Abstract
Dramatic changes in neocortical electroencephalogram (EEG) rhythms are associated with the sleep–waking cycle in mammals. Although amphibians are thought to lack a neocortical homologue, changes in rest–activity states occur in these species. In the present study, EEG signals were recorded from the surface of the cerebral hemispheres and midbrain on both sides of the brain in an anuran species, Babina daunchina, using electrodes contacting the meninges in order to measure changes in mean EEG power across behavioral states. Functionally relevant frequency bands were identified using factor analysis. The results indicate that: (1) EEG power was concentrated in four frequency bands during the awake or active state and in three frequency bands during rest; (2) EEG bands in frogs differed substantially from humans, especially in the fast frequency band; (3) bursts similar to mammalian sleep spindles, which occur in non-rapid eye movement mammalian sleep, were observed when frogs were at rest suggesting sleep spindle-like EEG activity appeared prior to the evolution of mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The electroencephalogram (EEG) is characterized by a frequency range from 0.05 to 500 Hz and shows predictable changes during the sleep–waking cycle in mammals (Buzsaki and Draguhn 2004). Moreover, adjacent EEG frequency bands presumably generated by distinct neuronal networks are typically correlated with specific brain states and predominate during different behavioral states (Klimesch 1999; Kopell et al. 2000; Engel et al. 2001; Csicsvari et al. 2003) such as sensory registration, perception, movement and cognitive processes related to attention, learning and memory (Klimesch 1999; Basar et al. 2000, 2001; Thut and Miniussi 2009).
In humans the EEG has been manually divided into broad frequency bands by visual inspection (Nunez and Cutillo 1995) while for studies in non-human mammals the EEG band divisions have been defined arbitrarily or determined empirically based on presumed homologies with the frequency bands observed in humans (Lancel and Kerkhof 1989; Bakalian and Fernstrom 1990; Grasing and Szeto 1992). In frogs, functionally significant EEG frequency ranges have been recognized (Smolin 1962; Servít et al. 1965; Hobson 1967a, b; Kostowski 1967; Hobson et al. 1968; Sato 1969; Ono et al. 1980; Blisard et al. 1994) although only a few studies classified the frequency boundaries and these were based by default on those adopted in human studies (Balasandaram et al. 1997; Aristakesyan and Karmanova 2007). Neurobehavioral studies have shown considerable differences in rest–activity patterns among anuran species. The nocturnally active bullfrog (Rana catesbiana) exhibits neither the electroencephalographic nor the threshold criteria for mammalian sleep in the laboratory (Hobson 1967a) and remains vigilant when at diurnal rest in the field. In contrast, tree frogs (Hyla squirella, H. cinera and H. septentrionalis) remain immobile with eyes closed and head pointing toward the tips of leaves when resting by day and exhibit elevated thresholds to sensory stimulation during resting compared to those in the active state. Thus, it has been proposed that tree frogs sleep on the basis of behavioral criteria (Hobson et al. 1968).
EEG phenomena including sleep spindles and slow waves, such as delta waves, typify non-rapid eye movement (NREM) sleep in mammals (Bjorvatn et al. 1998). Nevertheless, the properties of the EEG during different vigilance states in frogs still remain unclear. The problem is complicated by the fact that although EEG recordings during active and rest states are available in some anuran as well as other species these are difficult to compare with those of mammals including humans due to lack of reliable methods for identifying the boundaries of EEG frequency bands.
The present study addresses both issues. First we sought to determine if a rigorous multivariate approach based on factor analysis could be used to classify frog EEG frequency bands. Factor analysis has been used successfully to identify differences in functionally relevant EEG bands in humans and rats which could not be identified by manual visual inspection of EEG patterns (Klimesch 1999; Corsi-Cabrera et al. 2000, 2001). The present study is the first to apply this method to anurans.
Second, we sought to identify EEG spectral components specifically associated with behaviorally defined vigilance states in a frog species, the music frog (Babina daunchina), whose rest–activity cycle can be monitored under controlled laboratory conditions and for which extensive studies of natural behavior have previously been conducted (Cui et al. 2010). To do this we recorded behavioral data and multi-channel electrocorticogram (ECoG) signals from Babina and identified functionally relevant EEG frequency bands as a function of behavioral state using factor analysis.
Materials and methods
Animals
Five male and five female adult music frogs captured from the Emei mountain area of Sichuan, China, in July 2010 were used in these experiments. Subjects were housed by sex in two opaque plastic tanks (45 × 35 cm and 30 cm deep) containing mud and water. The tanks were placed in a room under controlled temperature conditions (23 ± 1°C) and maintained on a 12:12 light–dark cycle (lights on at 08:00 h). The animals were fed fresh live crickets every 3 days. Mean masses were 10.2 ± 2.4 g (mean ± SD), and frogs were 4.6 ± 0.3 cm in length at the time of surgery.
