Abstract
We studied the photopic spectral sensitivity in the green-backed firecrown, Sephanoides sephaniodes, a South American hummingbird, and its possible ecological relationship with preferred flowers and body colouration. Avian colour vision is in general tetrachromatic with at least four types of cones, which vary in sensitivity from the near ultraviolet (UV) to the red wavelength range. Hummingbirds represent an important family of birds, yet little is known about their eye sensitivity, especially about the role of photoreceptors and their oil droplet complements. The photopic electroretinogram shows a main sensitivity peak at 560 nm and a secondary peak in the UV, and may be explained by the presence of four single cones (λ max at ~370, 440, 508 and 560 nm), and a double cone (λ max at 560 nm) screened by oil droplets. The flowers preferred by the firecrown are those in which the red–green wavelength region predominates and have higher contrast than other flowers. The crown plumage of males is highly iridescent in the red wavelength range (peak at 650 nm) and UV; when plotted in a high-dimensional tetrachromatic space, it falls in a “red + UV” purple hue line, suggesting a potential significant communication signal for sexual differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among birds, the hummingbirds (Trochilidae) show a unique pattern of radiation and diversification, including behavioural specializations associated with their way of life (Greenewalt 1960; Bleiweiss 1998). This group is highly dependent on flowers as a food source, and the apparent fit between bird-pollinated flowers (ornithophilous) and hummingbirds has led to the hypothesis that ornithophilous flowers have evolved to match the preferences of hummingbirds for shape, colour and reward (Grant and Grant 1968; Raven 1972). At the behavioural level, training experiments have shown that they can discriminate between close wavelengths over a wide range of the spectrum. Hummingbirds visit flowers of all colours in proportion to nectar quality, suggesting that colour vision is a learned predictor of reward and not an exclusive agent of attraction (Bené 1941; Miller and Miller 1971; Stiles 1976; Huth and Burkhardt 1972; Goldsmith and Goldsmith 1979; Goldsmith et al. 1981; Waser et al. 1996; Melendez-Ackerman et al. 1997; Altshuler 2003). The photoreceptor complement, including oil droplets, underlying colour vision in these birds is not completely known.
Avian retinae show a more complex array of photoreceptors than mammals, including single and double cones with visual pigments of maximum sensitivity (λ max) at long-wavelength-sensitive (LWS) cone visual pigments with λ max between 540–570 nm, medium-wavelength-sensitive (MWS) cone with λ max 500–507 nm, short-wavelength-sensitive (SWS) cone with λ max 430–460 nm and violet-sensitive (VS) cone with λ max 400–420, or ultraviolet-sensitive (UVS) cone with λ max 360–370 nm (Hart 2001; Hart and Vorobyev 2005; Hart and Hunt 2007). Among vertebrates, the shortest wavelength range shows the highest variability in λ max, and birds are no exception. Parrots and most passerines possess UVS cones, whereas most non-passerines have VS cones (Bowmaker et al. 1997; Hart 2001). A recent comparison of the gene sequence in birds suggests that UVS and VS opsins differ only in a few amino acids (e.g. Phe86 or Ser86) (Carvalho et al. 2007) and that VS is the ancestral avian prototype from which UVS evolved independently several times in birds (Ödeen and Håstad 2003).
A special trait of bird cones is the presence of oil droplets in their inner segment, which form cut-off filter transmitting longer wavelengths with optical properties that depend on their carotenoid content and concentration. In general, UVS or VS cones contain a transparent (T) oil droplet with no carotenoids; SWS galloxanthin (pale colour); MWS zeaxanthin [yellow, described in the chicken as a mixture of 15:15′ cis (“cisC”) and all trans (“transC”) isomers]; and LWS astaxanthin (red) in high concentrations. Double cones have LWS visual pigments and are screened by a variety of galloxanthin and ε-carotene mixtures (Goldsmith et al. 1984; Bowmaker et al. 1997; Vorobyev et al. 1998; Vorobyev 2003; Hart and Vorobyev 2005).
In tropical ecosystems, ornithophilous plant varieties interact with a large number (10–30) of hummingbird species. However, in areas such as Chiloé Island (Chile), a South American temperate forest ecosystem (Smith-Ramirez 1993; Armesto et al. 1996; Fraga et al. 1997; Aizen et al. 2002), the green-backed firecrown, Sephanoides sephaniodes (Trochilidae), accounts for about 20% of the pollination of the entire flora. Although red colouration predominates in a temperate forest ecosystem, the firecrown visits flowers of diverse colouration, shape, and nectar concentration (Smith-Ramirez 1993). If hummingbirds are not restricted to visiting red flowers, why are so many ornithophilous flowers red? It has been proposed that red colouration might be a way to enhance discrimination (Pickens 1930; Chittka and Waser 1997). However, to our knowledge this possibility has not been directly evaluated in hummingbirds.
