Abstract
Florida manatees inhabit the coastal and inland waters of the peninsular state. They have little difficulty navigating the turbid waterways, which often contain obstacles that they must circumnavigate. Anatomical and behavioral research suggests that the vibrissae and associated follicle–sinus complexes that manatees possess over their entire body form a sensory array system for detecting hydrodynamic stimuli analogous to the lateral line system of fish. This is consistent with data highlighting that manatees are tactile specialists, evidenced by their specialized facial morphology and use of their vibrissae during feeding and active investigation/manipulation of objects. Two Florida manatees were tested in a go/no-go procedure using a staircase method to assess their ability to detect low-frequency water movement. Hydrodynamic vibrations were created by a sinusoidally oscillating sphere that generated a dipole field at frequencies from 5 to 150 Hz, which are below the apparent functional hearing limit of the manatee. The manatees detected particle displacement of less than 1 μm for frequencies of 15–150 Hz and of less than a nanometer at 150 Hz. Restricting the facial vibrissae with various size mesh openings indicated that the specialized sensory hairs played an important role in the manatee’s exquisite tactile sensitivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manatees possess a unique arrangement of specialized sensory hairs, classified as vibrissae, present on the face and across the body. Anatomical and neurophysiological evidence in conjunction with behavioral assessments from other species as well as manatees suggest that vibrissae play an important role in detecting environmental stimuli.
Each vibrissal apparatus is known as a follicle–sinus complex (FSC), which includes a blood-filled sinus, bounded by a dense connective tissue capsule, is robustly innervated, and provides haptic feedback to the animal (Dykes 1975; Rice et al. 1986). Vibrissae are located primarily on the mystacial region of terrestrial and non-sirenian aquatic mammals, and are commonly referred to as whiskers. They can possess a number of mechanoreceptors such as Merkel cells, lanceolate endings, and free nerve endings (Zelena 1994). A deep vibrissal nerve containing 100–200 axons is found in rodents (Rice et al. 1986), whereas a number of aquatic mammals possess several main nerves, and a higher number of axons per follicle (Dehnhardt et al. 1999; Reep et al. 2001; Sarko et al. 2007). Ringed seals have between 1,000 and 1,500 axons per vibrissa (Hyvärinen 1995) and bearded seals exhibit a similar range, with a maximum of 1,650 (Marshall et al. 2006). The Australian water rat, which lives on land but hunts for prey in water, displays a count of 500 axons per follicle, intermediate between terrestrial and aquatic species (Dehnhardt et al. 1999). Manatees have up to 250 axons per FSC of the facial region (Reep et al. 2001).
Aquatic mammals face a unique challenge that terrestrial mammals do not. The increased density of water in comparison with air causes a constant deflection of vibrissae during any movement. Hanke et al. (2010) noted that harbor seals possess vibrissae that have an undulated surface structure. This specialization results in reduced vibrissal vibration, and thus a reduction in self-noise during swimming. The efference copy mechanism that has been documented in some fishes (Bell 1982; Coombs et al. 2002), allowing the organism to differentiate between externally generated stimuli versus those resulting from its own actions, could also be utilized by aquatic mammals.
To obtain information about their environment, aquatic mammals have developed adaptations of vibrissal systems. Walruses use their stiff vibrissae to explore the benthic substrate in search of invertebrates and are able to discriminate different objects at a small scale (Fay 1982; Kastelein and van Gaalen 1988). Seals and sea lions have been found to discriminate fine differences in objects (Dehnhardt 1994; Dehnhardt and Kaminski 1995; Dehnhardt and Dücker 1996). Seals can detect low-frequency hydrodynamic stimuli (Dehnhardt et al. 1998), and sea lions (Gläser et al., 2011) as well as seals (Dehnhardt et al. 2001; Schulte-Pelkum et al. 2007) can follow hydrodynamic trails generated by swimming conspecifics or as might be generated by prey. Manatees use their facial vibrissae to investigate food items and novel objects (Hartman 1979; Marshall et al. 1998; Bachteler and Dehnhardt 1999; Reep et al. 2002). They may also use them to detect hydrodynamic stimuli.
Manatees have only vibrissae and no other hair type distributed over their bodies. This arrangement appears to be unique among mammals, although rock hyraxes, a terrestrial relative of manatees, have a similar distribution, but with the vibrissae intermixed with pelage (D. Sarko, pers. comm.). Vibrissae are ~30 times denser on the facial region than on the post-facial body. The lips of the manatee are very mobile and prehensile. The vibrissae on the upper lip (U2 field) and lower lip (L1 field) are everted during grasping of objects, including plants ingested during feeding. This oral grasping has been termed oripulation (Marshall et al. 1998; Reep et al. 1998). The number of axons per follicle decreases when traveling further from the oral cavity (Reep et al. 2001). Vibrissae on the oral disk, classified as bristle-like hairs (BLHs) that are intermediate in stiffness and innervation, are used in non-grasping investigation of objects and food items (Hartman 1979; Marshall et al. 1998).
