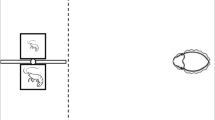Abstract
Moving shadows signify imminent threat to foraging juvenile crayfish, and the animals respond with one of two discrete anti-predatory behaviors: They either freeze in place or rapidly flex their tails, which quickly propels them away from the approaching danger signal. Although a freeze might be the more risky choice, it keeps the animal near the expected food reward, while a tail-flip is effective in avoiding the shadow, but puts critical distance between the animal and its next meal. We manipulated the satiation level of juvenile crayfish to determine whether their behavioral choices are affected by internal energy states. When facing the same visual danger signal, animals fed to satiation produced more tail-flips and fewer freezes than unfed animals, indicating that intrinsic physiological conditions shape value-based behavioral decisions. Escape tail-flip latencies, however, were unaffected by satiation level, and an increase in food quality only produced a minor behavioral shift toward more freezing in both fed and unfed animals. Thus, satiation level appears to be the dominant factor in regulating decision making and behavioral choices of crayfish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All living organisms are driven by their need to survive, and many come equipped with mechanisms to integrate information into adaptive decisions that will foster survival. These decisions often involve trade-offs between the value of a potential resource, such as food or mate, against other risks, including a predatory or conspecific attack (e.g., Lima and Dill 1990). Since decisions are made in the nervous system and activation of discrete neural circuitry determines behavioral action, there is great urgency to better understand the underlying neural basis. Specifically concerned with value-based decisions, this has led to a new research field termed “neuroeconomics” (Glimcher and Rustichini 2004; Camerer et al. 2005). Mechanisms related to decision making in humans have been uncovered using functional magnetic resonance imaging (fMRI), and some studies have linked both intrinsic factors such as current emotional state, sleep deprivation, and perceived social status (Harrison and Horne 2000; Sanfey et al. 2003; Heekeren et al. 2004; Marsh et al. 2009), as well as extrinsic factors, such as risk, time, and reward quality to decision making (Doya 2008). In addition, in human studies it was found that activity in certain decision-related brain areas correlates not only with the presence of a reward, but it scales with its magnitude (Sanfey et al. 2006), while yet other brain areas are associated with decision making under uncertainty (Platt and Huettel 2008).
However, studies in humans are limited to noninvasive methodology, which restricts the quality and amount of information that can be gathered (Logothetis 2008), and since evidence suggests that risk assessment and decision-making processes are produced in similar fashion across taxa, non-human primates, rodents, birds, and other vertebrate species have been used to study neuroeconomics (Glimcher et al. 2004). However, the neuronal pathways that connect decision-making neurons to other brain areas and to motor neurons that drive behavior often remain elusive (Kristan 2008).
Thus, using invertebrate models to better understand decision making provides many unique advantages. The nervous systems are of lower complexity and contain large, accessible, and individually identified neurons that link directly to behavioral output (Herberholz and Marquart 2012; Gaudry and Kristan 2012; Dickinson et al. 2015; Crossley et al. 2016). When threatened, crayfish often activate escape tail-flips that propel the animals away from the danger signal and are mediated by well understood neural circuits, two of which are controlled by identified giant interneurons (Wine and Krasne 1972; Edwards et al. 1999; Herberholz et al. 2004). Tail-flips in response to salient visual danger signals are generated by the medial giant (MG) interneurons, which upon activation, are sufficient to generate the entire behavioral escape sequence (Liden and Herberholz 2008). During a shadow-evoked tail-flip, the MG neurons produce an action potential large enough to be detected outside the animal by a pair of bath electrodes located in the water, which permits simultaneous behavioral and neuronal analysis in freely behaving juvenile crayfish (Herberholz et al. 2001; Liden and Herberholz 2008).
Studies of escape behavior produced in response to moving shadows are relatively sparse. However, different insect and crustacean species have been productive models for identifying discrete visual pathways for perception of looming danger signals (e.g., a fast-expanding disk presented on a monitor), and in some cases individual “trigger” neurons and their downstream connections to motor control units for escape (e.g., Fotowat and Gabbiani 2007; Oliva et al. 2007; Card and Dickinson 2008; Herberholz and Marquart 2012). Moreover, the internal state of the animal (i.e., arousal) and the biochemistry involved in the behavior have been investigated in a few studies (e.g., Rind et al. 2008), and more naturalistic predator signals have been used in both the laboratory and the field (Hemmi and Tomsic 2012; Santer et al. 2012). In mice, it has been shown that looming stimuli displayed above the animals elicit either flight or freezing and this was dependent on the parameters (e.g., speed) of the stimulus (Yilmaz and Meister 2013). In addition, it has recently been reported that a looming stimulus (simulating an attacking predator) reliably induced flight responses into a refuge in the cage, whereas a black disk sweeping across an overhead screen (simulating an aerial predator cruising above the mouse) primarily evoked freezing behavior; thus, the animals made discrete behavioral decisions depending on the significance of the perceived threat (De Franceschi et al. 2016). It has also been reported that fruit flies (Drosophila) exposed to overhead shadows produced freezing behavior, and flies exposed to multiple shadow repetitions were slower to return to a food source than animals exposed to only a few shadow repetitions (Gibson et al. 2015).
