effect. To further confirm the effect of SS or GABAAR, SS and Mus with saturated concentration were selected to repeat part of the experiment. After 5 mM SS and 100 μM Mus treatment for 30 min, there was no statistical difference between 5 mM SS+100 μM Mus group and 5 mM SS group (F = 22.016, P = 0.375 > 0.05), nor was between 5 mM SS+100 μM Mus group and 100 μM Mus group (P = 0.361 > 0.05) (Fig. 5). That is to say, the effects of SS and GABAAR modulation operated by the same mechanism.
Sodium salicylate (SS) down-regulated the Fyn-dependent phosphorylation of Y1472, but the downregulation was not related to the CK2 (Casein Kinase 2) dependent phosphorylation of S1480
To study the potential mechanism of SS-mediated NMDAR internalization, and verify how it regulates the effect of GABAAR on NMDAR in SGNs, phosphorylation levels were determined to reveal the possible mechanisms. Mus down-regulated Fyn protein expression by 20.73% (F = 34.688, P = 0.010 < 0.05); conversely, Bic promoted Fyn protein expression by 29.07% (P = 0.001 < 0.05). Among the treatments, the combined effect of 0.5/5 mM SS and Mus was more significant than the effect of using Mus alone, decreasing by 17.48% (P = 0.004 < 0.05) and 48.65% (P = 0.000 < 0.05), respectively. The combined treatment of 0.5/5 mM SS and Bic reversed the effect of using Bic alone, decreasing by 20.91% (P = 0.010 < 0.05) and 33.06% (P = 0.000 < 0.05), respectively. On the other hand, Mus also down-regulated the expression of the P-Y1472 protein by 25.65% (F = 30.821, P = 0.002 < 0.05), and Bic upregulated the expression of the P-Y1472 protein by 38.48% (P = 0.000 < 0.05). Among the treatments, the combined effect of 0.5/5 mM SS and Mus was more significant than the effect of using Mus alone, decreasing by 25.82% (P = 0.003 < 0.05) and 29.95% (P = 0.000 < 0.05), respectively. The combination of 0.5 mM SS/5 mM SS and Bic reversed the effect of using Bic alone, decreasing by 25.43% (P = 0.002 < 0.05) and 54.60% (P = 0.000 < 0.05), respectively (Fig. 6). However, under the influence of SS and GABAAR activity, there was no significant change in CK2 protein expression between the treatment group and the control group (F = 0.742, P = 0.655 > 0.05), nor was there a change in P-S1480 protein expression (F = 1.230, P = 0.337 > 0.05) (Fig. 7). The results suggested that NMDAR internalization regulated by SS or GABAAR is associated with Fyn and Y1472.
Western blot analyzed protein of Fyn and P-Y1472 after 0.5 or 5 mM SS, 10 μM Mus and 20 μM Bic co-application for 30 min. *P < 0.05, compared with control group; #P < 0.05, compared with Mus group; &P < 0.05, compared with Bic group (n = 3 for all groups, \(\mathop {\overline{x} }\limits^{{}}\) ± s)
Fyn-dependent phosphorylation of Y1472 regulated NMDA receptor 2B subunit (NR2B) internalization
To further verify the correlation between NR2B internalization and Fyn-dependent phosphorylation of Y1472, PP2 (an inhibitor of Fyn) was added on the basis of SS, GABAAR agonist and inhibitor. The results show that there was no significant change in NR2B surface protein expression among all groups (F = 0.436, P = 0.884 > 0.05), nor was P-Y1472 protein expression (F = 0.340, P = 0.939 > 0.05) (Fig. 8). Therefore, this further suggested that SS and GABAAR regulate NMDAR internalization through Fyn-dependent phosphorylation of Y1472.
Discussion
GABAAR and NMDAR are important inhibitory and excitatory receptors, respectively, in the central nervous system and are also highly expressed in the peripheral auditory system (Niedzielski and Wenthold 1995; Yao et al. 2015). It has been found that GABAAR reduces the response of NMDAR. For example, in inferior colliculus, GABAAR activation inhibits the current of NMDAR through the Ca2+/CaMKII signaling pathway (Cong et al. 2011). The activated GABAAR inhibits the Ca2+ influx of postsynapse and causes the hyperpolarization of neuronal membrane (Ohkuma et al. 1994; Miura et al. 1997), which thereby may inhibit the function of NMDAR via attenuation of the increased interaction of Src, NR2A and scaffold protein PSD-95, consequently protecting neurons from apoptotic neuronal death (Zhang et al. 2007). GABAAR overactivation potentiates the effects of NMDA blockade in inducing locomotor hyperactivity during this period (Oliveira-Pinto et al. 2015). As for the SGNs, evidence implicates the GABAAR in mediating the inhibitory effect of GABA specifically. GABA-mediated (Cl-) currents have been recorded from SGNs isolated from neonatal (E14-P5) mice (Lin et al. 2000), neonatal (P3) rat (Malgrange et al. 1997). Recordings of afferent activity from high frequency regions of the intact guinea pig cochlea, show that GABA application decreases Glu- and ACh-induced increases in afferent spiking specifically through activation of GABAAR (Felix and Ehrenberger 1992; Arnold et al. 1998). However, cultured SGNs (P2) ECl is -18 mV (Zhang et al. 2015). Given this, it is probable that activating GABAAR will depolarize SGNs. In this study, we found that the activation of GABAAR in SGNs could downregulate the expression of NR2B surface protein without affecting total protein expression (Figs. 2, 3). But the exact mechanism for the protective action produced by GABAAR activation on the NMDAR, remains to be elucidated.