A previous study with the same two Florida manatees used in the current research investigated their ability to perform active touch discrimination of texture gratings using the facial vibrissae. Weber fractions (just-noticeable-differences), the proportion change in size needed for the subject to detect a difference between objects, were measured and compared to those of other species. Both manatees demonstrated low Weber fractions. One subject was able to detect differences in size down to 0.025 of a standard with 2.0-mm gratings and the other subject down to 0.075 (Bauer et al. 2012). The present study sought to test the hypothesis that manatees use their facial vibrissae not only for active touch but also to detect hydrodynamic stimuli. We conducted three experiments to test this hypothesis. The first generated a manatee tactogram, tactile detection thresholds across a set of low frequencies. A second test restricted vibrissae to assess their involvement in detection of hydrodynamic stimuli. A third experiment assessed vibrissae sensitivity using a signal detection format.
Materials and methods
Subjects
The subjects were two male Florida manatees (Trichechus manatus latirostris) housed at Mote Marine Laboratory and Aquarium in Sarasota, Florida, USA. Buffett and Hugh, 21 and 24 years of age, respectively, at the initiation of the study, had an extensive training history in the context of husbandry and sensory research (Colbert et al. 2001, 2009; Bauer et al. 2003; Mann et al. 2005; Bauer et al. 2012; Gaspard et al. 2012).
Equipment
A dipole vibration shaker (Data Physics—Signal Force, Model V4, San Jose, CA, USA) with a 5.7-cm diameter rubberized sphere connected via a 35.6-cm, rigid, stainless steel extension rod was used to generate the stimuli. The dipole shaker generates a localized flow that decreases in amplitude as 1/distance3, as opposed to a monopole source that decreases in amplitude as 1/distance2 (Kalmijn 1988). To minimize any vibrational transfer between the shaker apparatus and the stationing apparatus, the stationing apparatus and the shaker mount were separated and buffered with shock absorbing foam.
The stimuli were generated digitally by a Tucker-Davis Technologies (TDT) Enhanced Real-Time Processor (RP2.1, Alachua, FL, USA; sample rate 24.4 kHz), attenuated with a TDT Programmable Attenuator (PA5) to control level, and amplified with a Samson Power Amplifier (Servo 120a, Hauppauge, NY, USA). The signal generating equipment was controlled by a program in MATLAB (The MathWorks, Natick, MA, USA) in conjunction with a graphical user interface (TDT Real-Time Processor Visual Design Studio) created specifically for this research. A digital output on an RP2.1 was used to control the LED that indicated the start of a trial. A separate D/A channel was used to generate the acoustic secondary reinforcer, which was presented through an underwater speaker (Clark Synthesis, Model AQ-39, Littleton, CO, USA) when the manatee was correct on a trial. The speaker was located >1 m away from the subject and also presented noise (151 dB re 1 μPa; 0–12.2 kHz bandwidth) constantly through the session to mask any auditory artifacts from the generation of the hydrodynamic stimulus. These signals were amplified by a separate amplifier (American Audio, Model VLP 300, Los Angeles, CA, USA) to avoid crosstalk.
For stimuli analysis and calibration, a 3-dimensional accelerometer (Dimension Engineering, Model DE-ACCM3D, Akron, OH, USA) was embedded into the sphere to measure its movement. MATLAB was used to calculate, plot, and log the stimulus for each trial. This accelerometer was used to monitor the shaker operation during testing. To calculate particle motion from the dipole for threshold measurements, a 3-D accelerometer was mounted to a neutrally buoyant, spring-mounted geophone. The outputs from all three channels were recorded simultaneously by the RP2.1. The rms acceleration of the unattenuated stimulus for each stimulus frequency was calculated from these recordings. The magnitude of acceleration from all three axes was calculated as the square root of the sum of squares of each axis. The acceleration at the threshold was calculated by scaling the acceleration measured at no attenuation by the attenuation at threshold. For sinusoidal signals, particle velocity is the particle acceleration divided by 2πf, and particle displacement is particle velocity divided by 2πf (where f = frequency in Hertz). The sensitivity of the accelerometer was verified by comparing its output when directly vibrated with the output of a laser vibrometer (Polytec, Model CLV 1000, Irvine, CA, USA) pointed at the accelerometer. The laser vibrometer could not be used in the manatee tank because it only measures motion in one direction along the laser beam.