Crayfish have many predators, including birds, fish, and mammals, all of which attack from above and are likely to cast shadows (Englund and Krupa 2000; Davis and Huber 2007; Tablado et al. 2010; Wolff et al. 2016). We recently demonstrated that foraging juvenile crayfish that approach an expected food reward will respond to an approaching shadow (that moves within a certain velocity range) with only one of two discrete and incompatible behaviors: They will either freeze in place, or produce an escape tail-flip, a rapid flexion of the tail that propels the animal backwards, away from both the approaching shadow and the expected food reward (Liden and Herberholz 2008). Altering extrinsic environmental factors, such as the velocity of an approaching shadow and the quality of the expected food reward influenced escape decisions, i.e., freezing or tail-flipping (flight) in crayfish (Liden et al. 2010). Crayfish were less likely to tail-flip away from faster shadows and selected freezing instead, and once the shadow speed exceeded their reaction time for a successful tail-flip escape, they almost never activated this behavior and defaulted to freezing instead. In addition, when a stronger food reward was presented (a higher concentrated food odorant in the water), crayfish also decided to freeze more and suppress tail-flipping. When the shadow signal was made more dangerous by adjusting its velocity, however, animals reversed to more tail-flipping despite the presence of higher food value (Liden et al. 2010). Freezing keeps the animal closer to the expected food, while tail-flipping propels it away, and it takes animals much longer to eventually reach the food source in the tank after a tail-flip. Because freezing is hypothesized to be the more risky option, these results support the notion that crayfish carefully balance costs (predation risk) and benefits (food reward) before selecting the most adaptive behavioral action, suggesting that they are capable of economic decision making (Liden et al. 2010). Thus, our earlier work has shown that crayfish make fast decisions based on the integration of multimodal sensory information, and they adjust their decisions based on calculating the values of different behavioral outcomes. The current paper expands on this concept.
Our new research presented here for the first time investigates the combined effects of altering crayfish food satiation levels, an intrinsic state, and varying levels of food quality, an extrinsic factor, on crayfish decision making in response to threatening shadows. We found that satiated animals were more likely to exhibit tail-flip escape responses when exposed to shadows, propelling them farther away from the food source, while unfed animals were more likely to freeze and stay close to the food. When we increased the value of the expected food reward, we found that animals tail-flipped less, though this effect was only mildly affected by their hunger levels. Together this suggests that under our experimental conditions, energy state of the animal is the dominating factor in regulating behavioral decisions.
Materials and methods
Animals
Juvenile crayfish (Procambarus clarkii), sized 3–4cm in length from rostrum to telson, were obtained from a commercial supplier (Atchafalaya Biological Supply Co.). They were housed in large aquaria (H: 31 cm, L: 32 cm, L: 76 cm) in groups of up to 50 animals. Animals were fed every Monday and Thursday, scattering approximately one food pellet (Ocean Nutrition Formula One) per animal into the aquarium. All animals spent a minimum of one week in communal aquaria and experienced a minimum of two communal feedings before they were used in experiments. Temperature in the room was controlled, and the light/dark cycle was set for 12h/12h. Each animal was only used once, and no animal was injured in any of the experiments.
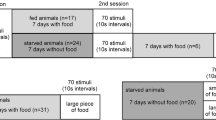
One day before the experiments, animals were taken from communal aquaria, measured and sexed, and isolated in smaller plastic tanks (H: 10.5 cm, W: 11 cm, L: 17.5 cm), which were filled with 1L of water and covered with a plastic lid. Each tank contained an air stone, which was connected to an air pump to provide oxygenation. Upon isolation, animals were assigned to one of two groups (“fed” or “unfed”). Animals belonging to the fed group were given three food pellets (mass = 50 mg) at the time of isolation, while the unfed animals were not given any food. Animals were kept in isolation overnight and then tested the next day. Animals were tested either two or three days after their last communal feeding, which did not affect the experimental results.
Experimental setup
The shadow experimental setup was identical to the version previously described (Liden and Herberholz 2010; Fig. 1a). The experimental tank (H: 22 cm, L: 36.5 cm, W: 21.5 cm) was separated into a tunnel chamber (H: 3.5 cm, L: 24 cm, W: 5.5 cm), which was covered by a clear plastic lid, and a starting chamber (H: 10 cm, L: 6.5 cm, W: 10 cm). The starting chamber was separated from the tunnel by a removable door (H: 7.8 cm, W: 7.5 cm, L: 0.6 cm), which was made of a translucent plastic with a hole drilled through the center of the top of the door. A string was tied through the hole as a handle to remove the door. In the tunnel, opposite from the starting chamber, was an opening in the plastic lid where a polyethylene inflow tube (0.5 cm diameter) was attached.
