Abstract
Big brown bats form large maternity colonies of up to 200 mothers and their pups. If pups are separated from their mothers, they can locate each other using vocalizations. The goal of this study was to systematically characterize the development of echolocation and communication calls from birth through adulthood to determine whether they develop from a common precursor at the same or different rates, or whether both types are present initially. Three females and their six pups were isolated from our captive breeding colony. We recorded vocal activity from postnatal day 1 to 35, both when the pups were isolated and when they were reunited with their mothers. At birth, pups exclusively emitted isolation calls, with a fundamental frequency range <20 kHz, and duration >30 ms. By the middle of week 1, different types of vocalizations began to emerge. Starting in week 2, pups in the presence of their mothers emitted sounds that resembled adult communication vocalizations, with a lower frequency range and longer durations than isolation calls or echolocation signals. During weeks 2 and 3, these vocalizations were extremely heterogeneous, suggesting that the pups went through a babbling stage before establishing a repertoire of stereotyped adult vocalizations around week 4. By week 4, vocalizations emitted when pups were alone were identical to adult echolocation signals. Echolocation and communication signals both appear to develop from the isolation call, diverging during week 2 and continuing to develop at different rates for several weeks until the adult vocal repertoire is established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microchiropteran bats use vocalizations for two separate purposes, to navigate by means of echolocation and to communicate and socialize with other bats. Echolocation signals are highly stereotyped and species-specific, and are often clearly correlated with the type of foraging strategy employed by a species (Aldridge and Rautenbach 1987; Neuweiler 2000; Pavey et al. 2001; Schnitzler and Kalko 2001; Schnitzler et al. 2003; Siemers and Schnitzler 2004; Wund 2005). Social vocalizations are more variable in their temporal and spectral features than echolocation signals and are used to express territoriality, courtship, aggression, isolation, and other social displays (Gould 1971; Gould et al. 1973; Kanwal et al. 1994; Heckel and Helversen 2003;). The characteristics of social vocalizations depend on species, sex, context, and individual identity (Masters et al. 1995; Kazial et al. 2001; Kazial and Masters 2004).
Both echolocation and communication vocalizations change and mature as young bats grow from infancy to adulthood. At birth, an infant bat is incapable of emitting either adult echolocation signals or mature social communication vocalizations. However, before young bats begin to fly and hunt insects at 4–5 weeks of age, they must be capable of producing high frequency echolocation signals and processing the information contained in the echoes. They must also be capable of emitting complex social vocalizations to communicate and interact with other bats.
Infant vocalizations have been recorded in several bat species (Eptesicus fuscus: Gould 1971; Myotis lucifugus: Konstantinov 1973; Moss et al. 1997; Rhinolophus ferrumequinum: Matsumura 1979; Rhinolophus rouxi: Rübsamen 1987; Hipposideros speoris: Habersetzer and Marimuthu 1986; Pteronotus parnelIii: Vater et al. 2003) and exhibit many similarities across species. In general, young bats emit multi-harmonic sounds that are lower in frequency than adult vocalizations. In many species, these appear to be isolation calls that a young bat emits to attract its mother (Gould 1971). It has been suggested that isolation calls of infant bats are precursors to adult echolocation signals (Moss 1988; Fenton 1995; Moss et al. 1997). However, it is equally likely that isolation calls are precursors to communication vocalizations, or precursors to both. It is also possible that the vocalizations emitted immediately after birth may exhibit differences that would permit their classification into early echolocation signals and early communication signals. We hypothesized that the isolation calls emitted at birth are the precursors to both echolocation and social vocalizations and that these two types diverge at some point in early development, possibly maturing at different rates. We predicted that this divergence occurs simultaneously, but that echolocation signals mature at a faster rate than social vocalizations. To assess this hypothesis, we investigated the development of vocalizations from postnatal day 1 until the bats reached adulthood in order to characterize the full repertoire of vocalizations and to assess the relationship between echolocation and communication signals over time.
Methods
Animals
Nine big brown bats (E. fuscus) were used in this study, three adult females and six pups. The adult bats were all bred and raised in captivity, and were descendants of bats originally captured in North Carolina and maintained in a breeding colony at the University of Washington. Three pregnant females were isolated from the colony approximately 2–3 weeks prior to giving birth. These bats were monitored daily and kept on a 15/9 h reverse light/dark cycle. The females gave birth to healthy twins, one male and one female in each pair. Eptesicus fuscus mates promiscuously, so it is likely that some of the twins had different fathers (Vonhof et al. 2006). Forearm length (FAL) was measured daily to monitor growth of the infant bats.