To ensure that the test subjects were not cued during testing, a number of protocols and measurements were conducted. A 3-D accelerometer was routinely attached to the stationing apparatus to ensure that there was no vibrational transfer from the shaker during presentation trials. The subjects’ minimum angle of visual resolution (MAR) [(Buffett’s MAR = 21 arc minutes and Hugh’s MAR = 66 arc minutes (Bauer et al. 2003)] precluded detection of the maximum sphere movement at threshold (0.0095 cm), which subtended a visual angle of less than 0.02 arc minutes for each manatee. The research trainer responsible for verifying the position of the manatee and providing the primary reinforcement was blind to whether the ensuing trial was a stimulus-present or stimulus-absent trial. This trainer was also out of sight of the manatee and remained motionless until the trial sequence was complete.
Experiment I: Tactogram
The tactogram established the tactile thresholds for frequencies ranging from 5 to 150 Hz. The upper limit was selected to minimize the possibility that detection of the stimuli by hearing confounded tactile measurements.
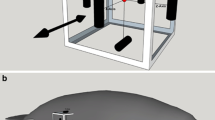
The manatees were trained using operant conditioning methods. A go/no-go procedure was used: If the stimulus was detected, the manatee responded by withdrawing from the horizontal stationing bar and touching a response paddle 1 m to the subject’s left with its muzzle, and if no stimulus was detected, the manatee remained at station for a minimum of 10 s (Fig. 1). They were reinforced for correct responses to either signal present trials (hits) or signal absent trials. They were not rewarded for incorrect responses to signal present trials (misses) or signal absent trials (false alarms-FA). Correct responses were followed by an auditory secondary reinforcer, a digitized whistle from an underwater speaker, followed by primary reinforcement, preferred food items of pieces of apples, carrots, beets, and monkey biscuits. After a correct response on a signal present trial, the intensity of the stimulus was attenuated 3 dB. A staircase method for stimulus presentation (Cornsweet 1962) was used in which eight reversals determined a threshold measurement. If the manatee was incorrect on a signal present trial, the intensity level of the stimulus was increased by 3 dB. Four “warm-up” trials were conducted prior to testing to assess the motivation and performance levels of the manatees with the stimulus at the same frequency and highest level that was to be tested. A criterion of 75 % correct on “warm-up” trials had to be met for testing to occur during that particular session. If the subject failed to meet criterion on the first set of warm-up trials, a second warm-up set was conducted. Testing was not conducted if the subject failed to meet criterion on the second warm-up block. Sessions were rejected if the FA rate was greater than 0.25 to avoid biased detection thresholds.
The subjects were trained to station by placing their postnasal crease on a horizontal PVC bar (2.5 cm diameter) at a depth of 0.75 m which facilitated a replicable positioning of the test subject’s muzzle to be 10 cm both forward and below, on the midline, from the stimulus generating sphere (Fig. 2). A tri-cluster LED signaled the initiation of every trial, illuminating for a duration of 1 s, followed by a 0.5-s delay prior to both signal present and signal absent windows. During testing the stimuli were generated by a 5.7-cm sinusoidally oscillating sphere with the embedded accelerometer driven by a computer-controlled calibrated vibration shaker (Data Physics, V4) (during training a 4.9-cm sphere without the accelerometer was used). The sphere was connected to the shaker via a rigid stainless steel rod. The shaker and attachment rod were oriented vertically in the water column. The stimuli were 3 s in duration with cos2 rise-fall times of 300 ms and ranged from 5 to 150 Hz. Signal present versus signal absent trials were counterbalanced using a 1:1 ratio. Daily sessions (weekdays) were conducted with each session focused on a single frequency, encompassing 12–72 trials. A single frequency was tested over the course of two separate staircase sessions conducted on consecutive days to confirm thresholds. If the thresholds were not within 6 dB of each other, a third session was conducted and the thresholds were averaged. An underwater speaker presented masking noise throughout the session to mask any auditory artifacts generated by the shaker. The speaker also presented the secondary reinforcer when the manatee was correct on a trial.
Experiment II: Restriction Tests
To determine if the vibrissae contributed to detection of the hydrodynamic stimuli, tests in which vibrissae were restricted with variable size mesh netting were conducted using the same procedure as in Experiment I. The mesh was arranged on a stainless steel ring slightly larger than the manatees’ muzzles and mounted on the stationing apparatus. The subjects were trained to insert their muzzle into the mesh, which restricted a percentage of their facial vibrissae exposed to particle flow (Fig. 3). The threshold testing was conducted under four masking conditions, ~10, ~25, ~65, and ~100 % of vibrissae restricted determined by the sizes of the openings in the mesh (Table 1). The number of vibrissae protruding through the mesh were counted during three separate placements and verified by a second counter for each mesh condition to determine the percentage occluded. Exact statistics for the effect of restriction on thresholds were calculated by calculating a correlation coefficient (r) between fraction of restriction and threshold. This was done separately for each frequency. The probability (p exact) of an r coefficient the same or higher using the same data was calculated by permuting all of the values and calculating r for each permutation. The joint probability of all frequencies having an r coefficient the same or higher was calculated by multiplying all p exact values.