Considering this situation and the scarce data available on hummingbird colour vision, in general, we evaluated firecrown retinal visual capacities and their relation with chromatic signals present in their habitat. Specifically we determined: (1) the photopic spectral sensitivity from the electroretinogram (ERG), and with a mathematical model we estimated the most likely chromatic mechanisms (cone visual pigments + oil droplets) contributing to the ERG; (2) the spectral reflectance of plumage and flowers visited; and (3) colour contrast of flowers against foliage with respect to firecrown visual capacity.
Materials and methods
Bird capture and maintenance
Non-reproductive birds (mean body mass ± SD = 4.9 ± 0.4 g, n = 6 females; 6.9 ± 0.4 g, n = 3 males) were captured with mist nets in central Chile (33°17′S, 71°11′W) in June–July, 2003. Birds were maintained in individual 60 × 60 × 60 cm cages, at 25°C under a 12:12 light:dark cycle and fed a 0.6 M sucrose solution, fruit flies (Drosophila melanogaster), and water ad libitum. Between experiments, a vitamin and protein supplement was added to the sucrose solution (Vimiprotein-L®, Rhone-Poulenc Rorer, 0.3 g/50 ml of solution).
Electroretinogram recording
Animals were anaesthetized with 75 mg/ml Ketamine and 5.82 mg/ml of Xilazine, 1 μl per body gram, and few drops of Lidocaine (1%) + Atropine (1%) were locally applied in the eyes.
The optical system consisted of a stabilized power supply with a quartz lamp (250 W, ORIEL), and a monochromator (1,200 lines mm−1 grating, ORIEL, 20 nm half-bandwidth). A short-pass filter (Schott UG11 bandpass filter) to isolate the UV band and long-pass filters (Schott RG500, RG540, RG680) to eliminate stray light from the monochromator were used. An electronic shutter (Uniblitz, Vincent Associates) was utilized to control flash duration and an optical quartz wedge (0–5 OD) was inserted to attenuate the flash intensity. The monochromator, optical wedge, and shutter were under computer control and adjusted to deliver short flashes at wavelengths from 300 to 700 nm in 20 nm steps. The eye was kept light-adapted by a quartz tungsten lamp (150 W) producing a background illumination of 240 μW cm−2 sr−1 at the cornea. The light stimulus was concentrated at the cornea using a quartz lens condenser. Before each experiment, the lamp’s photon flux was calibrated for wavelengths from 300 to 700 nm with a photocell (Photometer S370, UDT Instruments) positioned at the corneal level. The monochromator’s wavelength was calibrated using an Ocean Optics spectrometer (S2000). The ERG signal was recorded using a pair of Ag/AgCl electrodes, one placed at the cornea and the other under the skin, and amplified at high gain (DP-301, Warner Instruments). The photopic spectral sensitivity S(λ) was calculated from r peak/I; where I is the flash photon flux, and r peak is the b-wave peak response evoked by a series of dim flashes (n = 50–100). During the ERG and colouration experiments, the body temperature was kept at 32º C with a commercial bed warming device. For further details of the methodology, see Chávez et al. (2003).
Spectral reflectance and eye transmission
The reflectance of feathers, flowers, and foliage background were measured with a S2000 spectrometer between 250 and 750 nm, acquired with an I/O interface card (National Instruments, DAQCard-700) and stored in a computer. A white standard source (Spectralon™ 99%) was used for reflectance calibration. Sample patches were illuminated by a xenon flash lamp (Ocean Optics) through a fused silica fibre optic (400 μm diameter) with six external concentric fibre bundles. A single central internal fibre was used to collect the reflected light. Since reflectance measurements are very sensitive to the incidence and angle of the light, the fibre was positioned at a right angle to the surface of the patch with a reading acceptance angle of 20º for all measurements. A light radiance sensor placed 1–2 cm from the sample allowed measurements of a surface area of 0.1–0.4 cm2. The maximal colour iridescence from male crown plumage was obtained by varying systematically the position of the detecting probe until a red brilliant colour became visible. Plumage samples from dorsal and ventral body regions, as well as flowers visited by firecrowns were measured under controlled laboratory conditions. The eye transmittance was measured using a quartz cuvette device adapted to the size of the eye (Chávez et al. 2003).