Experimental setup and design. a Schematic of the experimental tank. The crayfish is depicted in a location that would initiate the release of the shadow. b Five still images from a video recording illustrating an exemplary trial. The animal leaves the start compartment and begins foraging (b1), the animal reaches the bath electrodes (b2), the shadow sweeps across the tank (b3), the animal tail-flipped and is in a more rearward position before it re-initiates foraging (b4), the animal reaches the food odorant release point at the end of the tank (b5)
A “regular” (1×) stock solution of food odorant was created by dissolving 1 g of food pellets in 1L of deionized (DI) water for one hour using a stir plate (Corning Inc.) and subsequently filtering the water using filter paper to remove any particulates. A stronger concentration of stock solution was prepared by using 4 g of food pellets dissolved in 1L of DI water (= high food odorant; 4 × concentration). The experimental solution of food odorant was always created by mixing 400 mL of the stock solution with 9.6 Lof DI water in a large reservoir (H: 33.5 cm, W: 24 cm. L: 24 cm). An inflow tube led from the reservoir into the experimental tank, and the flow was regulated by a flow meter (Cole Parmer Instrument Company) which was set to 190 mL/min. The solution entered into the tunnel via the inflow tube, moved along the tunnel toward the starting chamber, and exited via an outflow tube (1.5 cm diameter), located at the back of the starting chamber. The outflow tube emptied into a plastic bucket, placed on the floor, which kept the volume of water in the tank constant and at a consistent flow rate.
The side of the tank containing the outflow tube, the starting chamber floor, and the tunnel walls and floor were all painted white. The sides of the experimental tank to the left of and behind the inflow tube were painted black. The side of the tank to the right of the inflow tube (and facing the light source; see below) was unpainted and covered with a translucent piece of paper (21.6 cm × 35.6 cm, 92 brightness) in order to prevent the crayfish from seeing outside of the tank.
The experimental tank was placed inside a metal frame (H: 61 cm, W: 5 cm L: 51 cm). In order to block any possible external light, a large piece of cardboard (H: 94 cm, L: 93 cm) was affixed to the front of the frame. A window was cut out of the cardboard (H: 20 cm, L: 33 cm) in order to allow the light source to reach the tank, without any extra light passing through to other areas around the tank. Two photodiodes (Allied Electronics) were mounted to the cardboard just above the tank, spaced exact1y 175 mm apart. The first diode captured the shadow when it first appeared in the tank (and became visible to the animal), while the second diode captured the leading edge of the shadow at a position in the tank where bath electrodes were placed for neuromuscular recordings when the animal tail-flipped.
Bath electrodes were used as described in earlier publications (e.g., Herberholz et al. 2001; Liden and Herberholz 2008). A pair of copper wire bath electrodes (24 AWG, 0.25 mm insulation except for the tips; Belden CDT Inc.) was attached on opposite sides inside the tunnel to record field potentials generated during tail-flips. The electrodes were located 80 mm distance from the tunnel entrance and 160 mm from the end of the tunnel. The bath was grounded using a ground wire. The bath electrodes were connected to an extracellular amplifier (A-M Systems), and the amplified signals (1000×) were filtered, digitized, and recorded with Axoscope software (Axon Instruments) on a personal computer. MG tail-flips can be readily identified with bath electrode recordings (due to their initial large and phasic muscle potentials and preceding giant neuron action potentials), and the response latency can be determined by measuring the time between activation of the first photodiode signal and the signal from the bath electrodes (Liden et al. 2010).
The light source was a gooseneck illuminator (Fiber-Light MI-150) placed 65 cm away from the tank. This allowed light to evenly illuminate the entire side of the tank that was covered with the white sheet of paper. Using a Luxmeter (Milwaukee SM700) to measure light levels inside of the tank, the lighting was set to 25 Lux when pointing toward the ceiling, and 75 Lux when pointed directly toward the light source.
The shadow was generated by passing a rectangular piece of black plastic (H: 18 cm, W: 9 cm) through the focused light beam directed at the experimental tank. The plastic sail was moved along a track parallel to the longer side of the tank using a single-axis programmable stepper motor control system (OES Inc., Allegra 1-10). The sail was set to move along the track at a speed that produced a constant shadow of 2 m/s inside the experimental tank. The shadow velocity was measured and confirmed before experiments were performed using the measurements from the two photodiodes located immediately above the tank. Shadows of 2 m/s were visible to the animals for 87.5 ms before they reached the animal’s position in the tank (where the bath electrodes are located).