Audio recordings
We recorded vocalizations from each pup for 5 weeks, starting with the earliest vocalizations on postnatal day 1, and compared vocalizations emitted when the young bats were alone to those emitted when they were in the presence of their mothers. Each day, bats were gently removed from the colony and placed in a sound-attenuating chamber (123 × 154 × 103 cm, Industrial Acoustics Co., Inc.). After a 15-min rest period, the pups’ vocalizations were recorded for about 5 min using a Pettersson Elektronik AB D 980 ultrasound detector. Following these initial recordings, we introduced mothers to the recording chamber and recorded for an additional 15 min to study the mother–infant interactions. A low light-sensitive digital video camera was used to observe the behavior of the bats while they were vocalizing in the acoustic chamber and to verify vocalization by an individual when more than one bat was in the chamber at the same time. All vocalizations were recorded when the bats were most active, approximately 2–4 h after light offset.
Sound analysis
The spectral and temporal structure of the vocal emissions was analyzed using Batsound Pro software version 3.3 (Pettersson Elektronik Inc., sampling rate of 200 kHz). All vocalizations emitted by pups consisted of downward frequency-modulated (FM) sweeps that were sometimes followed by a tail that varied in duration, bandwidth and spectrotemporal structure. A syllable was defined as a single acoustic event; a phrase was defined as a series of syllables emitted in rapid succession. Syllables were classified based on the type of tail that followed the initial downward FM sweep and phrases were classified based on the number and type(s) of syllables, using a nomenclature similar to that used for mustached bats and horseshoe bats (Kanwal et al. 1994; Ma et al. 2006). We performed a quantitative analysis of 2,273 vocalizations that had the best signal to noise ratio and minimal interference from external noises and echoes produced by the walls and floor of the acoustic chamber. For each vocalization, we measured number of syllables, syllable duration, maximum frequency, minimum frequency, bandwidth, and power spectrum of the fundamental harmonic.
Results
Over the course of the 5-week study, the young bats showed distinct developmental changes in their in locomotor and vocal behaviors. Forearm length increased from 16 to 46 mm and bats began to fly by week 5 using echolocation to orient. Vocal repertoire changed in parallel with growth and behavioral changes.
Classification of vocalizations
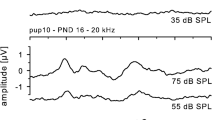
Like many other bats, newborn Eptesicus pups vocalized when separated from their mothers (Gould 1971, Fig. 1a). These vocalizations were stereotyped single syllable multiharmonic downward FM sweeps about 50 ms in duration with a fundamental bandwidth of approximately 10–20 kHz. This type of vocalization, termed an “isolation call”, was the only type emitted for the first three postnatal days. Starting on the fourth postnatal day, the pups began to emit other types of vocalizations, which were classified based on the spectrotemporal features of the tail that followed the initial FM sweep of each syllable. Because divergence of vocalization types first occurred in the middle of week 1, we considered the first half of the week separately from the second half in some analyses. FM syllables (FM) were rapid downward sweeps (<20 ms) with 2–3 harmonics (Fig. 1b) and no tail. FM syllables resembled adult echolocation signals and were emitted when the young bats were alone. Syllables with tails were emitted when the young bats were in the presence of their mothers (except in week 1), and could also be evoked by touching the pup.
Spectrograms of five single syllable vocalization types. a Isolation calls b FM calls c UP-tail d CF-tail e SFM-tail. Spectrograms of three double syllable phrases. f FM–FM g UP–FM h UP–UP. Spectrograms of two triple syllable phrases. i FM–FM–FM j UP–UP–FM k Mother–pup interaction during week 2; mother’s calls are single short FM syllables; pup’s vocalizations alternate with mother’s and are FM with variable tail
Syllables in which the initial FM component was followed by a tail that increased in frequency by at least 3 kHz and was greater than 10 ms in duration were classified as up-tail syllables (UP-tail, Fig. 1c). Those in which the initial FM component was followed by a constant frequency (CF) component with duration of more than 30 ms were classified as CF-tail syllables (CF-tail, Fig. 1d). Those in which the initial FM component was followed by a sinusoidally frequency-modulated (SFM) tail more than 30 ms in duration, were classified as SFM-tail syllables (SFM-tail, Fig. 1e). The young bats often emitted multi-syllable phrases, most often containing two or three syllables (Fig. 1f–j).