Surgery
All experiments were conducted after September 2010 when the reproductive season ended for music frogs (Cui et al. 2010). The animals were deeply anesthetized by intraperitoneal injection of pentobarbital sodium (3 mg/100 g) and the degree of anesthesia was evaluated through the toe pinch response.
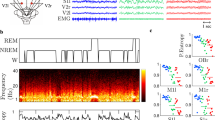
Four cortical EEG electrodes, composed of miniature stainless steel screws (φ 0.8 mm), were implanted on the frog skull: the left and right sides of the telencephalon and mesencephalon (R1, R2, R3 and R4), and referenced to the electrode above the cerebellum (P, the corresponding electrode pairs were abbreviated as PR1, PR2, PR3 and PR4, respectively) (Fig. 1). Ten seconds of typical EEG activity after re-referencing to the average reference during the waking (i.e. active) and rest periods are shown along with each placement of the electrodes (Fig. 1). R1 and R2 were implanted bilaterally 2.2 mm anterior to the lambda (i.e. the point where skull sutures intersect) and 1.5 mm lateral to the midline, respectively, while R3 and R4 were implanted bilaterally 2.3 mm posterior to the lambda and 1.5 mm lateral to the midline. P was implanted 3.9 mm posterior to the lambda at the midline (Fig. 1). One end of a formvar-insulated (except at the two ends) nichrome wire (φ 0.1 mm) served as a ground electrode and was fixed subcutaneously about 5 mm posterior to P.
Electrode placements and 10 s of typical EEG tracings during the awake (active) and resting vigilance states for each channel after re-referencing to the average reference. The intersection of the three dashed lines in bold in the frog head denotes the intersection of suture lines corresponding to lambda. The bold line in the lower right of the picture denotes the period in which spindles are present
All electrode leads were formvar-insulated nichrome wires with one end intertwined tightly around the screws except for the ground electrode which was fixed subcutaneously and directly, and with the other end tin soldered to the female-pins of an electrical connector (GongZhan, PHD2004S; DongGuang, China). Electrodes were fixed to the skull with dental acrylic. The connector was covered with self-sealing membrane (Parafilm® M; Chicago, USA) for water-proofing and located about 1 cm above the head of the animal. For best suspension, six formvar-insulated nichrome wires were wound together around a pencil to form a loose spring (Laming 1982). One end of each wire was tin soldered to the male-pin of the electrical connector; the other ends were connected to the cable of the signal acquisition system (Chengyi, RM6280C; Sichuan, China). The junction points were kept in a box covered with silver paper and earthed.
Each frog was housed singly for 2 days for recovery before the following experiments were performed. After the end of the experiments, all frogs were euthanized by overdose of intraperitoneal pentobarbital sodium and electrode localizations were confirmed by injecting hematoxylin dye through the skull holes in which the screws were installed previously.
Data acquisition
The experiments were performed in a soundproof and electromagnetic shielding chamber in which the background noise was 23.0 ± 1.7 dB (mean ± SD) with an opaque plastic tank (80 × 60 cm and 55 cm deep) containing mud and water. Lights and temperature in the chamber were maintained as in the home-cages. A video camera with infrared light source and motion detector was appended centrally above the tank about 1 m for monitoring the behavior of the subject from outside of the chamber. In order to eliminate the effects of digestion on the results, the subject was not fed during the experimental period.
The procedure for data collection consisted of electrophysiological and behavioral recordings on two sequential days. On the first day the subject was placed in the experiment tank and connected to the signal acquisition system for habituation; neurophysiological and behavioral data were collected on the subject on the second day. The signal acquisition system was set to record continuously from evening at 20:00 to the following morning at 8:00. The experimental design was based on the facts that (1) music frogs remain active all day except for noon and midnight, with the highest level of activity occurring at dawn and dusk; (2) the protosleep of fish and amphibians includes three immobility forms: type P-1, type P-2 and type P-3, and the P-3 form occurs predominantly during darkness in amphibians and is the closest equivalent of the slow wave sleep of birds and mammals (Karmanova 1996). Band-pass filters, set to 0.16–100 Hz, were used for EEG signals with the notch filter of the amplifiers set to eliminate possible interference at 50 Hz. A sampling frequency of 1,000 Hz was used.