A GoPro Hero3 Black edition video camera was used to record the animal’s behavior during each trial. The GoPro was attached to a monitor (Dell), placed to the side of the experimental tank to allow observation of the animal’s behavior throughout the experiment. Digital files were downloaded onto a personal computer and analyzed on a large TV (Sony WEGA) screen.
Experimental procedure
Immediately prior to placing the animal in the experimental tank, the tank was filled with DI water until water started to trickle out of the outflow tube. An animal was removed from its isolation tank and placed into the starting chamber of the experimental tank. The animal was given 10 min to acclimate before the flow of food odorant was turned on, AxoScope recordings and GoPro video recordings were started, and the door was removed from the starting chamber. In a typical trial (Fig. 1b), the animal would leave the start compartment after some delay and move along the tunnel toward the end where the highest concentration of food odorant was present. Once the animal’s eyes passed the bath electrodes, the computer program was triggered that advanced the plastic sail and produced the shadow. The animal either tail-flipped or froze in response to the shadow. Tail-flips were easily identified because they propelled the animal backwards, sometimes into the start compartment. Freezing was also unambiguously identified because animals immediately stopped forward locomotion and remained motionless for a few seconds. All animals independent of their response to the shadow eventually resumed forward walking. The trial ended when the animal had reached the end of the tunnel where the food odorant release point was located, or after ten minutes from the start of the trial, whichever occurred first.
Animals were excluded from data analysis if they were not moving when the shadow was released, their eyes were more than 10 mm past the bath electrodes when the shadow was released, or if they were turned more than 45° toward one side of the tunnel when the shadow was released. All parts of the experimental tank were washed in between trials.
Each animal was used only once and exposed to only one shadow. A total of 133 animals were used in all experiments, 15 of which were excluded because of the above criteria, leaving 118 animals for analysis.
Data analysis
Video recordings from the GoPro camera were carefully analyzed for the following behavioral measurements: (a) behavioral frequencies of freezing and tail-flipping, (b) time to enter the tunnel (in seconds), i.e., how long it took animals to leave the start compartment and begin foraging, (c) time to reach bath electrodes (in seconds); how long it took the animals to walk to the bath electrodes, and (d) time to reach end (in seconds); how long it took the animals to walk to the end of the tunnel after shadow exposure. Response latencies (in milliseconds) for tail-flips were calculated from photodiode and bath electrode measurements as the duration between the signal of the photodiode that recorded the first appearance of the shadow in the tank and the neuromuscular response.
Unless otherwise stated, data are presented as means with standard deviation (mean ± SD). Statistical software (IBM SPSS version 23.0; SPSS Inc.) was used for analysis, and each applied statistical test is specified in the text.
Results
Behavioral latencies of foraging activity
We first looked at the effects that feeding status has on foraging activity. We measured two time points that give indication of the animals’ motivation to reach the food odorant release point: time to leave the start compartment after the beginning of the trial, and time to reach the pair of bath electrodes in the tank, at which time the shadow signal is released. After the animals leave the start compartment, they must walk a distance of 8 cm to reach the bath electrodes (Fig. 1).
We first analyzed the foraging behavior of fed (N = 29) and unfed animals (N = 30) in the regular concentration of food odorant (Fig. 2a). The average time for fed animals to enter the tunnel was 76.9 ± 84.1 s, whereas unfed animals entered the tunnel and started approaching the food odorant release point more quickly (55.13 ± 45.19 s) although not significantly (Mann–Whitney U Test: p = 0.638). The average time for fed animals to reach the bath electrodes was 123.9 ± 117.3 s and unfed animals also reached this point more quickly (91.2 ± 70.8 s) although the difference did not produce statistical significance (Mann–Whitney U Test: p = 0.321). It is notable that unfed animals reached the bath electrodes earlier than fed animals not only because they started foraging earlier, but also because they walked more quickly. It took fed animals on average 47.0 ± 92.4 s from entering the tunnel to reaching the bath electrodes, while it took unfed animals only 36.1 ± 41.7 s to cover the same distance in the tank (Mann–Whitney U Test: p = 0.371).