Experiment III: Signal Detection Tests
Threshold measures are influenced by decision criteria. An alternative way to address sensitivity while controlling for these criteria is to use a signal detection analysis (Gescheider 1997). Detection testing was conducted under two conditions, with and without the fine mesh (0.397 mm), at 25 Hz at 0.21 μm displacement, a 3.35× (10.5 dB) attenuation from the starting level during threshold testing. Fifty trials were conducted under each condition (25 signal present; 25 signal absent). Values for d’ and C were calculated. In signal detection theory d’ is an unbiased sensitivity parameter. C is an index of the decision criterion. Unbiased responses are indicated by C values approaching zero. Values of C < 0 indicate a greater probability of reporting a signal present when it is not, a false alarm, and values >0 indicate a greater probability of reporting a signal absent when it is in fact present (Gescheider 1997).
Results
Experiment I: Tactogram
Results for the behavioral tactogram highlight the sensitivity and frequency dependence of the detection of hydrodynamic stimuli (Table 2). Threshold values were calculated in terms of displacement, velocity, and acceleration as it is unknown which stimulus parameters the manatees detect. Both subjects displayed thresholds below 1 μm of particle displacement for frequencies above 10 Hz. At 150 Hz Buffett and Hugh detected particle displacement near and below 1 nm, respectively. Sensitivity was positively correlated with frequency with a decrease in sensitivity for stimuli at 10 Hz and below, highlighted by the failure to detect the stimulus at 5 Hz by one subject (Fig. 4). Both manatees demonstrated similar thresholds, suggesting that the combined tactogram may be a reasonable representation of the abilities of manatees generally. A number of sessions were videotaped underwater to view the side profile of the manatees and showed that they did not appear to flare their muzzle to expose their perioral vibrissae during testing, with the BLHs composing the dominant class of facial vibrissae exposed to the stimuli.
Threshold values for displacement, velocity, and acceleration detection for both manatee test subjects—Buffett (solid diamond, solid line) and Hugh (open circle, dashed line). Threshold values for a harbor seal (X) have been included for comparison (Dehnhardt et al. 1998). Both the x-axis and y-axis are represented with logarithmic scales
Experiment II: Restriction Trials
Data from the restriction trials demonstrated that the thresholds increased as a greater number of vibrissae were restricted (smaller mesh hole size) (Table 3). The correlation coefficients between the fraction of the restricted vibrissae and the displacement threshold are all positive and most are high (Table 3). The probability of all frequencies having correlation coefficients this high by chance was extremely low (Table 3). At the higher frequencies, the thresholds did not show as much of an effect of restriction (Fig. 5). Interestingly, the manatees were unable to detect the stimuli at lower frequencies as a greater percentage of the vibrissae were restricted, as Buffett demonstrated no response to the stimuli at 25 Hz (1.69 μm displacement) and Hugh could not detect the stimuli at 25 or 50 Hz (0.44 μm displacement) when the 35 μm mesh was employed.
Experiment III: Signal Detection
A signal detection analysis was conducted with trials run at the same frequency (25 Hz) and level (0.21 μm displacement) highlighting the restriction of vibrissae as the only difference between tests. The d’ and C values were calculated for both the ‘no mesh’ and ‘fine mesh’ conditions (Table 4). The value of d’ decreased from 1.801 to 0.909 when the fine mesh was added into the procedure, restricting >65 % of the facial vibrissae. This indicated that the mesh was reducing the sensitivity, therefore suggesting the importance of the vibrissae in detecting hydrodynamic stimuli. While C increased slightly for the mesh condition, indicating a more conservative response, the false alarm rate was the same for both conditions. The positive C values for both conditions demonstrate that the manatee’s decisions were conservative, which suggests that the tactile thresholds may be an underestimate.
Discussion
The thresholds determined for the facial vibrissae of manatees demonstrate remarkable sensitivity, highlighted by the detection of particle displacement approaching and below 1 nm at 150 Hz. Dehnhardt et al. (1998), in a study which served as a model for this one, tested the ability of a harbor seal (Phoca vitulina) to detect hydrodynamic stimuli. Comparing the data, the manatees were more sensitive by an order of magnitude (Fig. 4) and more recent research has established that the California sea lion (Zalophus californianus) has an intermediate sensitivity (Dehnhardt and Mauck 2008). Manatees often inhabit bodies of water with very little water motion, and this high sensitivity may allow them to sense their environment through detection of boundary layers, in-water obstacles, and changes in water currents. Signals with a high frequency could be related to the manatee swimming through a boundary, but also could be related to movement of vibrissae during normal swimming, and provide an indication of swimming speed.