Data processing
EEG data were first re-referenced to an average reference. Since fish and amphibians are not considered sleep taxa (Tauber 1974), for the present study the vigilance states of the frog were divided into two categories: awake (or active) and rest. Representative and artifact-free EEG segments (120 s) were selected from the electrophysiological data acquired on the second day for each state, electrode pair and subject. In order to ensure the accuracy of factor analysis, half of the representative EEG segments for the awake state were selected randomly from the 20:00–21:30 period and the other half were selected randomly from the 6:30–8:00 period. The representative EEG segments for the resting state were selected from the longest periods (85 ± 35 min for the nine frogs during 0:00–3:00, mean ± SD) when frogs did not show any spontaneous movement. This 120-s time window was used to acquire data for all four channels. In order to obtain clean data, for the awake state the representative EEG segments were selected from the sixth second after the end of any movement (i.e. 5 s after the end of any movement) and for resting state EEG segments were selected in the middle of the longest period of immobility. Since artifact-free data could not be obtained for one frog from electrode placements PR3 and PR4, the statistical tests and factor analysis were based on the data obtained from the other nine frogs.
After band-pass filtering (1–45 Hz) and downsampling at 512 Hz, a 120-s segment was divided into 60 two-second epochs for each channel, each of which was detrended (i.e. the linear trend was removed) using an algorithm which computes the least-squares fit of the data. Power spectra with 1.0-Hz resolution were computed by Welch’s method with the Hamming window. To compare EEG power differences among the four channels and between the active and resting states, the power spectra of all 60 two-second epochs were first log-transformed and then averaged for each channel, each state and each frog. These average values (i.e. one value for each channel, state and frog) were further analyzed statistically.
Since there are significant differences in EEG power spectra among various brain regions, especially between anterior and posterior areas (Saastamoinen et al. 2007; Young and McNaughton 2009; Fang et al. 2010), factor analysis was performed for the telencephalon and the mesencephalon, respectively, in this study. For each vigilance state and each frog, the log-transformed power spectra of 60 epochs were averaged between both sides of each brain region, and then averaged in blocks of 5.
Using these methods, 12 average values were computed for each state, each brain region and each subject. To obtain frequencies covariant with each other and extract broad frequency bands correlated with each state, these 12 values were pooled for all 9 frogs and used for factor analysis with EEG frequencies as variables and principal component analysis (PCA) as the extraction method for the factors. The following criteria were used: the Kaiser–Guttman criterion (eigenvalues higher than 1 for eigenvectors); the scree slope method; factor loading set at 0.55, i.e. only frequency bands for which the index is above 0.55 are included. Both varimax rotation and promax rotation were performed. Because there were few differences between the results obtained from these two methods, only results based on varimax rotation were reported in the present paper.
Factor analysis
Factor analysis refers to a set of statistical methods used to detect underlying patterns in the relationships among numbers of observed variables (Ismail 2008) and is an extremely powerful tool for reducing multivariate datasets to fewer underlying dimensions (Hair et al. 1998). Generally 5–10 subjects per variable are used in most studies (Kline 1994), although in practice, the adequacy of sample size can be determined by the Kaiser–Meyer–Olkin (KMO) test. A minimum KMO score of 0.50 is considered necessary to reliably use factor analysis for data analysis, while scores over 0.80 are considered to be robust (Hair et al. 1998). Bartlett’s test of sphericity should reach statistical significance in order to acquire reliable results.
Although it is often impracticable in animal studies to meet conditions concerning the ratio of subject numbers to variable (Kline 1994), an alternative approach is to substitute samples of EEG for individual subjects. Corsi-Cabrera et al. (2000, 2001) studied frequency bands of EEG using channels instead of subjects as inputs in factor analysis thereby increasing the ratio of data inputs to variable several fold, whereas whether the inputs passed the KMO test and the Bartlett’s test of sphericity was not reported (Corsi-Cabrera et al. 2000, 2001). Because the skull of music frog is too small to implant many electrodes, the above substitution was not suited for the current study. Since factor analysis could also be used for defining frequency bands on EEG data based on “n epochs of a single subject” (Klimesch 1999), such pooled data derived from the nine frogs in which 12 average values were obtained for each subject were used as inputs in factor analysis for each state and each location.
Statistical analyses
To evaluate differences in power spectra, 3-way within-subject ANOVA (i.e. 3-way repeated measures ANOVA) with the factors of ‘state’, ‘frequency’ and ‘electrode placement’ was employed. Both main effects and interactions were examined. Simple effects analysis would be applied when the interaction was significant. For significant ANOVAs, data were further analyzed for multiple comparisons using the least-significant difference (LSD) test. Estimations of effect size for ANOVAs were determined with partial \( \eta^{2} \) (partial \( \eta^{2} \) = 0.20 is a small effect size, 0.50 is a medium effect size and 0.80 is a large effect size) (Cohen 1992). SPSS software (release 13.0) was utilized for the statistical analysis and a significance level of p < 0.05 was used in all comparisons.