Behavioral latencies during foraging activity in fed and unfed crayfish. Results show the times it took fed and unfed animals to leave the gate and reach the bath electrodes after the start of the trial in regular (1×) food odorant concentration (a) and in high food odorant (4×) concentration (b). **p ≤ 0.01
When we analyzed behavioral latencies for fed (N = 29) and unfed (N = 30) animals in the higher food odorant concentration, we found a very similar pattern; however, the differences between the two groups were more pronounced (Fig. 2b). Fed animals started foraging activity (i.e., left the start compartment) after 73.8 ± 93.5 s, but it took unfed animals only 51.2 ± 56.4 s, a difference that approached statistical significance (Mann–Whitney U Test: p = 0.081). Moreover, while fed animals needed 108.7 ± 93.5 s to reach the bath electrodes, unfed animals arrived at this point in the tank much earlier (68.4 ± 56.0 s), a significant difference between the two groups (Mann–Whitney U Test: p = 0.038). Unfed animals also moved more quickly than fed animals once they started foraging. It took fed animals 34.9 ± 36.3 s from entering the tunnel to reaching the bath electrodes, while it took unfed animals only 17.2 ± 9.7 s to walk the same distance in the tank (Mann–Whitney U Test: p = 0.005).
Next, we combined the two groups (fed and unfed) and looked for differences between the two food odorant concentrations across all animals (Fig. 3). Irrespective of feeding status, we found that animals in the regular odorant concentration (N = 59) started foraging after 65.8 ± 67.8 s and reached the bath electrodes after 107.3 ± 97.1 s, whereas animals in the higher food odorant concentration (N = 59) started foraging slightly earlier (62.3 ± 77.1 s), and they reached the bath electrodes sooner (88.2 ± 86.5 s). Thus, animals initiated foraging activity with similar latencies (Mann–Whitney U Test: p = 0.471) in both concentrations of food odorant, but they reached the bath electrodes sooner in the higher concentration although the difference was not significant (Mann–Whitney U Test: p = 0.187). The earlier arrival at the bath electrodes in the higher food odorant concentration is due to the animals’ higher walking speed in this condition. In regular food odorant, animals needed 41.4 ± 70.8 s to walk to the bath electrodes after they started foraging, but they covered this distance in 25.9 ± 27.6 s when exposed to the higher food odorant concentration, which is significantly faster (Mann–Whitney U Test: p = 0.026).
Behavioral responses to shadow signals
The frequency of tail-flips was highest in the animals that were fed during isolation. This was found to be true for animals tested using both the regular (1×) and the increased (4×) food odorant concentration (Fig. 4). Fifty-nine percent of the fed animals (N = 29) in the regular food odorant concentration group tail-flipped and 41% froze in response to the shadow stimulus, while only 27% of the unfed animals (N = 30) tail-flipped and 73% displayed freezing behavior instead. There is a significant difference in behavioral frequencies between the two groups (Fisher Exact Test: p = 0.018; Fig. 4a). For animals tested in the higher concentration of food odorant (4×), 55% of the fed animals (N = 29) tail-flipped and 45% froze, while only 23% of the unfed animals (N = 30) tail-flipped and 77% froze instead. As before, the measured frequencies between the two groups are significantly different (Fisher Exact Test: p = 0.017; Fig. 4b).
Since we monitored feeding activity by counting how many food pellets were consumed before the shadow experiment, we were able to compare food intake between animals that tail-flipped and animals that did not. We found that fed animals that tail-flipped (across both regular and high odorant concentrations; N = 33) had consumed on average more pellets (2.30 ± 0.85) than animals that froze (1.94 ± 0.99; N = 25); however, this difference was not statistically significant (Mann–Whitney U Test: p = 0.155).
Next, we analyzed the effect of food odorant concentration across all animals (fed and unfed). Fewer tail-flips and more freezing behavior were observed in animals tested in the higher concentration (4×) compared to the regular concentration (1×), and this was true independent of the animals’ feeding status (Fig. 5). When we combined both fed and unfed animals, we found that 42% of animals tail-flipped in regular food odorant concentration and 58% froze in response to the shadow danger signal (N = 59). In higher food odorant concentration, only 39% of animals tail-flipped and 61% froze when they responded to the shadow (N = 59). Thus, fewer tail-flips were observed in higher food odorant, which is what we expected, but the difference in behavioral frequencies between the two food odorant concentrations was fairly small and nonsignificant (Fisher Exact Test: p = 0.851).
Neural responses to shadow signals
Lastly, we determined latencies for tail-flip responses by measuring the difference (in milliseconds) between the signal recorded by the first photodiode, which was activated by the moving shadow when it first became visible to the animal, and the animal’s neuromuscular response during the tail-flip, which was recorded by the bath electrodes. We found no difference in escape latencies between fed animals (81.22 ± 11.22 ms; N = 21) and unfed animals (81.75 ± 23.38 ms; N = 10) across both odorant concentrations (Mann–Whitney U Test: p = 0.268), indicating that although fed animals produced a significantly higher frequency of tail-flips, they did not produce faster tail-flips in response to the shadow signal. Since the shadow was programmed to take approximately 87.5 milliseconds to reach the animals’ position in the tank (velocity = 2 m/s), both fed and unfed animals produced tail-flips that were on average fast enough to escape the approaching shadow. However, 33% of the fed animals (7/21) and 30% of the unfed animals (3/10) initiated tail-flips after the shadow reached their position in the tank.