The vocalizations emitted by the mothers were multiharmonic single or double syllable FM sweeps with a fundamental that swept from about 50–24 kHz in about 4 ms but differed slightly from one mother to another. (Fig. 4c) There were no clear similarities between the frequency characteristics of the pups’ calls and the calls of their mother during early development. Figure 1k provides an example of a mother–infant interaction. In general, mothers emitted syllables that consisted of a rapid downward FM sweep (1–4 ms) spanning about 50–25 kHz. The young bats appeared to respond to this vocalization with several different types of vocalizations. As the young bats grew older, their vocalizations became more similar to those of their mother. Vocalizations of siblings were more similar than those of unrelated individuals in terms of their spectral characteristics.
Vocal behavior as a function of age
Week 1 (Forearm length: 16–28 mm)
During the first days after birth, the young bats were hairless and spent most of their time nursing. Their movements were limited and uncoordinated. When removed from their mothers, they would often remain in one corner of the recording chamber. For the first 3 days after birth, the vocalizations emitted by the infant bats in the presence of their mothers appeared identical to those emitted when they were alone, and were all classified as isolation calls (Fig. 2a, b).
By the middle of the first week (days 4–6), the young bats began to emit multiple types of vocalizations in the presence of their mothers. At this point, some multi-syllable phrases were emitted, with an increased variety of syllable types. In the absence of their mothers, 95% of the vocalizations emitted during days 4–6 were single syllables. Of these, 57% were isolation type and 43% were FM. In the presence of their mothers, 98% of the vocalizations were single syllable. Of these, 58% were isolation type, 12% were FM, 16% were CF-tail, 12% were UP-tail, and 3% were SFM-tail (Fig. 3b shows pooled data for all days of week 1). The double syllable phrases were either a pair of FM’s (80%) or an UP-tail followed by an FM (20%). The fundamentals swept from about 25–10 kHz with the greatest energy around 17 kHz (Fig. 4a). Figure 4b shows the average durations of the different types of syllables.
Development of spectral features of vocalizations emitted in the absence and presence of mothers. Black symbols indicate vocalizations that were emitted in solitude; gray symbols indicate vocalizations that were emitted in the presence of mothers. Each data point indicates the mean value ± SD for all calls emitted during weeks 1–5. a Development of peak frequency for different syllable types. b Durations of different syllable types over time. c Table of spectrotemporal characteristics of mothers’ FM vocalizations and those of their offspring (a and b) during week 5. Numbers designate mothers, numbers plus letters designate pups
Week 2 (FAL: 25–37 mm)
During week 2, the pups became more active and began to explore the cage when left alone. Most of their vocalizations were different from the isolation calls emitted in week 1 and were clearly different depending on whether the pups were alone or with their mother (Fig. 2c).
When the pups were alone they emitted biosonar-like single syllable FM sweeps (78%), double syllable FM–FM phrases (18%), and a few isolation calls (4%, Fig. 3a, c). The fundamental harmonic swept from about 33–18 kHz with a peak energy frequency of approximately 25 kHz (Fig. 4a). The duration of the single FM syllables and isolation calls decreased considerably compared to other call types, the duration of which remained virtually unchanged (Fig. 4b).
When the door of the recording chamber opened and the young bat heard its mother vocalize, it would begin to emit several different types of vocalizations (Fig. 2d). The percent usage of each type varied among individuals. Overall, 75% of the vocalizations were single syllables, and the remainder double syllable phrases (Fig 3d). Of the single syllables, 12% were isolation calls, 18% were FM, 37% had CF-tails, 25% had UP-tails, and 8% had SFM-tails (Fig. 3b). Double syllable phrases included FM–FM (78%), UP-tail–FM (20%), and FM–UP-tail (2%). Maximum, minimum and peak energy frequencies were similar to those of the vocalizations emitted when the bats were alone (Fig. 4a).
Week 3 (FAL: 35–43 mm)
By week 3, the pups were more agile. They attempted to fly and produced aggressive vocalizations when bothered. At this point, the pups appeared to be using echolocation to orient in the cage (Fig. 2e). The fundamental harmonics of all vocalizations shifted to a higher frequency range (44–16 kHz). Peak energy frequencies of vocalizations other than FM syllables increased to about 25 kHz. Peak energy frequencies of FM syllables increased to 35 kHz, the first point where there is a clear difference in vocalization types (Fig. 4a). By days 13–15, double syllable phrases were a major component of the pups’ repertoire (Fig. 3c, d).