Results
EEG power variations among different frequencies, different electrode placements and different vigilance states
For EEG power, the results of ANOVA revealed that both the main effects were significant for the factor ‘state’ [F(1,8) = 15.537; p < 0.05, partial \( \eta^{2} \) = 0.660] and the factor ‘frequency’ [F(44,352) = 328.014; p < 0.001, partial \( \eta^{2} \) = 0.976], respectively; the interaction between ‘state’ and ‘frequency’ was significant [F(44,352) = 1.957; p < 0.001, partial \( \eta^{2} \) = 0.197]; and the interaction between ‘frequency’ and ‘electrode placement’ was also significant [F(132,1056) = 1.664; p < 0.001, partial \( \eta^{2} \) = 0.172]. Because the two interactions were significant, simple effect analysis was further applied.
For the factor ‘frequency’, simple effect analysis revealed that EEG power of a given frequency was significantly lower than those of smaller frequencies as a whole for each state and each channel, while it was significantly higher than those of larger frequencies (p < 0.05).
For the factors ‘electrode placement’ and awake (active) state, simple effect analysis showed that the EEG power of PR1 was significantly higher than those of PR2, PR3 and PR4 for 1, 3, 12–13, 17–23 and 25–26 Hz (p < 0.05; Fig. 2a); EEG power of PR1 was significantly higher than that of PR2 and PR3 for 2 and 44 Hz (p < 0.05; Fig. 2a); EEG power of PR1 was significantly higher than that of PR3 for 7, 28 and 45 Hz (p < 0.05; Fig. 2a); EEG power of PR4 was significantly higher than that of PR2 for 1–2 Hz (p < 0.05; Fig. 2a), while EEG power of PR4 was significantly higher than PR3 for 21–23, 26, 28 and 44–45 Hz (p < 0.001; Fig. 2a).
Means and standard deviations of EEG power spectra for the four electrode locations during the awake (a) and rest (b) states, respectively. For a given frequency, asterisk denotes that EEG power for PR1 was significantly higher than those for PR2, PR3 and PR4 (p < 0.05), filled star denotes that EEG power for PR1 was significant higher than those for PR2 and PR3 (p < 0.05), open star denotes that EEG power for PR1 was significant higher than those for PR3 and PR4 (p < 0.05), open triangle denotes that EEG power for PR1 was significant higher than that for PR3 (p < 0.05), filled triangle denotes that EEG power for PR1 was significant higher than that for PR4 (p < 0.05), while the filled square denotes that EEG power for PR4 was significant higher than that for PR2 (p < 0.05). Only the data from 1 to 15 Hz were shown for a better demonstration. PR1, PR2, PR3 and PR4 the four electrode pairs
For the factor ‘electrode placement’ and rest state, simple effect analysis showed EEG power of PR1 was significantly higher than those of PR2, PR3 and PR4 for 3 Hz (p < 0.05; Fig. 2b); EEG power of PR1 was significantly higher than that of PR3 and PR4 for 7 and 12 Hz (p < 0.05; Fig. 2b); EEG power of PR1 was significantly higher than that of PR3 for 8–9 Hz (p < 0.05; Fig. 2b), while EEG power of PR1 was significantly higher than PR4 for 13 Hz (p < 0.001; Fig. 2b).
For the factor ‘state’, EEG power during the awake state was significantly higher than during rest for 2, 4–6, 8, 16–34 and 36–45 Hz in PR1 (p < 0.05; Fig. 3a), for 2–6, 8, 17–25, 27–29, 31–33 and 36–42 Hz in PR2 (p < 0.05; Fig. 3b), for 2–8, 17–34 and 36–45 Hz in PR3 (p < 0.05; Fig. 3c), and for 2–6, 8, 15–34 and 36–45 Hz in PR4 (p < 0.05; Fig. 3d).
Means and standard deviations of EEG power spectra during the awake and rest states for PR1 (a), PR2 (b), PR3 (c) and PR4 (d), respectively. Asterisk denotes that EEG power during the awake state was significantly higher than during the rest state for a given frequency (p < 0.05). Only the data from 1 to 15 Hz were shown for a better demonstration
EEG frequency bands for different states
Both varimax rotation and promax rotation were performed for factor analysis in the current study. Because there were few differences between them, the results based on varimax rotation were reported only. Factor analysis showed that there were four independent factors or eigenvectors for the awake state accounting for 90.557 and 82.245% of the total variance for the telencephalon and the mesencephalon, respectively, and three eigenvectors for the resting state explaining 89.407 and 82.523% of the total variance for the two brain regions, respectively (Tables 1, 2). Consequently, the EEG bands identified by means of factor analysis were different for each physiological state.