Discussion
The three escape behaviors that can be witnessed across the animal kingdom are freezing, fleeing, and defensive attack (Eilam 2005). In response to a moving shadow stimulus, juvenile crayfish will display two of these behaviors, freezing or fleeing, in a robust and discrete manner (Liden and Herberholz 2008). Fleeing is expressed as a powerful tail-flip, which is produced by a pair of medial giant (MG) interneurons. The MGs, if excited, will consistently result in a fixed motor output, the MG-mediated tail-flip (Edwards et al. 1999). In the presence of a natural predator (i.e., a dragonfly nymph), MG-mediated tail-flips are a highly effective escape response to predator attacks, making their use a desirable motor action that ensures survival (Herberholz et al. 2004). However, tail-flipping (like other forms of fleeing) is an energetically costly behavior and, importantly, in foraging animals it adds critical distance between the animal and its next meal. Thus, freezing, while potentially more risky, is an alternative behavioral choice that allows preserving important needs such as feeding opportunities.
Satiation level affects motivation to forage
Our current study shows that the crayfish’s decisions are strongly affected by the internal (energy) state of the animal. The first significant finding of our study is the observed difference in foraging activity between fed and unfed animals. Animals that received food the night before the experiment initiated foraging activity later and walked slower toward the food odorant release point. This indicates that our manipulation of food satiation level was successful because fed animals displayed a lower motivation to find food than unfed animals, and this was most apparent when the food odorant concentration, i.e., the food value, was high. This is an important result as it sets the stage for the behavioral frequencies measured in response to shadow exposure. Animals that were fed large amounts of food before the experiment tail-flipped significantly more and froze significantly less than animals that were not given any food. This shows that crayfish integrate internal hunger level into the decision on how to respond to the shadow danger signal, and when staying close to the expected food reward becomes a less desirable option for satiated animals, they choose the safer strategy and tail-flip away from both the approaching shadow and nearby food source. A hungry crayfish, however, makes an entirely different and potentially more risky choice in response to the same danger signal: Because food is now much more desirable, it suppresses tail-flipping and selects freezing instead to stay near the food. Riskier foraging decisions are common among hungry animals because of their immediate energy needs. This shifts the trade-off between finding a meal and becoming a meal toward the more risky behavior. For example, in insects, fish, and mammalian species, it was found that hungry individuals will be less vigilant, spend less time in a refugee, and/or occupy more risky habitats (Lima 1998).
Neural underpinnings of behavioral choice
In the present study, the decision to abandon the expected food reward in favor of safety is based on the threshold of a single neuron. Although activation of the MG-mediated tail-flip is extremely fast (within tens of milliseconds after the shadow becomes visible), the decision process that determines whether it becomes activated depends on the integration of multiple internal and external factors. Altering the properties of external stimuli such as shadow speed and quality of the expected food reward can change the behavioral frequencies for freezing or tail-flipping, implying that the animal is integrating multiple sensory modalities before the desired motor pattern is selected (Liden et al. 2010). In addition, as we have now shown, selection of the appropriate behavioral choice is shaped by internal energy states, which must be integrated into the neural machinery that determines the behavioral action.
A study by Krasne and Lee (1988) tested the threshold of the lateral giant (LG) interneurons in feeding and non-feeding crayfish. The LG neurons are homologues of the MG neurons, and while the MGs are activated by mechanosensory and visual stimuli to the front of the animal and drive the animal backward, the LGs are activated by strong, phasic mechanosensory stimuli to the tail; once activated the LGs propel the animal upwards and forward away from the point of stimulation (Edwards et al. 1999). In actively feeding crayfish, the excitability of the LGs decreases compared to animals that are not feeding, indicating that the animal actively suppresses escape when a valuable resource is encountered (Krasne and Lee 1988). Although the study did not test the effect of hunger on LG excitability, it made some important suggestions about the underlying mechanism for the recorded changes in LG threshold. The LG circuit, which is located in the abdomen, receives GABAergic tonic inhibition from descending interneurons that originate in the crayfish brain (Vu and Krasne 1993). Tonic inhibition, which is common to many species, acts on LG’s dendrites and allows subtle up- and down-regulation of LG’s excitatory state (Vu et al. 1993). In the example of the feeding crayfish, the animal’s experience of feeding may cause an increase in tonic inhibition that reduces the probability for a tail-flip. In our current study, a similar mechanism can be suggested. Although we measured the excitability of the MG neurons, which produce tail-flips in response to shadows, it seems plausible that tonic GABAergic inhibition onto MG neurons increases or decreases in parallel with an increase or decrease in energy level. In other words, in a hungry crayfish approaching a potential meal, MG’s excitability to danger signals could be downregulated via GABAergic modulation to avoid escape tail-flipping in favor of freezing. Of course, further research is needed to understand if suppression of MG tail-flipping automatically leads to freezing and what relationship exists between the neural circuits that control these two discrete behavioral choices. In addition, the involvement of other neurotransmitters such as serotonin and octopamine (the invertebrate homologue of norepinephrine) must be considered given their prominent role in modulating crayfish behavior and neural function (Krasne and Edwards 2002).