In the absence of their mothers, pups vocalized when orienting and moving around the chamber, producing: single (62%), double (35%), and triple syllable phrases (3%), all of which consisted entirely of FM syllables with relatively short durations (Fig. 3a, c). Between weeks 2 and 3, maximum frequencies of the fundamental increased by about 10 kHz, minimum frequencies increased by about 4 kHz, and peak energy frequencies increased by about 7 kHz (Fig. 4a).
Once the mother was introduced into the recording chamber, the young bats emitted a variety of different vocalizations, including single, double, and triple syllable phrases. Single syllables included FM (52%), isolation calls (12%), CF-tails (23%), UP-tails (10%), or SFM-tails (3%, Fig. 3b). 75% of double syllable phrases were FM–FM, 20% were UP-tail–FM, and the remainder were FM–UP-tail. The majority of the triple syllable phrases were FM–FM–FM (85%). By week 3, the durations of the FM syllables reached average adult values (Fig. 4b).
Week 4 (FAL: 41–45 mm)
At this age, the forearm lengths of the young bats were nearly adult size (average adult forearm length is 47 mm). Pups were seldom found nursing but were often found next to their mothers and siblings. During week 4, the young bats clearly used echolocation signals for orientation. When first placed into the recording chamber, 53% of their vocalizations were single syllable, 39% were double syllable, and 8% were triple syllable (Figs. 2g, 3c). After an initial bout of vocalization, bats stopped vocalizing and perched in a corner of the cage. The fundamental of the FM syllables swept from about 50–22 kHz, close to the range of adult echolocation calls. The frequency of peak energy (about 33 kHz) also approached the value for adult calls (35 kHz).
The pups continued to emit a variety of vocalization types in the presence of the mother (Fig. 2h) but the use of syllable types other than FM decreased. 74% of all vocalizations emitted in the presence of the mother consisted entirely of FM syllables (Fig. 3b, d). Of the vocalizations emitted when the mother was present, 52% were single syllable, 41% were double syllable, and 6% were triple syllable (Fig. 3c). The frequency range and peak energy of the fundamental for calls containing only FM syllables were 5–7 kHz higher than for the few calls that ended in an UP-tail, CF-tail, or SFM-tail (Fig. 4a).
Week 5 (FAL: 44–46 mm)
By postnatal week 5, forearm lengths of the pups were comparable to those of their mothers and other adults. Pups were often found away from their mothers, hanging with other bats. They were more independent and perched away from their mothers after a brief initial communication and recognition.
As in the previous week, young bats emitted echolocation signals when alone in the recording chamber. When first placed in the chamber, pups emitted single syllable (58%), double syllable (34%), and triple syllable (8%) FM phrases (Fig. 3c) but stopped vocalizing as soon as they settled in a corner. These vocalizations were virtually identical to adult echolocation signals with a maximum frequency of about 50 kHz, a minimum of about 24 kHz, and peak energy frequency around 35 kHz (Petrites et al. 2009).
The young bats called to their mothers less frequently than during previous weeks, and the types of vocalizations emitted were more similar to those emitted when alone (Fig. 2j). The majority were single syllable (56%) or double syllable (40%) FM phrases (Fig. 3b, d). Pups emitted fewer tailed syllables either by themselves or in combination with other syllables.
Discussion
The present study demonstrates that echolocation and communication signals develop from isolation calls starting on postnatal day 1. Consistent with our hypothesis, infant bats do not emit any differentiated vocalizations at birth, but during the first week the two vocalization types begin to diverge. In accordance with our prediction, the maturation of echolocation signals occurs prior to the social vocalizations. By the time, bats reach maturity at 5 weeks of age; both types of emissions match those of adults.
Development of vocal behavior
Our data suggest that there are three stages in the development of vocal behavior in big brown bats. The first stage occurs during the first 4–5 postnatal days, during which time bats were completely helpless and unable to move toward their mother when separated. Instead, the mother always recognized and approached her young, attracted by their isolation calls. At this stage, the vocalizations consisted entirely of isolation calls.
The second developmental stage was marked by an abrupt change from emitting only isolation calls to emitting multiple vocalization types. This stage lasted between 16 and 18 days. When the young bats were alone, they oriented using what appeared to be echolocation signals but in a lower frequency range than those used by adults. In the presence of their mothers, the bats appeared to hear her vocalizations and responded by emitting a variety of vocalization types and approaching their mothers.
The third developmental stage occurred after about 20–25 postnatal days, when there was a decline in the young bats’ motivation to call for their mothers. At this stage, their vocalizations resembled those of their mothers, and may have served mainly echolocation/orientation functions and/or general communication functions.