For the telencephalon and the awake state, as shown in Table 1, the KMO test of sampling adequacy for all variables was 0.955. Similarly, the Bartlett’s test of sphericity was 10,226.010 with significant levels of p < 0.0001. Both the Kaiser–Guttman criterion and the scree slope method (Fig. 4a) yielded four independent broad bands for the awake state: a delta band from 1 to 4 Hz, a theta band from 6 to 8 Hz that covaried with frequency 14 Hz, an alpha band from 9 to 17 Hz that covaried with 5 Hz and frequencies from 20 to 21 Hz, and a beta band from 16 to 45 Hz (Table 1). The gamma band could not be identified as an independent eigenvector for the awake state.
For the mesencephalon and the awake state, the KMO test was 0.946 (Table 2). The Bartlett’s test was 8,095.148 with significant levels of p < 0.0001. Both the Kaiser–Guttman criterion and the scree slope method (Fig. 4c) yielded four independent broad bands for the awake state: a delta band from 1 to 4 Hz, a theta band from 6 to 8 Hz that covaried with frequencies 13–14 Hz, an alpha band from 9 to 17 Hz that covaried with 5 Hz and frequencies from 19 to 22 Hz, and a beta band from 17 to 45 Hz. Similarly, the gamma band could not be identified for the awake state either.
For the telencephalon and rest, the KMO test was 0.957, while the Bartlett’s test was 10,831.502 with significance level of p < 0.0001. The Kaiser–Guttman criterion identified four independent broad bands in which one band included only two components. The combination of the Kaiser–Guttman criterion and the scree slope method (Fig. 4b), however, showed that three independent factors could be identified by factor analysis for rest: a slow band from 1 to 5 Hz that covaried with frequencies from 9 to 10 Hz and 15 Hz, an intermediate band from 11 to 14 Hz that covaried with frequencies from 6 to 7, 16 to 17, and 19 Hz, and a fast frequency band from 17 to 45 Hz that covaried with 6–8 Hz (Table 1). The gamma band was not identified as an independent eigenvector for rest.
For the mesencephalon and rest, the KMO test was 0.952, while the Bartlett’s test was 8,206.559 with significance level of p < 0.0001. The Kaiser–Guttman criterion identified four independent broad bands in which one band included four discrete components. The combination of the Kaiser–Guttman criterion and the scree slope method (Fig. 4d), however, showed that three independent factors could be identified by factor analysis for rest: a slow band from 1 to 6 Hz that covaried with frequencies from 8 to 11 Hz and 15 to 16 Hz, an intermediate band from 12 to 14 Hz, and a fast frequency band from 17 to 45 Hz that covaried with 7 Hz (Table 2). Similarly, the gamma band was not identified for rest either.
Discussion
Four-channel EEG recordings on both sides of the telencephalon and the mesencephalon were obtained during the awake (active) and rest states and analyzed by means of factor analysis for the telencephalon and the mesencephalon, respectively, in the present study. Although there are some differences between the results obtained from the telencephalon and the mesencephalon, four EEG bands in the awake state and three including sleep spindle components (12–14 Hz) in the rest state were recognized. These EEG phenomena distinguish the two vigilance states but differ from EEG patterns in humans and other mammals.
Validity of EEG factor analysis for anurans
Some significant differences in EEG power spectra were found among various brain regions, especially between antero-posterior ones (Fig. 2). This is consistent with the idea that EEG properties differ along the antero-posterior and left–right axes of the brain in anurans as well as humans and other mammals (Saastamoinen et al. 2007; Young and McNaughton 2009; Fang et al. 2010). Because of these differences, the averaged EEG power spectra between both sides of the telencephalon and between both sides of the mesencephalon might be more suited for factor analysis. In the present study, 12 values averaged in blocks of 5 for the telencephalon or the mesencephalon were submitted to factor analysis as inputs and passed the KMO test and Bartlett’s test of sphericity. In addition, the outputs of the factor analysis conducted here for Babina resembled results of other studies on frogs (Smolin 1962; Hobson 1967a, b; Kostowski 1967; Sato 1969; Ono et al. 1980). Taken together these results support the idea that factor analysis is a useful tool for studying EEG phenomena in anuran species.
EEG frequency bands for different vigilance states
For the awake state, four EEG bands were extracted from both the telencephalon and the mesencephalon for Babina by factor analysis, and there were few differences between the two brain regions. These bands are similar to the energy distribution in the EEG in R. tigrina (Balasandaram et al. 1997) although different from the energy distribution in human EEG bands in the awake state, especially in fast frequency band (Merica and Fortune 2004; Thut and Miniussi 2009). For rest, three EEG bands were extracted from both the telencephalon and the mesencephalon by factor analysis that correspond most closely to immobile patterns previously designated catatonia type (P-2) and catalepsy type (P-3) in frogs (Aristakesyan and Karmanova 2007).