Modelling the electroretinogram
The ERG results from a composite contribution of several spectral mechanisms and accurate information on the participation of different visual pigments cannot be directly extracted. To reproduce the α -band and β-band absorbance peak of visual pigments, we followed the methods of Govardovskii et al. (2000) and Stavenga et al. (1993). A function determining the β-band position is described in Palacios et al. (1998). The filter effect of an oil droplet is given by:
where D(λ) corresponds to the normalized absorbance spectrum of the carotenoid and c to its peak absorbance. Therefore, the spectral response of the ERG is reproduced by
where n = 5 is the number of different cone types, i is their corresponding index, k i is their relative contribution, P i represents the absorption spectra of cones and S i are the associated oil droplets. The long-wavelength increase in sensitivity by self-screening for axial absorbance is expected to be between 0.1–0.3 (see Fig. 9 and text in van Roessel et al. 1997) and was ignored in our analysis.
In our model calculation we assumed that the firecrown has four single and one double cone types, as found in most common birds (see Goldsmith et al. 1984; Bowmaker et al. 1997; review in Hart 2001). As a starting point, a reasonable estimation of cone λ max may be predicted from the average λ max value collected from 25 different species of birds (see Table 2 in Hart and Vorobyev 2005). A realistic fit resulted when the model was constrained to: cones with λ max varying ±5 nm from the average λ max value and their contribution set to be at least 1%; varying the β-band amplitude between 0.1 and 1 times α-band, (Palacios and Goldsmith 1993; Palacios et al. 1998); oil droplet peak absorbance varying as: galloxanthin (0.1–2.0); ε-carotene (0.1–2.0); zeaxanthin (2.0–5.0); astaxanthin (8.0–10) (T.H. Goldsmith personal communication and Goldsmith and Butler 2003).
A non-linear fit from the MATHEMATICA software was used to minimize the least-squares function (downhill simplex algorithm, Nelder and Mead 1965).
Chromatic contrast of flowers
A receptor noise limited model (Vorobyev and Osorio 1998; Vorobyev et al. 1998) was used to calculate the chromatic contrast of flowers with respect to their foliage background. First, we calculated q i , the receptor quantum catches (for single cones, with λ max at LWS, MWS, SWS and UVS as estimated in the previous section); double cones were not considered for this analysis (Vorobyev and Osorio 1998):
where R i (λ) is the spectral sensitivity of the photoreceptor i, S(λ)is the reflectance spectrum of a surface and I(λ) is the standard illuminant D65. Von Kries normalization factors k i are used to account for chromatic adaptation. They were calculated as functions of a background spectrum S b(λ) that was obtained by averaging the measured foliage spectra (samples 5, 10, 17, 19, 21 from Table 2).
A threshold distance of stimuli (ΔS t) for a tetrachromatic receptor space was calculated as a function of the difference in quantum catch Δq i between background and stimulus (Vorobyev and Osorio 1998):
where e i is the limiting Weber fraction assigned to each receptor class
and υ i is the standard deviation of the noise of an individual cone and η i is the number of cones per receptive field (Vorobyev et al. 1998). ΔS indicates units of just noticeable differences (JND): values <1 JND specify that two wavelengths are undifferentiated, and values >1 JND indicate wavelengths that can be discriminated (for other details in the calculation, see Vorobyev and Osorio 1998).
Tetrachromatic colour space
Loci of sampled plumages and flowers in a tetrachromatic space were calculated by considering
where r i is the relative contribution of quantum catches for single cones with λ max at LWS, MWS, SWS, and UVS as estimated in the previous section, as weights on the vectors of a tetrahedron.