Although tail-flip frequencies were strongly affected by satiation level, latencies for activating the MGs and corresponding behavior were not. Both fed and unfed animals exhibited almost identical tail-flip latencies, which were fast enough (on average) to escape from collision with the approaching shadow. The measured latencies are in line with previously reported values (Liden et al. 2010), and also similar to escape latencies measured in copepods, another aquatic crustacean, in response to shadows that appeared almost instantly (Buskey and Hartline 2003). Fast shadows, such as the one used in the current study, produce escape latencies that are probably close to the minimum time it takes to process visual information and activate the MG neurons. Alternatively, this result may suggest that excitability of visual input pathways to the MG neurons is unaffected by hunger state, and MG firing threshold is regulated postsynaptically, maybe via the above mentioned tonic GABAergic inhibition.
Interplay between satiation level and expected food reward
Increasing the concentration of food odorant in our study did not significantly change the frequencies of tail-flipping and freezing; it did, however, produce a small reduction in tail-flips and a small corresponding increase in freezing as we would have expected. The reduction in tail-flipping was not different for fed and unfed animals. Previous work using a similar manipulation of food quality resulted in a significant change in behavior of animals that were somewhat similar to our unfed group (Liden et al. 2010). However, the increase in food odorant concentration was 2.5 times higher compared to our current study, and animals did not receive any food during a longer isolation period (one week). Thus, it is possible that greater changes in food odorant concentration and/or longer periods of starvation would have caused a more pronounced effect in our current study.
It is certainly notable that the observed changes in neural and behavioral threshold must be based not only the animal’s current hunger state, but also the quality of the chemical food signal, and the properties of the shadow. Thus, before crayfish decide to tail-flip or freeze, they integrate their current physiological state with environmental cues of multiple sensory modalities, and they make cost-benefit calculations that lead to the most adaptive and desirable behavioral output. Given the accessibility of the MG neurons for neurophysiological and neuropharmacological investigation (Liu and Herberholz 2010), this opens up exciting new avenues for future research into the cellular and circuit-level mechanisms underlying value-based decision making. Together with recent demonstration of binary decisions (fleeing vs. freezing) in response to visual danger signals in mammals (De Franceschi et al. 2016), this could ultimately lead to a better understanding of neuronal processes that govern economic behavior in many species, including humans.
References
Buskey EJ, Hartline DK (2003) High-speed video analysis of the escape responses of the copepod Acartia tonsa to shadows. Biol Bull 204:28–37
Camerer C, Loewenstein G, Prelac D (2005) Neuroeconomics: How neuroscience can inform economics. J Econ Lit 43:9–64
Card G, Dickinson MH (2008) Visually mediated motor planning in the escape response of Drosophila. Curr Biol 18:1300–1307
Crossley M, Staras K, Kemenes G (2016) A two-neuron system for adaptive goal-directed decision-making in Lymnaea. Nat Commun 7
Davis KM, Huber R (2007) Activity patterns, behavioural repertoires, and agonistic interactions of crayfish: a non-manipulative field study. Behaviour 144:229–247
De Franceschi G, Vivattanasarn T, Saleem AB, Solomon SG (2016) Vision guides selection of freeze or flight defense strategies in mice. Curr Biol 26:2150–2154
Dickinson KJ, Wainwright ML, Mozzachiodi R (2015) Change in excitability of a putative decision-making neuron in Aplysia serves as a mechanism in the decision not to feed following food satiation. Behav Brain Res 281:131–136
Doya K (2008) Modulators of decision-making. Nat Neurosci 11:410–416
Edwards DH, Heitler WJ, Krasne FB (1999) Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci 22:153–161
Eilam D (2005) Die hard: a blend of freezing and fleeing as a dynamic defense—implications for the control of defensive behavior. Neurosci Biobehav Rev 29:1181–1191
Englund G, Krupa JJ (2000) Habitat use by crayfish in stream pools: influence of predators, depth and body size. Freshwater Biol 43:75–83
Fotowat H, Gabbiani F (2007) Relationship between the phases of sensory and motor activity during a looming-evoked multistage escape behavior. J Neurosci 27:10047–10059