Comparing the thresholds as a function of displacement, velocity, or acceleration shows a much larger range for displacement than velocity or acceleration. We do not know which modality or modalities the vibrissae sense. Studies with rat whiskers suggest that they are velocity-sensitive because thresholds varied as a function of stimulus amplitude or frequency, but not as a function of amplitude × frequency (Adibi et al. 2012).
As a greater percentage of the vibrissae were limited, the manatees’ thresholds increased and the subjects were not able to detect the stimuli at the lower frequencies when they were completely restricted. These results strongly suggest that tactile senses, including those mediated by the vibrissae, were responsible for the observed thresholds, and not some other sense such as vision or hearing. MARs for both animals (Bauer et al. 2003) were above the angle of resolution necessary to see the distance moved by the stimulus sphere displacement. Auditory thresholds of manatees are highest at low frequencies (Gerstein et al. 1999; Gaspard et al. 2012). Note that one of the two manatees tested by Gerstein (1999) could detect the acoustic signals from 15 to 400 Hz with thresholds from 93 to 111 dB re 1 μPa. However, Gerstein et al. (1999) suggested that under 400 Hz the manatee was detecting the stimulus tactually, rather than by hearing, based on response characteristics.
In the restriction experiments, there was convergence of sensitivity at the higher frequencies as it appeared that the mesh had less effect at these frequencies. The mechanism of detection may change at these frequencies, and could involve follicle-associated mechanoreceptors and surface Merkel cells. The increase of thresholds during restriction testing and the decrease in d’ with the inclusion of the mesh netting during signal detection tests indicates that the vibrissae were a key component to the detection of low-frequency vibratory stimuli.
It is not known what cues manatees use for orientation as they navigate through their environment and migrate between summer and winter refugia. They spend a significant portion of time in turbid waters, especially during travel, but they have poor visual acuity (Mass et al. 1997, 2012; Bauer et al. 2003) and do not echolocate. Previous work has shown that the perioral bristles play a dominant role during feeding and oripulation (Hartman 1979; Marshall et al. 1998; Bachteler and Dehnhardt 1999; Bauer et al. 2012). The BLHs of the oral disk may serve as a sensory array to detect hydrodynamic stimuli, in addition to their use in direct contact tactile scanning (Bauer et al. 2012). The anatomical differentiation between the stout perioral bristles and the more pliant BLHs supports the likelihood of a role for the latter in passive detection of hydrodynamic stimuli (Sarko et al. 2007). Furthermore, our failure to observe the subjects flare their lips to expose the perioral bristles during exposure to the vibratory stimuli suggests that they do not play a role in passive detection, leaving the BLHs as the most likely vibrissae to be involved in facial sensitivity to hydrodynamic flow.
Bearded seals and ringed seals possess FSCs innervated by more than 1,000 axons per vibrissa (Hyvärinen 1995; Marshall et al. 2006) with rodents demonstrating significantly less innervation at 100–200 per FSC (Rice et al. 1986). The Australian water rat, since it does not live exclusively in an aquatic environment, and displays an intermediate number of axons per follicle (~500), seems to optimize its existence in both mediums (Dehnhardt et al. 1999). The increased innervation of aquatic species highlights the specialization required to exist in a complex environment. The facial region of the manatee is densely populated with approximately 2,000 vibrissae with over 100,000 associated axons innervating these FSCs and approximately 600 are the BLHs located on the oral disk (Reep et al. 1998; 2001). This axonal innervation, up to 250 axons per facial vibrissae, is comparable to the specialized nasal region of the star nosed mole (Catania and Kaas 1997).
The FSCs of manatees possess Merkel endings that are found within the ring sinus and at the rete ridge collar in post-facial and bristle-like hairs (BLHs) which may allow for the extraction of multiple features of a stimulus, potentially including the intensity, direction, velocity, and acceleration of hair deflection (Rice et al. 1997; Ebara et al. 2002; Sarko et al. 2007). Merkel cells in the post-facial FSCs are highly innervated in contrast to the facial vibrissae (Sarko et al. 2007), possibly implicating the facial vibrissae in “active” touch and the post-facial FSCs in a “passive” detection system. Sarko et al. (2007) discovered a “tangle” nerve ending unique to manatees that might act as a low threshold mechanoreceptor, indicating a possible increase in sensitivity of manatees to minute stimuli. Vibrissae on non-mystacial regions have been demonstrated to play a crucial role in some species. Naked mole rats use modified hairs located on their bodies for orientation and some squirrels and jerboas possess tactile hairs on their extremities that could provide feedback about landing sites after jumps (Sokolov and Kulikov 1987; Crish et al. 2003). These peripheral specializations of the manatee somatosensory system are supported by larger regions of the somatosensory brainstem, thalamus, and cortical regions featuring specialized neuronal aggregations (Rindenkerne) which are analogous to the barrel cortex in rodents (Reep et al. 1989; Marshall and Reep 1995).