Results
ERG profile of firecrown eyes
The photopic spectral sensitivity was determined using the b-wave amplitude evoked by dim flashes of different wavelengths between 360 and 700 nm. Figure 1a shows a normalized response-photon flux function evoked at 560 nm by 10-ms flashes of increasing intensities under photopic conditions. At the highest intensities, the ERG saturates and the continuous line corresponds to the best fit using a Hill function. These experiments, including other wavelengths, showed no significant differences between females (n = 3) and males (n = 2) (P > 0.05). Subsequent ERG data were pooled for analyses. An important step for determining correct eye sensitivity is to characterize the transmission of the eye (cornea, lens). Figure 1b shows a relatively flat-eye transmission beyond 400 nm, which drops to 30% at 300 nm. A 50% transmittance criterion value λ T0.5 = 315 nm was estimated from a regression function.
a Normalized b-wave amplitude of the ERG (n = 4) to 10 ms flashes at 560 nm with increasing intensity of 2.3, 11.4, 22.7, 57.1, 114, and 228 photons μm−2 at the cornea. Each data point results from the average of 50 trials. The continuous line is the best fit using a Hill function: r/r max = I n/(I n + σ n), with σ = 63 photons μm−2 and n = 1.2, where σ is the number of photons required for a half-saturating response. b Eye transmittance (lens + cornea) for n = 2 individuals λ T0.5 ≈ 315 nm. c Regression function (y = −2.4804 + 0.88349 *x, r 2 = 0.847) comparing λ max for UVS or VS and λ T0.5 eye transmittance obtained from data extracted from 25 species of birds (see Table 2 in Hart and Vorobyev 2005)
Figure 2a shows the mean photopic spectral sensitivity of five animals, corrected for eye transmission. The ERG is dominated by a maximum around 560 nm, with relatively low sensitivity at wavelengths shorter than 450 nm.
a Mean photopic spectral sensitivity function for firecrown (n = 5, ±SD). The continuous line is the best fit (r 2: 0.994) using a linear combination of cone visual pigments + oil droplets. b Receptor relative participation to the ERG obtained after modelling. In single cones, the LSW is screened by astaxanthin, MWS by zeaxanthin and SWS by galloxanthin, and UVS was assumed with oil droplets that lack pigmentation. The principal and accessory member of double LWS cones were associated with a mixture of ε-carotene and galloxanthin
A further step was to estimate the putative chromatic mechanisms that contribute to the spectral sensitivity (see Materials and methods). An uncertainty for the model is the presence of a UVS or VS cones. Although we tested both options, we decided on the latter based on the following arguments: (1) previous ERG eye-cup experiments in three species of hummingbirds described the presence of a UV mechanism (Chen and Goldsmith 1986); (2) some hummingbirds discriminate UV from visible light (Huth and Burkhardt 1972; Goldsmith 1980); and (3) Fig. 1c shows a practical predictor for the presence of an UVS or VS cone visual pigment obtained after plotting λ T0.5 versus UVS or VS λ max from the data in Table 1 of Hart and Vorobyev (2005). The firecrown’s λ T0.5 = 315 nm matches a relatively UV clear eye and suggests the presence of a UVS cone.
Figure 2a (continuous line) shows the most parsimonious simulation result (r 2: 0.994) (see Table 1 for numerical data) and Fig. 2b the respective contributions of different chromatic mechanisms to that fit. Furthermore, simulations using a VS (λ max 420) instead of a UVS cone or limiting the ERG input to double cones gave a worse fit (r 2: 0.967, r 2: 0.979, respectively).
Flowers visited and plumage reflectance
Visited flowers and foliage strongly reflect beyond 500 nm in the green–red region compared to the blue–UV wavelength region (Fig. 3a).
a Reflectance spectra samples of flowers and foliage visited by firecrown (top). Tristerix aphyllus (1), Tristerix tetrandus (1), Sphacele salviae (2), Eucalyptus globulus (5), Fuchsia magellanica (3), Lobelia polyphyla (3), Hibiscus sp. (rosenblow) an introduced ornamental Malvaceae (3), Phycella bicolor (6), Sophora macrocarpa (2), Minulus luteus (3), Chlorae nudilabia (2), and Drimys winteri (2). In parentheses is the averaged number of measured samples. b Loci of flower and plumage spectra on a tetrahedron corresponding to avian receptor spaces for eyes with oil droplets and without oil droplets. Samples are numbered according to Table 2. A black dotted line shows the trajectory of monochromatic loci over the 300–650 nm spectral range
A tetrahedron colour space (after Goldsmith 1990; Vorobyev et al. 1998) was created to compare firecrown results with other species. In Fig. 3b only single cones were used, since double cones are not believed to be involved in bird colour vision (Vorobyev and Osorio 1998). Each locus in Fig. 3b represents a floral spectrum (Fig. 3a) in tetrachromatic coordinates (see Table 2 for numerical data). The majority of flower and foliage loci are plotted in the red/green part of the chromatic diagram; a few samples (loci 1, 13, 20, 21) fall in the blue region, and only Lobelia polyphyla (petal) and Eucalyptus globules (fruits) are in the UV (loci 2, 8). The importance of the screenings of oil droplets is to expand the colour space, enhancing chromatic discriminability (Fig. 3b) (Vorobyev et al. 1998).