Gaudry Q, Kristan WB Jr (2012) Decision points: the factors influencing the decision to feed in the medicinal leech. Front Neurosci 6(:):101
Gibson WT, Gonzalez CR, Fernandez C et al (2015) Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in Drosophila. Curr Biol 25:1401–1415
Glimcher PW, Rustichini A (2004) Neuroeconomics: the consilience of brain and decision. Science 306:447–452
Harrison Y, Horne JA (2000) The impact of sleep deprivation on decision making: a review. J Exp Psychol 6:236–249
Heekeren S, Marrett P, Bandettini A, Ungerleider LG (2004) A general mechanism for perceptual decision-making in the human brain. Nature 431:859–862
Hemmi JM, Tomsic D (2012) The neuroethology of escape in crabs: from sensory ecology to neurons and back. Curr Opin Neurobiol 22:194–200
Herberholz J, Marquart GD (2012) Decision making and behavioral choice during predator avoidance. Front Neurosci 6:125
Herberholz J, Issa FA, Edwards DH (2001) Patterns of neural circuit activation and behavior during dominance hierarchy formation in freely behaving crayfish. J Neurosci 21:2759–2767
Herberholz J, Sen MM, Edwards DH (2004) Escape behavior and escape circuit activation in juvenile crayfish during prey–predator interactions. J Exp Biol 207:1855–1863
Krasne FB, Edwards DH (2002) Modulation of the crayfish escape reflex—physiology and neuroethology. Integr Comp Biol 42:705–715
Krasne FB, Lee SC (1988) Response-dedicated trigger neurons as control points for behavioral actions: selective inhibition of lateral giant command neurons during feeding in crayfish. J Neurosci 8:3703–3712
Kristan WB (2008) Neuronal decision-making circuits. Curr Biol 18:928–932
Liden WH, Herberholz J (2008) Behavioral and neural responses of juvenile crayfish to moving shadows. J Exp Biol 211:1355–1361
Liden WH, Phillips ML, Herberholz J (2010) Neural control of behavioral choice in juvenile crayfish. Proc R Soc B 277:3493–3500
Lima SL (1998) Nonlethal effects in the ecology of predator-prey interactions. Bioscience 48:25–34
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Liu YC, Herberholz J (2010) Sensort activation and receptive field organization of the lateral giant escape neurons in crayfish. J Neurophysiol 104:675–684
Logothetis N (2008) What we can do and what we cannot do with fMRI. Nature 453:869–878
Marsh AA, Blair KS, Jones MM, Soliman N, Blair RJR (2009) Dominance and submission: the ventrolateral prefrontal cortex and responses to status cues. J Cog Neurosci 21:713–724
Oliva D, Medan V, Tomsic D (2007) Escape behavior and neuronal responses to looming stimuli in the crab Chasmagnathus granulatus (Decapoda: Grapsidae). J Exp Biol 210:865–880
Platt ML, Huettel SA (2008) Risky business: the neuroeconomics of decision making under uncertainty. Nat Neurosci 11:398–403
Rind FC, Santer RD, Wright GA (2008) Arousal facilitates collision avoidance mediated by a looming sensitive visual neuron in a flying locust. J Neurophysiol 100:670–680
Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD (2003) The neural basis of economic decision-making in the ultimatum game. Science 300:1755–1758
Sanfey AG, Loewensteinb G, McClurec SM, Cohen JD (2006) Neuroeconomics: cross-currents in research on decision-making. Trends Cogn Sci 10:108–116
Santer RD, Rind FC, Simmons PJ (2012) Predator versus prey: locust looming-detector neuron and behavioural responses to stimuli representing attacking bird predators. PLoS One 7:e50146
Tablado Z, Tella JL, Sánchez-Zapata JA, Hiraldo F (2010) The paradox of the long-term positive effects of a North American crayfish on a European community of predators. Conserv Biol 24:1230–1238
Vu ET, Krasne FB (1993) Crayfish tonic inhibition: prolonged modulation of behavioral excitability by classical GABAergic inhibition. J Neurosci 13:4394–4402
Vu ET, Lee SC, Krasne FB (1993) The mechanism of tonic inhibition of crayfish escape behavior: distal inhibition and its functional significance. J Neurosci 13:4379–4393
Wine JJ, Krasne FB (1972) The organization of escape behavior in the crayfish. J Exp Biol 56:1–18
Wolff PJ, Taylor CA, Heske EJ, Schooley RL (2016) Predation risk for crayfish differs between drought and nondrought conditions. Freshw Sci 35:91–102
Yilmaz M, Meister M (2013) Rapid innate defensive responses of mice to looming visual stimuli. Curr Biol 23:2011–2015
Acknowledgements
This work was supported by a grant (IOS-0919845) from the National Science Foundation to J.H. We would like to thank Ms. Alexis Exum for her help with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schadegg, A.C., Herberholz, J. Satiation level affects anti-predatory decisions in foraging juvenile crayfish. J Comp Physiol A 203, 223–232 (2017). https://doi.org/10.1007/s00359-017-1158-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-017-1158-8