Development of echolocation signals
In this study, we investigated the developmental transitions of vocalizations emitted when the young bats were alone, the assumption being that these were precursors to echolocation signals. These vocalizations more closely resembled typical adult echolocation signals than did other vocalization types, and were emitted when the bats displayed orientation behaviors. This signal type was part of the young bats’ repertoires by the end of the first postnatal week. This finding is consistent with reports of Eptesicus and other bat genera emitting echolocation signals in the 2nd postnatal week (Konstantinov 1973; Gould 1975; Matsumura 1979; Moss et al. 1997). Later in development, it became apparent from the bat’s movements that it was using echolocation to orient within the cage.
The echolocation signals emitted by young big brown bats differed from those of adult bats in that the frequency range was lower and the duration was longer. These changes are probably due to the maturation of the laryngeal musculature and the fine-tuning of the vocal apparatus throughout postnatal development.
Development of communication signals
To understand the development of communication signals, we investigated the developmental transitions of vocalizations emitted by young bats in the presence of their mothers. Communication signals were emitted in response to the mother’s presence and/or vocalization but not when the pups were alone. Our findings suggest that the vocalizations emitted by the young bats in the presence of their mothers were precursors to adult communication sounds since many of them resembled social vocalizations of adults (Gould 1975). Although the frequency range of all of the signals emitted in the presence of the mothers increased, the overall duration and frequency-time structure remained roughly the same throughout development. Many of these vocalizations consisted of FM sweeps with long tails (>30 ms), the duration of which did not change over time. Adult big brown bats use similar vocalizations during social interactions such as mating (Gould et al. 1973; Gould 1975; Monroy et al. 2005; Farrar et al. 2006). Thus, the vocal repertoire that young bats use to attract their mothers may develop into adult communication vocalizations with similar structure but different uses.
Recent work on the sac-winged bat (Saccopteryx bilineata), suggested that the variation of infant vocalizations is actually a form of babbling in a manner similar to human infants (Knornschild et al. 2006). Babbling allows the young to practice the adult vocal repertoire during development. It is suggested that sac-winged bats utter a variety of different sounds to develop fully functional adult vocalizations. Our data show that Eptesicus emits different vocalization types in the presence of the mother which all seem to serve the same purpose, to attract attention. This variability in vocalization structure may be a form of babbling in which young Eptesicus pups practice producing adult vocalizations.
Parallel development of communication and echolocation
The two modes of vocalization, echolocation and communication, appear to develop in parallel. Our data suggest that both echolocation and communication signals develop from the isolation call that is emitted at birth and then diverge during the 2nd postnatal week. It appears that the echolocation signals develop at a more rapid rate than the communication signals. By week 3, echolocation signals had reached maximum, minimum, and peak frequency values comparable to those of adults. Although the peak and minimum frequencies stabilize, the maximum frequency continues to increase by approximately 10 kHz up to week 5. These results also suggest that the rate at which communication develops does not depend exclusively on the maturation of the laryngeal muscles. If so, one would expect the rate of increase in frequencies to match across all vocalization types. Instead, the frequency spectrum of communication vocalizations developed more slowly than that of sonar signals, even though individuals were capable of emitting higher frequencies in the sonar signals.
Future studies on the development of bat vocalizations could provide insight regarding the respective roles of learning and genetic control in development of mammalian vocalizations. While this study did not compare twins born of one father versus two fathers; future studies could use this approach to examine the role of genetics in determining vocalization patterns.