At present, considerable data have shown that GABAAR hypofunction and NMDAR hyperfunction are key events in ototoxicity induced by SS. On the one hand, salicylate increases the arachidonate content of the whole cochlea in vivo (Ruel et al. 2008), salicylate and arachidonate enable the Ca2+ influx and the neural excitatory effects of NMDA on SGNs (Sahley et al. 2013). On the other hand, SS inhibits GABAAR-mediated postsynaptic currents, reducing synaptic transmission, and attenuates GABAergic inhibitory effects in rat hippocampal CA1 region (Xu et al. 2005; Wang et al. 2006; Zou and Shang 2012). In our study, SS decreased NR2B surface protein expression through affecting GABAAR, but the total protein did not significantly change (Fig. 4). Furthermore, there was no superposition of SS and GABAAR activity at saturation concentration, which proved SS or GABAAR activity is the same effect (Fig. 5). Previous studies suggested that activated NMDAR may be left from the cell membrane by internalization, thus avoiding reactivation. For instance, cortical neurons induce NMDAR internalization through the c-Jun phosphorylation pathway to alleviate the excitatory toxicity caused by excessive NMDA stimulation (Vaslin et al. 2007). Excitatory stimulation of hippocampal neurons in rats induces a reduction in the proportion of NR2B subunits and an elevation in the proportion of NR2A subunits (Bellone and Nicoll 2007). Neuronal suppression enhances NMDAR channel currents and increases NR2B subunits content (Lee et al. 2010). Therefore, we speculated that the imbalance between GABAAR and NMDAR functional regulation through a receptor internalization mechanism mediates the excitotoxicity of SS.
In addition, this study showed that the application of 0.5/5 mM SS significantly decreased Fyn and P-Y1472 protein expression (Fig. 6), and PP2 could reverse this process (Fig. 8). The above suggested that SS inhibits Fyn-mediated Y1472 phosphorylation. Consistent with the results, studies on cardiomyocytes and monocytes cultured in vitro also found that SS inhibits angiotensin- or platelet-derived growth factor activation of Src (which belongs to the same Src kinase family as Fyn) in a concentration-dependent manner, and the most obvious effect is 5–20 mM (Wang and Brecher 2001; Perez et al. 2002). Other studies have found that the internalization of NMDAR generally occurs in a manner similar to the production of clathin, which is mainly mediated through the interaction between the NR2 subunit C-terminal internalized motif YEKL and AP2. In rat cerebellum and hippocampus (Yaka et al. 2002; Prybylowski et al. 2005), neuronal activity induces the phosphorylation of the YEKL motif Y1472 site of NR2B by Fyn, thus inhibiting AP2-mediated NMDAR internalization. Moreover, the phosphorylation of NR2B-S1480 by CK2 destroys the binding of the receptor and membrane-associated guanylate kinase (MAGUK), causing NMDAR not to bind to Fyn, reducing the phosphorylation of NR2B-Y1472 and promoting internalization (Sanz-Clemente et al. 2010). However, only the changes in Fyn and Y1472 could be detected in this experiment (Figs. 6, 7). Therefore, we speculated that SS mediates NMDAR internalization relying on GABAAR, and the Fyn-dependent phosphorylation of Y1472 participates in the regulation of this process.
In conclusion, we assessed firstly that Fyn-dependent phosphorylation of Y1472 is a pathway of SS action on SGNs. The inter-receptor crosstalk acts as a compensatory, counterbalancing mechanism to dampen NMDAR-mediated excitotoxicity, which not only breaks the traditional idea that the receptor is a single and independent functional unit but also provides a new idea for studying the intrinsic mechanisms of ototoxicity. However, there are still many components in the molecular mechanism of SS toxicity, and further studies on cell electrophysiology and molecular pharmacology are needed.