Behavioral studies with mottled sculpin (Cottus bairdi) using a dipole found acceleration thresholds of about 0.18 mm/s2 for 10–100 Hz (Coombs and Janssen 1989a, b, 1990). This is about 4–20 times more sensitive than the manatee facial vibrissae thresholds over the same frequency range. Several studies have investigated the ability of fish to detect particle displacement; however, these were primarily measured in primary auditory afferents, perceived as acoustic stimuli. Oscars (Astronotus ocellatus) detected article displacement of 1.2–1.6 nm (RMS) at 100 Hz (Lu et al. 1996). Similar sensitivity was demonstrated by goldfish (Carassius auratus) and toadfish (Opsanus tau) with a detection of particle displacement less than 1 nm (RMS) (Fay and Olsho 1979; Fay 1984; Fay et al. 1994). Particle displacement sensitivity for the manatees at 100 Hz was 1.9 and 3.1 nm (Table 2). Although the detection modality sometimes differed in fish, the manatees were able to detect the particle displacement at slightly higher levels.
Blind cavefish sense objects in the water by detecting alterations in self-produced hydrodynamic stimuli as they go near or pass them (von Campenhausen et al. 1981; Weissert and von Campenhausen 1981; Hassan 1989). Future research should investigate whether manatees utilize their own self-generated hydrodynamic stimuli in a similar manner to the blind cave fish, which detect reflected bow waves or interruptions of the pressure waves, gaining information about their typically turbid environment. The vibrissae of manatees are anatomically specialized and behaviorally utilized to detect hydrodynamic stimuli, supporting and strengthening the hypothesis that the vibrissae act as a sensory array analogous to the lateral line system of fish.
Abbreviations
- BLH:
-
Bristle-like hair
- f:
-
Frequency (Hz)
- FA:
-
False alarm
- FSC:
-
Follicle–sinus complex
- MAR:
-
Minimum angle of resolution
References
Adibi M, Diamond ME, Arabzadeh E (2012) Behavioral study of whisker-mediated vibration sensation in rats. Proc Natl Acad Sci USA 109:971–976
Bachteler D, Dehnhardt G (1999) Active touch performance in the Antillean manatee: evidence for a functional differentiation of facial tactile hairs. Zoology 102:61–69
Bauer GB, Colbert DE, Gaspard JC, Littlefield B, Fellner W (2003) Underwater visual acuity of Florida manatees (Trichechus manatus latirostris). Int J Comp Psych 16:130–142
Bauer GB, Gaspard JC III, Colbert DE, Leach JB, Stamper SA, Mann D, Reep R (2012) Tactile discrimination of textures by Florida manatees (Trichechus manatus latirostris). Mar Mammal Sci doi: 10.1111/j.1748-7692.2012.00565.x
Bell CC (1982) Properties of a modifiable efference copy in an electric fish. J Neurophysiol 47:1043–1056
Catania KC, Kaas JH (1997) Somatosensory fovea in the star-nosed mole: behavioral use of the star in relation to innervation patterns and cortical representation. J Comp Neurol 387:215–233
Colbert D, Fellner W, Bauer GB, Manire CA, Rhinehart HL (2001) Husbandry and research training of two Florida manatees. (Trichechus manatus latirostris) Aquat Mamm 27:16–23
Colbert DE, Gaspard JC, Reep R, Mann DA, Bauer GB (2009) Four-choice sound localization abilities of two Florida manatees, Trichechus manatus latirostris. J Exp Biol 212(13):2105–2112
Coombs S, Janssen J (1989a) Peripheral processing by the lateral line system of the spotted sculpin (Cottus bairdi). In: Coombs S, Görner P, Munz H (eds) The mechanosensory lateral line system: neurobiology and evolution. Springer, Berlin Heidelberg New York, pp 299–319
Coombs S, Janssen J (1989b) Waterflow detection by the mechanosensory lateral line. In: Stebbins WC, Berkley M (eds) Comparative perception. John Wiley, New York, pp 89–123
Coombs S, Janssen J (1990) Behavioral and neurophysiological assessment of lateral line sensitivity in the mottled sculpin, Cottus bairdi. J Comp Physiol A 167:557–567
Coombs S, New JG, Nelson M (2002) Information-processing demands in electrosensory and mechanosensory lateral line systems. J Physiol 96:341–354
Cornsweet TN (1962) The staircase method in psychophysics. Am J Psychol 75:485–491