References
Aldridge HDJN, Rautenbach IL (1987) Morphology, echolocation and resource partitioning in insectivorous bats. J Anim Ecol 56:763–778
Farrar D, Monroy J, Casseday JH, Covey E (2006) Mother-infant communication in the big brown bat, Eptesicus fuscus. Integr Comp Biol:45, poster P2.5
Fenton M (1995) Natural history and biosonar signals. In: Popper A, Fay R (eds) Hearing by bats. Springer, New York, pp 37–86
Gould E (1971) Studies of maternal-infant communication and development of vocalizations in the bats Myotis and Eptesicus. Commun Behav Biol 5:263–313
Gould E (1975) Experimental studies of the ontogeny of ultrasonic vocalizations in bats. Dev Psychobiol 8:333–346
Gould E, Woolf NK, Turner DC (1973) Double-note communication calls in bats: occurrence in three families. J Mammal 54:998–1001
Habersetzer J, Marimuthu G (1986) Ontogeny of sound in the echolocating bat Hipposideros speoris. J Comp Physiol A 158:247–257
Heckel G, Helversen OV (2003) Genetic mating system and the significance of harem associations in the bat Saccopteryx bilineata. Mol Ecol 12:219–227
Kanwal JS, Matsumura S, Ohlemiller K, Suga N (1994) Analysis of acoustic elements and syntax in communication sounds emitted by mustached bats. J Acoust Soc Am 96:1229–1254
Kazial KA, Masters WM (2004) Female big brown bats, Eptesicus fuscus, recognize sex from caller’s echolocation signals. Anim Behav 67:855–863
Kazial KA, Burnett SC, Masters WM (2001) Individual and group variation in echolocation calls of big brown bats, Eptesicus fuscus (Chiroptera: Vespertilionidae). J Mammal 82:339–351
Knornschild M, Behr O, von Helversen O (2006) Babbling behavior in the sac-winged bat (Saccopteryx bilineata). Naturwissenschaften 93:451–454
Konstantinov AI (1973) Development of echolocation in bats in postnatal ontogenesis. Period Biol 75:13–19
Ma J, Kobayasi K, Zhang S, Metzner W (2006) Vocal communication in adult greater horseshoe bats, Rhinolophus ferrumequinum. J Comp Physiol A 192:535–550
Masters WM, Raver KAS, Kazial KA (1995) Sonar signals of big brown bats, Eptesicus fuscus, contain information about individual identity, age and family affiliation. Anim Behav 50:1243–1260
Matsumura S (1979) Mother-infant communication in a horseshoe bat (Rhinolophus ferrumequinym nippon): development of vocalization. J Mammal 60:76–84
Monroy J, Farrar D, Casseday JH, Covey E (2005) Mating display behavior of the big brown bat, Eptesicus fuscus. NASBR, Sacramento, poster 21
Moss CF (1988) Ontogeny of vocal signals in the big brown bat, Eptesicus fuscus. In: Nachtigall P, Moore P (eds) Animal sonar systems: processes and performance. Plenum Press, New York, pp 115–120
Moss C, Redish D, Gounden C, Kunz TH (1997) Ontogeny of vocal signals in the little brown bat, Myotis lucifugus. Anim Behav 54:131–141
Neuweiler G (2000) The biology of bats. Oxford University Press, New York
Pavey CR, Grunwald J, Neuweiler G (2001) Foraging habitat and echolocation behaviour of schneider’s leafnosed bat, Hipposideros speoris, in a vegetation mosaic in Sri Lanka. Behav Ecol Sociobiol 50:209–218
Petrites AE, Eng OS, Mowlds DS, Simmons JA, Delong CM (2009) Interpulse interval modulation by echolocating big brown bats (Eptesicus fuscus) in different densities of obstacle clutter. J Comp Physiol A 195:603–617
Rübsamen R (1987) Ontogenesis of the echolocation system in the rufous horseshoe bat, Rhinolophus rouxi (auditory and vocalization in early postnatal development). J Comp Physiol A 161:899–904
Schnitzler HU, Kalko EKV (2001) Echolocation by insect-eating bats. Bioscience 51:557–569
Schnitzler HU, Moss CF, Denzinger A (2003) From spatial orientation to food acquisition in echolocating bats. Trends Ecol Evol 18:386–394
Siemers BM, Schnitzler HU (2004) Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature 429:657
Vater M, Kössl M, Foeller E, Coro F, Mora E, Russell IJ (2003) Development of echolocation calls in the mustached bat, Pteronotus parnellii. J Neurophysiol 90:2274–2290
Vonhof MJ, Barber D, Fenton ME, Strobeck C (2006) A tale of two siblings: multiple paternity in big brown bats (Eptesicus fuscus) demonstrated using microsatellite markers. Mol Ecol 15:241–247
Wund M (2005) Learning and the development of habitat-specific bat echolocation. Anim Behav 70:441–450
Acknowledgments
This paper is dedicated to the memory of Gerhard Neuweiler. We thank Dawn Farrar for her technical assistance and for analyzing data. All procedures were approved by the animal care and use committee of the University of Washington and followed NIH guidelines. This research was supported by National Institute of Health grants DC-00607 and DC-00287, UW Royalty Research Grant No. 3544 and National Science Foundation grant IOS0719295.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monroy, J.A., Carter, M.E., Miller, K.E. et al. Development of echolocation and communication vocalizations in the big brown bat, Eptesicus fuscus . J Comp Physiol A 197, 459–467 (2011). https://doi.org/10.1007/s00359-010-0614-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-010-0614-5