For the Babina awake state, EEG frequencies from 1 to 5 Hz were grouped into a single broad band termed delta which corresponds to the predominant frequency range observed in the forebrain of Rana nigromaculata (Sato 1969). Furthermore, this band is also consistent with the range of slow wave activity obtained in humans (Corsi-Cabrera et al. 2000) and rats (Corsi-Cabrera et al. 2001) by factor analysis. Frequencies from 6 to 8 Hz were defined as the theta band which, as in mammals, has been associated with the spontaneous rhythmic activities of the hippocampus in frogs (Servít et al. 1965) with peaks of power spectra between 5 and 9 Hz (Ono et al. 1980). The 5–8 Hz frequency band is predominant in tree frogs, H. septentrionalis, during waking (Hobson et al. 1968) while the normal pattern of activity of the telencephalon in R. esculenta, consists mainly of energy in the 4–8 Hz band (Kostowski 1967).
The alpha band in active Babina is the 9–17 Hz band and appears similar to the main EEG component of the active state in other frog species (Smolin 1962; Hobson 1967a). Factor analysis identified fast frequencies (17–45 Hz) as a beta band, consistent with the rhythmic waves in the 15–40 Hz EEG frequency range typically manifest in response to olfactory stimulation in frogs and toads (Hobson 1967b). No gamma band was identified in Babina as an independent band. This may reflect the fact that no counterpart to the mammalian neocortex has been identified in amphibia thus resulting in simpler EEG patterns (Ono et al. 1980). In addition, because the frequency of theta band depends on body temperature with cold water reducing the frequency in rats (Whishaw and Vanderwolf 1971; Pan and McNaughton 1997), the differences in frequency patterns in anurans could, then, depend in part on the fact that their brains will be at a much lower temperature than homeotherms.
For the telencephalon, the theta band covaried with frequency 14 Hz, while the alpha band covaried with frequencies from 20 to 21 Hz. For the mesencephalon, the theta band covaried with frequencies 13–14 Hz, while the alpha band covaried with frequencies from 19 to 22 Hz. Because phase synchronizations may occur for harmonic frequency relationships (Pletzer et al. 2010), these covariations of the theta and alpha bands with other frequency bands might be the effect of harmonic frequency relationships rather than EEG functional binding.
For the rest state, three EEG bands were extracted from the mesencephalon by factor analysis: a slow band (1–11 Hz), an intermediate band (12–14 Hz) and beta band (17–45 Hz). For the telencephalon, there were also three corresponding EEG bands; however, theta band (6–8 Hz) was covaried with beta band (17–45 Hz). It has long been argued that fish and amphibians do not sleep and that the first appearance of true sleep occurred in reptiles (Tauber 1974). In contrast, the protosleep of fish and amphibians includes three forms that have been termed by Karmanova (1996) as: immobility of the catalepsy type (P-1) that occurs only at the day time; immobility of the catatonia type (P-2) observed at the twilight and night time; and immobility of the catalepsy type (P-3) recorded predominantly during darkness (Karmanova 1996). In the P-2 state, the power of delta and theta waves increases gradually in the frog, R. temporaria (Aristakesyan and Karmanova 2007). In Babina, factor analysis groups delta and theta as the same eigenvector for the mesencephalon suggesting that this coordinated EEG pattern is specifically associated with rest. Theta band was covaried with delta and alpha bands in the mesencephalon, while this band was covaried with beta band in the telencephalon (Tables 1, 2). This difference is most likely a reflexion of the anatomical and functional differences between the telencephalon and the mesencephalon. There exist two alpha rhythm patterns (Merica and Fortune 2004) and one is associated with the sleep state and is grouped by the same eigenvector with delta and theta for the rest state in Babina.
Sleep spindles during the Babina rest state
EEG power spectra during the awake state were significantly higher than those during rest for both slow wave and fast wave activities (Fig. 3). These results are consistent with previous studies showing that high voltage slow waves during the awake state are more prominent than during rest (Hobson 1967a; Hobson et al. 1968) and that high-frequency EEG components are inhibited substantially during rest periods at and during the night (P-2) (Karmanova et al. 1987). Of particular interest, the intermediate band consisting of 12–14 Hz waves identified in the resting state resembles the sleep spindles of humans (Rechtschaffen and Kales 1968).