Crish S, Rice FL, Park T, Comer C (2003) Somatosensory organization and behavior in naked mole rats I: body vibrissae form a stereotyped sensory array that mediates orientation to tactile stimuli. Brain Behav Evol 62:141–152
Dehnhardt G (1994) Tactile size discrimination by a California sea lion (Zalophus californianus) using its mystacial vibrissae. J Comp Physiol 175:791–800
Dehnhardt G, Dücker G (1996) Tactile discrimination of size and shape by a California sea lion (Zalophus californianus). Ani Learn Beh 24:366–374
Dehnhardt G, Kaminski A (1995) Sensitivity of the mystacial vibrissae of harbor seals (Phoca vitulina) for size differences of actively touched objects. J Exp Biol 198:2317–2323
Dehnhardt G, Mauck B (2008) Mechanoreception in secondarily aquatic vertebrates. In: Thewissen JGM, Nummela S (eds) Sensory evolution on the threshold—adaptations in secondarily aquatic vertebrates. University of California Press, Berkely, pp 295–314
Dehnhardt G, Mauck B, Bleckmann H (1998) Seal whiskers detect water movements. Nature 394:235–236
Dehnhardt G, Hyvarinen H, Palviainen A, Klauer G (1999) Structure and innervation of the vibrissal follicle-sinus complex in the Australian water rat, Hydromys chrysogaster. J Comp Neurol 411:550–562
Dehnhardt G, Mauck B, Hanke W, Bleckmann H (2001) Hydrodynamic trail-following in harbor seals (Phoca vitulina). Science 293:102–104
Dykes RW (1975) Afferent fibers from mystacial vibrissae of cats and seals. J Neurophysiol 38:650–662
Ebara S, Kumamoto K, Matsuura T, Mazurkiewicz JE, Rice FL (2002) Similarities and differences of mystacial vibrissal follicle-sinus complexes in the rat and cat: a confocal microscopic study. J Comp Neurol 449:103–119
Fay FH (1982) Ecology and biology of the Pacific walrus Odobenus rosmarus divergens. USFWS North American Fauna 74, Washington, DC
Fay RR (1984) The goldfish ear codes the axis of acoustic particle motion in three dimensions. Science 225:951–954
Fay RR, Olsho LW (1979) Discharge patterns oflagenar and saccular neurons of the goldfish eighth nerve: displacement sensitivity and directional characteristics. Comp Biochem Physiol 62:377–386
Fay RR, Edds-Walton PL, Highstein SM (1994) Directional sensitivity of saccular afferents of the toadfish to linear acceleration at audio frequencies. Biol Bull 187:258–259
Gaspard JC III, Bauer GB, Reep RL, Dziuk K, Cardwell A, Read L, Mann DA (2012) Audiogram and auditory critical ratios of two Florida manatees (Trichechus manatus latirostris). J Exp Biol 215:1442–1447
Gerstein ER, Gerstein L, Forsythe SE, Blue JE (1999) The underwater audiogram of the West Indian manatee (Trichechus manatus). J Acoust Soc Am 105:3575–3583
Gescheider GA (1997) Psychophysics: the fundamentals. Lawrence Ehrlbaum Associates, Mahwah, NJ
Gläser N, Wieskotten S, Otter C, Dehnhardt G, Hanke W (2011) Hydrodynamic trail following in a California sea lion (Zalophus californianus). J Comp Physiol A 197:141–151
Hanke W, Witte M, Miersch L, Brede M, Oeffner J, Michael M, Hanke F, Leder A, Dehnhardt G (2010) Harbor seal vibrissa morphology suppresses vortex-induced vibrations. J Exp Biol 213:2665–2672
Hartman DS (1979) Ecology and behavior of the manatee (Trichechus manatus) in Florida. Am Soc Mamm Special Pub 5:1–153
Hassan ES (1989) Hydrodynamic imaging of the surroundings by the lateral line of the blind cave fish Anoptichthys jordani. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line. Neurobiology and evolution. Springer, Berlin, pp 217–227
Hyvärinen H (1995) Structure and function of the vibrissae of the ringed seal (Phoca hispida L.). In: Kastelein RA, Thomas JA, Nachtigall PE (eds) Sensory systems of aquatic mammals. De Spil Publishers, The Netherlands, pp 429–445
Kalmijn AJ (1988) Hydrodynamics and acoustic field detection. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds) Sensory biology of aquatic animals. Springer, New York, pp 83–130
Kastelein RA, Van Gaalen MA (1988) The tactile sensitivity of the mystacial vibrissae of a Pacific walrus (Odobenus rosmarus divergens) Part 1. Aquatic Mamm 14:123–133
Lu Z, Popper AN, Fay RR (1996) Behavioral detection of acoustic particle motion by a teleost fish (Astronotus ocellatus): sensitivity and directionality. J Comp Physiol A 179:227–233