Abbreviations
- Bic:
-
Bicuculline
- GABAA :
-
Receptor agonist
- CK2:
-
Casein Kinase 2
- GABAAR:
-
γ-Aminobutyric acid A receptor
- Mus:
-
Muscimol, GABAA receptor antagonist
- NMDAR:
-
N-Methyl-d-aspartate receptor
- NR2B:
-
NMDA receptor 2B subunit
- PP2:
-
4-Amino-5-(4-chlorophenyl)-7-(t-butyl) pyrazolo[3,4-d] pyrimidine
- RQ:
-
Relative quantity
- SGNs:
-
Spiral ganglion neurons
- SS:
-
Sodium salicylate
References
Arnold T, Oestreicher E, Ehrenberger K, Felix D (1998) GABA(A) receptor modulates the activity of inner hair cell afferents in guinea pig cochlea. Hear Res 125(1–2):147–153
Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ (2003) Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci 24(3):818–830
Bellone C, Nicoll RA (2007) Rapid bidirectional switching of synaptic NMDA receptors. Neuron 55(5):779–785
Buesa I, Ortiz V, Aguilera L, Torre F, Zimmermann M, Azkue JJ (2006) Disinhibition of spinal responses to primary afferent input by antagonism at GABA receptors in urethane-anaesthetised rats is dependent on NMDA and metabotropic glutamate receptors. Neuropharmacology 50(5):585–594
Caperton KK, Thompson AM (2011) Activation of serotonergic neurons during salicylate-induced tinnitus. Otol Neurotol 32(2):301–307
Chen QX, Wong RK (1995) Suppression of GABAA receptor responses by NMDA application in hippocampal neurones acutely isolated from the adult guinea-pig. J Physiol 482(Pt 2):353–362
Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R (2010) Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res 265(1–2):63–69
Chen GD, Stolzberg D, Lobarinas E, Sun W, Ding DL, Salvi R (2013) Salicylate-induced cochlear impairments, cortical hyperactivity and re-tuning, and tinnitus. Hear Res 295:100–113
Chen H, Zeng Q, Yao C, Cai Z, Wei T, Huang Z, Su J (2016) Src family tyrosine kinase inhibitors suppress Nav1.1 expression in cultured rat spiral ganglion neurons. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 202(3):185–193
Cong DN, Tang ZQ, Li LZ, Huang YN, Wang J, Chen L (2011) Cross-talk between NMDA and GABA(A) receptors in cultured neurons of the rat inferior colliculus. Sci China Life Sci 54(6):560–566
de Almeida-Silva I, de Oliveira JA, Rossato M, Salata FF, Hyppolito MA (2011) Spontaneous reversibility of damage to outer hair cells after sodium salicylate induced ototoxicity. J Laryngol Otol 125(8):786–794
Felix D, Ehrenberger K (1992) The efferent modulation of mammalian inner hair cell afferents. Hear Res 64(1):1–5
Giovannini MG, Mutolo D, Bianchi L, Michelassi A, Pepeu G (1994) NMDA receptor antagonists decrease GABA outflow from the septum and increase acetylcholine outflow from the hippocampus: a microdialysis study. J Neurosci 14(3 Pt 1):1358–1365
Kurumaji A, McCulloch J (1989) Effects of MK-801 upon local cerebral glucose utilisation in conscious rats and in rats anaesthetised with halothane. J Cereb Blood Flow Metab 9(6):786–794
Lee MC, Yasuda R, Ehlers MD (2010) Metaplasticity at single glutamatergic synapses. Neuron 66(6):859–870
Lin X, Chen S, Chen P (2000) Activation of metabotropic GABAB receptors inhibited glutamate responses in spiral ganglion neurons of mice. NeuroReport 11(5):957–961
Malgrange B, Rigo JM, Lefebvre PP, Coucke P, Goffin F, Xhauflaire G, Belachew S, Van de Water TR, Moonen G (1997) Diazepam-insensitive GABAA receptors on postnatal spiral ganglion neurones in culture. Neuroreport 8(3):591–596
Miura M, Yoshioka M, Miyakawa H, Kato H, Ito KI (1997) Properties of calcium spikes revealed during GABAA receptor antagonism in hippocampal CA1 neurons from guinea pigs. J Neurophysiol 78(5):2269–2279
Niedzielski AS, Wenthold RJ (1995) Expression of AMPA, kainate, and NMDA receptor subunits in cochlear and vestibular ganglia. J Neurosci 15(3 Pt 2):2338–2353
Ohkuma S, Chen SH, Katsura M, Chen DZ, Kuriyama K (1994) Muscimol prevents neuronal injury induced by NMDA. Jpn J Pharmacol 64(2):125–128
Oliveira-Pinto J, Paes-Branco D, Cristina-Rodrigues F, Krahe TE, Manhaes AC, Abreu-Villaca Y, Filgueiras CC (2015) GABAA overactivation potentiates the effects of NMDA blockade during the brain growth spurt in eliciting locomotor hyperactivity in juvenile mice. Neurotoxicol Teratol 50:43–52