Slow wave activity and spindle activity have been considered orthogonally independent activities on the basis of the mutual exclusivity of the occurrence of sleep spindles and delta oscillations recorded intracellularly (Merica and Fortune 2004). Moreover, previous studies have shown that each expiration is followed by spindle-like waves in both frogs and toads (Segura and De Juan 1966; Hobson 1967a, b; Hobson et al. 1968; Hoffmann and Menescal De Oliveira 1990; Hoffmann et al. 1994), a phenomenon believed to reflect complex olfactory-evoked potentials engendered by stimulation of the olfactory mucosa by transpired air (Hobson 1967a, b). Since the frequencies of these spindle-like and respiration-related waves are below 12 Hz and since a prominent 12–14 Hz EEG component has not been identified in the awake state when much higher respiratory rates occur, it seems reasonable to hypothesize that 12–14 Hz oscillations constitute a primary rest/sleep pattern in frogs. In mammals, sleep spindles are one feature of NREM sleep. Thus the occurrence of this EEG signal in frogs with similar spectral properties in the resting state may reflect a primitive pattern of brain activity in the non-active state which appeared in ancestral amphibians.
In summary, factor analysis using averaged EEG power spectra derived from the telencephalon and the mesencephalon for two vigilance states in Babina indicates that: (1) EEG frequency bands in the frog are different from those in humans and rats including results based on factor analysis, especially in the fast frequency band; (2) there are four EEG bands in Babina during the alert, active state and three prominent EEG bands including one spectrally similar to mammalian sleep spindles during rest. These results suggest that at least some EEG properties of NREM sleep may have been present in ancestral amphibians.
References
Aristakesyan E, Karmanova I (2007) Effect of photostimulation on the wakefulness–sleep cycle in the common frog Rana temporaria. J Evol Biochem Physl Engl Transl 43(2):208–214
Bakalian MJ, Fernstrom JD (1990) Effects of l-tryptophan and other amino acids on electroencephalographic sleep in the rat. Brain Res 528(2):300–307
Balasandaram K, Ramalingam K, Selvarajan V (1997) Bioelectrical activity of brain in Rana tigrina (Daudin) in response to phosalone poisoning. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol 118(2):229–231
Basar E, Basar-Eroglu C, Karakas S, Schurmann M (2000) Brain oscillations in perception and memory. Int J Psychophysiol 35(2–3):95–124
Basar E, Basar-Eroglu C, Karakas S, Schurmann M (2001) Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 39(2–3):241–248
Bjorvatn B, Fagerland S, Ursin R (1998) EEG power densities (0.5–20 Hz) in different sleep–wake stages in rats. Physiol Behav 63(3):413–417
Blisard K, Fagin K, Falivena P, Privitera M, Olejniczak P, Harrington D, Taylor K, Scremin O (1994) Experimental seizures in the frog (Rana pipiens). Epilepsy Res 17(1):13–22
Buzsaki G, Draguhn A (2004) Neuronal oscillations in cortical networks. Science 304(5679):1926–1929
Cohen J (1992) A power primer. Psychol Bull 112(1):155–159
Corsi-Cabrera M, Guevara M, Del-Río-Portilla Y, Arce C, Villanueva-Hernández Y (2000) EEG bands during wakefulness, slow-wave and paradoxical sleep as a result of principal component analysis in man. Sleep 23(6):1–7
Corsi-Cabrera M, Pérez-Garci E, Del-Río-Portilla Y, Ugalde E, Guevara M (2001) EEG bands during wakefulness, slow-wave, and paradoxical sleep as a result of principal component analysis in the rat. Sleep 24(4):374–380
Csicsvari J, Jamieson B, Wise KD, Buzsaki G (2003) Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron 37(2):311–322
Cui J, Wang Y, Brauth S, Tang Y (2010) A novel female call incites male-female interaction and male–male competition in the Emei music frog, Babina daunchina. Anim Behav 80:181–187
Engel AK, Fries P, Singer W (2001) Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2(10):704–716
Fang G, Xia Y, Lai Y, You Z, Yao D (2010) Long-range correlations of different EEG derivations in rats: sleep stage-dependent generators may play a key role. Physiol Meas 31:795–808
Grasing K, Szeto H (1992) Diurnal variation in continuous measures of the rat EEG power spectra. Physiol Behav 51(2):249–254
Hair J, Anderson R, Tatham R, Black W (1998) Multivariate data analysis. Prentice–Hall, Englewood
Hobson J (1967a) Electrographic correlates of behavior in the frog with special reference to sleep. Electroencephalogr Clin Neurophysiol 22(2):113–121
Hobson J (1967b) Respiration and EEG synchronization in the frog. Nature 213:988–989
Hobson J, Goin O, Goin C (1968) Electrographic correlates of behaviour in tree frogs. Nature 220:386–387