Mann DA, Colbert DE, Gaspard JC, Casper BM, Cook MLH, Reep RL, Bauer GB (2005) Auditory temporal resolution of the manatee (Trichechus manatus latirostris) auditory system. J Comp Physiol A 191:903–908
Marshall CD, Reep RL (1995) Manatee cerebral cortex: cytoarchitecture of the caudal region in Trichechus manatus latirostris. Brain Behav Evol 45:1–18
Marshall CD, Huth GD, Edmonds VM, Halin DL, Reep RL (1998) Prehensile use of perioral bristles during feeding and associated behaviors of the Florida manatee (Trichechus manatus latirostris). Mar Mammal Sci 14:274–289
Marshall CD, Amin H, Kovacs KM, Lydersen C (2006) Microstructure and innervation of the mystacial vibrissal follicle-sinus complex in bearded seals, Erignathus barbatus (Pinnipedia: Phocidae). Anat Rec A 288:13–25
Mass AM, Odell DK, Ketten DR, Supin AY (1997) Ganglion layer topography and retinal resolution of the Caribbean manatee (Trichechus manatus latirostris). Dokl Biol Sci 355:392–394
Mass AM, Ketten DR, Odell DK, Supin AY (2012) Ganglion cell distribution and retinal resolution in the Florida manatee, Trichechus manatus latirostris. Anat Rec 295:177–186
Reep RL, Johnson JI, Switzer RC, Welker WI (1989) Manatee cerebral cortex: cytoarchitecture of the frontal region in Trichechus manatus latirostris. Brain Behav Evol 34:365–386
Reep RL, Marshall CD, Stoll ML, Whitaker DM (1998) Distribution and innervation of facial bristles and hairs in the Florida manatee (Trichechus manatus latirostris). Mar Mammal Sci 14:257–273
Reep RL, Stoll ML, Marshall CD, Homer BL, Samuelson DA (2001) Microanatomy of facial vibrissae in the Florida manatee: the basis for specialized sensory function and oripulation. Brain Behav Evol 58:1–14
Reep RL, Marshall CD, Stoll ML (2002) Tactile hairs on the postcranial body in Florida manatees: a mammalian lateral line? Brain Behav Evol 59:141–154
Rice FL, Mance A, Munger BL (1986) A comparative light microscopic analysis of the sensory innervation of the mystacial pad. I. Innervation of the vibrissal follicle-sinus complexes. J Comp Neurol 252:154–174
Rice FL, Fundin BT, Arviddson J, Aldskogius H, Johansson O (1997) Comprehensive immunoflorescence and lectin binding analysis of vibrissal follicle sinus complex innervation in the mystacial pad of the rat. J Comp Neurol 385:149–184
Sarko DK, Reep RL, Mazurkiewicz JE, Rice FL (2007) Adaptations in the structure and innervation of follicle-sinus complexes to an aquatic environment as seen in the Florida manatee (Trichechus manatus latirostris). J Comp Neurol 504:217–237
Schulte-Pelkum N, Wieskotten S, Hanke W, Dehnhardt G, Mauck B (2007) Tracking of biogenic hydrodynamic trails in harbour seals (Phoca vitulina). J Exp Biol 210:781–787
Sokolov VE, Kulikov VF (1987) The structure and function of the vibrissal apparatus in some rodents. Mammalia 51:125–138
von Campenhausen C, Riess I, Weissert R (1981) Detection of stationary objects in the blind cave fish Anoptichthys jordani (Characidae). J Comp Physiol A 143:369–374
Weissert R, von Campenhausen C (1981) Discrimination between stationary objects by the blind cave fish Anoptichthys jordani. J Comp Physiol A 143:375–382
Zelena J (1994) Nerves and mechanoreceptors. Chapman and Hall, London
Acknowledgments
The authors would like to thank the United States Fish and Wildlife Service (Permit MA837923-6/7); the Florida Fish and Wildlife Conservation Commission; University of Florida, College of Veterinary Medicine—Aquatic Animal Health Program; Mote Marine Laboratory staff, interns, volunteers, especially trainer Jann Warfield, that assisted with this research; Guido Dehnhardt and Wolf Hanke for their expertise and equipment loan during training; Ronnie and John Enander; the Thurell family; New College of Florida students; Peg Scripps Buzzelli Chair, New College Foundation. This work was supported by the National Science Foundation (IOS-0920022/0919975/0920117). All experimental procedures were approved by the Mote Marine Laboratory IACUC prior to implementation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaspard, J.C., Bauer, G.B., Reep, R.L. et al. Detection of hydrodynamic stimuli by the Florida manatee (Trichechus manatus latirostris). J Comp Physiol A 199, 441–450 (2013). https://doi.org/10.1007/s00359-013-0822-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-013-0822-x