Perez GM, Melo M, Keegan AD, Zamorano J (2002) Aspirin and salicylates inhibit the IL-4- and IL-13-induced activation of STAT6. J Immunol 168(3):1428–1434
Prybylowski K, Chang K, Sans N, Kan LL, Vicini S, Wenthold RJ (2005) The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron 47(6):845–857
Robello M, Amico C, Cupello A (1997) A dual mechanism for impairment of GABAA receptor activity by NMDA receptor activation in rat cerebellum granule cells. Eur Biophys J 25(3):181–187
Ruel J, Chabbert C, Nouvian R, Bendris R, Eybalin M, Leger CL, Bourien J, Mersel M, Puel JL (2008) Salicylate enables cochlear arachidonic-acid-sensitive NMDA receptor responses. J Neurosci 28(29):7313–7323
Sahley TL, Hammonds MD, Musiek FE (2013) Endogenous dynorphins, glutamate and N-methyl-d-aspartate (NMDA) receptors may participate in a stress-mediated Type-I auditory neural exacerbation of tinnitus. Brain Res 1499:80–108
Sanz-Clemente A, Matta JA, Isaac JT, Roche KW (2010) Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 67(6):984–996
Tang X, Zhu X, Ding B, Walton JP, Frisina RD, Su J (2014) Age-related hearing loss: GABA, nicotinic acetylcholine and Nmda receptor expression changes in spiral ganglion neurons of the mouse. Neuroscience 259:184–193
Torsney C, MacDermott AB (2006) Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci 26(6):1833–1843
Vaslin A, Puyal J, Borsello T, Clarke PG (2007) Excitotoxicity-related endocytosis in cortical neurons. J Neurochem 102(3):789–800
Wang Z, Brecher P (2001) Salicylate inhibits phosphorylation of the nonreceptor tyrosine kinases, proline-rich tyrosine kinase 2 and c-Src. Hypertension 37(1):148–153
Wang H, Ren WH, Zhang YQ, Zhao ZQ (2005) GABAergic disinhibition facilitates polysynaptic excitatory transmission in rat anterior cingulate cortex. Biochem Biophys Res Commun 338(3):1634–1639
Wang HT, Luo B, Zhou KQ, Xu TL, Chen L (2006) Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res 215(1–2):77–83
Wei L, Ding D, Salvi R (2010) Salicylate-induced degeneration of cochlea spiral ganglion neurons-apoptosis signaling. Neuroscience 168(1):288–299
Xu H, Gong N, Chen L, Xu TL (2005) Sodium salicylate reduces gamma aminobutyric acid-induced current in rat spinal dorsal horn neurons. NeuroReport 16(8):813–816
Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D (2002) NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci USA 99(8):5710–5715
Yao C, Cai Z, Wang R, Chen H, Huang Z, Qin J, Su J (2015) The effect of sodium salicylate on the expression of GABAa receptor subunits in cochlear spiral ganglion neurons. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 29(11):1024–1029
Zhang F, Li C, Wang R, Han D, Zhang QG, Zhou C, Yu HM, Zhang GY (2007) Activation of GABA receptors attenuates neuronal apoptosis through inhibiting the tyrosine phosphorylation of NR2A by Src after cerebral ischemia and reperfusion. Neuroscience 150(4):938–949
Zhang XD, Lee JH, Lv P, Chen WC, Kim HJ, Wei DG, Wang WY, Sihn CR, Doyle KJ, Rock JR, Chiamvimonvat N, Yamoah EN (2015) Etiology of distinct membrane excitability in pre- and posthearing auditory neurons relies on activity of Cl-channel TMEM16A. Proc Natl Acad Sci USA 112(8):2575–2580
Zou QZ, Shang XL (2012) Effect of salicylate on the large GABAergic neurons in the inferior colliculus of rats. Acta Neurol Belg 112(4):367–374
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (81360157, 81560174). We also thank the help from the Medical Scientific Research Center of Guangxi Medical University, and the Experimental Animal Center of Guangxi Medical University. All procedures performed in studies involving animals were in accordance with the ethical standards of the NSFC guidelines for the care and use of animals, as described in the “Materials and methods” section.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, P., Qin, D., Huang, X. et al. Neurotoxicity of sodium salicylate to the spiral ganglion neurons: GABAA receptor regulates NMDA receptor by Fyn-dependent phosphorylation. J Comp Physiol A 205, 469–479 (2019). https://doi.org/10.1007/s00359-019-01339-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-019-01339-z