Hoffmann A, Menescal De Oliveira L (1990) Changes in electric activity (EEG) of the telencephalon of conscious toads (Bufo paracnemis) caused by cholinergic stimulation of the mesencephalic tegmentum. Physiol Behav 47(5):857–861
Hoffmann A, Romero S, Menescal-de-Oliveira L (1994) The basal midbrain as a region modulating the level of alerting in the toad, Bufo paracnemis. Physiol Behav 55(2):301–306
Ismail K (2008) Unravelling factor analysis. Br Med J 11(4):99–102
Karmanova I (1996) Novel about peculiarities of sleep and organization of the wakefulness–sleep cycle in poikilothermal vertebrates. Zh Evol Biokhim Fiziol 32:511–535
Karmanova I, Aristakesyan E, Shilling N (1987) Neurophysiological analysis of hypothalamic mechanisms for the regulation of primary sleep and hypobiosis. Dokl Akad Nauk SSSR 294:245–248
Klimesch W (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev 29(2–3):169–195
Kline P (1994) An easy guide to factor analysis. Routledge, London
Kopell N, Ermentrout G, Whittington M, Traub R (2000) Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA 97(4):1867–1872
Kostowski W (1967) Pharmacological analysis of bioelectrical activity of the central nervous system of amphibia. I. The effect of some cholinergic and anticholinergic drugs on the frog’s EEG. Brain Res 6(4):783–785
Laming P (1982) Electroencephalographic correlates of behavior in the anurans Bufo regularis and Rana temporaria. Behav Neural Biol 34(3):296–306
Lancel M, Kerkhof GA (1989) Effects of repeated sleep deprivation in the dark-or light-period on sleep in rats. Physiol Behav 45(2):289–297
Merica H, Fortune R (2004) State transitions between wake and sleep, and within the ultradian cycle, with focus on the link to neuronal activity. Sleep Med Rev 8(6):473–485
Nunez PL, Cutillo BA (1995) Neocortical dynamics and human EEG rhythms. Oxford University Press, New York
Ono K, Baba H, Mori K, Sato K (1980) EEG activities during kindling in frog. Int J Neurosci 11(1):9–15
Pan WX, McNaughton N (1997) The medial supramammillary nucleus, spatial learning and the frequency of hippocampal theta activity. Brain Res 764(1–2):101–108
Pletzer B, Kerschbaum H, Klimesch W (2010) When frequencies never synchronize: the golden mean and the resting EEG. Brain Res 1335:91–102
Rechtschaffen A, Kales A (1968) A manual of standardized terminology, technique and scoring system for sleep stages of human subjects. UCLA Brain Information Service, Los Angeles
Saastamoinen A, Huupponen E, Varri A, Hasan J, Himanen S (2007) Systematic performance evaluation of a continuous-scale sleep depth measure. Med Eng Phys 29(10):1119–1131
Sato K (1969) On the effects of cutaneous stimulations upon EEG, respiratory and muscular activities of frog. Acta Med Nagasaki 13:14–27
Segura E, De Juan A (1966) Electroencephalographic studies in toads. Electroencephalogr Clin Neurophysiol 21(4):373–380
Servít Z, Machek J, Fischer J (1965) Electrical activity of the frog brain during electrically induced seizures. A comparative study of the spike and wave complex. Electroencephalogr Clin Neurophysiol 19(2):162–171
Smolin L (1962) The origin of the “spontaneous” electrical activity of the frog brain. Bull Exp Biol Med 52(6):1359–1362
Tauber E (1974) The phylogeny of sleep. In: Weitzman ED (ed) Advances in sleep research. Wiley, New York, pp 133–172
Thut G, Miniussi C (2009) New insights into rhythmic brain activity from TMS–EEG studies. Trends Cogn Sci 13(4):182–189
Whishaw I, Vanderwolf C (1971) Hippocampal EEG and behavior: effects of variation in body temperature and relation of EEG to vibrissae movement, swimming and shivering. Physiol Behav 6(4):391–397
Young C, McNaughton N (2009) Coupling of theta oscillations between anterior and posterior midline cortex and with the hippocampus in freely behaving rats. Cereb Cortex 19:24–40
Acknowledgments
This work was financially supported by grants from the Chinese Academy of Sciences ‘Bairenjihua’ (KSCX2-YW-R-077) to Yezhong Tang and Chinese Academy of Sciences ‘Xibuzhi-guang’ (09C302) and the National Natural Science Foundation of China (30900141) to Jianguo Cui. Permits for capturing animals were obtained from the local environmental department and all experiments were approved by Chengdu Institute of Biology and carried out according to international standards of animal care and use.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, G., Chen, Q., Cui, J. et al. Electroencephalogram bands modulated by vigilance states in an anuran species: a factor analytic approach. J Comp Physiol A 198, 119–127 (2012). https://doi.org/10.1007/s00359-011-0693-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-011-0693-y