To evaluate the hypothesis that red colouration might enhance detection, a chromatic distance between flowers, mostly collected in a temperate forest ecosystem in the south of Chile, and their foliage, was calculated considering the colour mechanisms predicted herein (see section Chromatic contrast of flowers). The results show that in bright daylight (D65 illuminant) all the flowers presented chromatic contrast values above the discrimination threshold, and discriminability for red flowers is slightly higher than for the white, yellow, or orange flowers tested here (Table 3 and methods for criteria). However, the significance of such differences needs to be further explored under ecological or behavioural considerations.
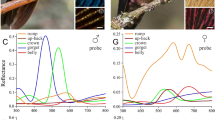
A potentially important visual signal in avian communication is their colourful plumage, which in some species includes iridescence properties. In firecrown males and females, the dorsal plumage shows a relatively low reflectance with 10% at 550 nm and dropping to 5% below 350 nm (Fig. 4a). However, the ventral part showed a monotonic increment of reflectance from UV to 25% in the red wavelength range. Figure 3b shows the dorsal reflectance (locus 23) located in the red/green region in a tetrahedron. The ventral reflectance (locus 22) shares similar tetrachromatic coordinates and can be expected to correspond to firecrown or avian “white” colour. An important source of iridescence in the red (peak 650 nm) wavelength range and a smaller, but significant iridescence source in the UV are present in the male head-crown, clearly suggesting a sexual dimorphic colour signature (Fig. 4b). In terms of tetrachromatic space, the crown iridescence locus (24) resides in a purple line formed by red and UV input, which is expected to produce a very distinct (non-spectral) chromatic hue. Spectral reflectance from female heads showed no iridescence properties and was similar to the dorsal trace in Fig. 4a.
a Spectral reflectance of ventral (dots) and dorsal (continuous) regions of the body for males and females. b Male head-crown measured from three different probe positions. The iridescent amplitude in the red and UV wavelength range (traces from bottom to top) depends on the angle established between the head and the probe. Head reflectance from females is similar to the dorsal spectrum shown in Fig. 4a
Discussion
Firecrown photopic spectral sensitivity appears to be dominated by the double cones that are thought to be involved in motion detection, brightness discrimination, polarized light detection and magnetic field orientation, but not in colour vision in birds (Osorio et al. 1999). Can we estimate the contribution of single cones to the ERG? We ran a series of simulations with a computational model. In a first approximation, the simulations match a pentachromatic system, four single cones and one double cone. One of the variants in the model was to decide between the participation of an UVS or VS cone; the simulation gave a better fit when a UVS cone with 5% participation was considered. This result is consistent with a previous electrophysiological description of a UVS receptor in the ruby-throated hummingbird (Archilochus colubris) (Chen and Goldsmith 1986). The estimated low participation of the UVS receptor may be misleading if we try to extrapolate its real importance for behaviour, as the black-chinned (Archilochus alexandri) hummingbird exhibits excellent behavioural wavelength discrimination in the UV range (Huth and Burkhardt 1972; Goldsmith and Goldsmith 1979; Goldsmith 1980, 1981).
The benefits of a UVS receptor for hummingbirds could be multiple, as UV signals are involved in several aspects of bird behaviour, e.g. the UV reflectance of the plumage participates in intraspecific (Bennett et al. 1997; Anderson et al. 1998; Sheldon et al. 1999) and interspecific interactions (Bleiweiss 2004). In this regard, the male firecrown’s head-crown shows strong iridescence in the red and UV, and there is evidence that carotenoid-based pigmentations (red, yellow, orange, in some cases with secondary peaks in the UV) are highly correlated with sexual dichromatism (Badyaev 2000; Osorio and Ham 2002) or are involved in aggressive displays associated with feeding territoriality or foraging behaviour (Stiles and Wolf 1970; Stiles 1982; Bleiweiss 1985).
To compare results of firecrown hummingbirds with other avian systems we plotted flowers and plumage reflectances in a tetrachromatic colour space (Fig. 3b). In the firecrown tetrahedron, oil droplets expanded the limits of pure colours and it is comparable to the colour space calculated for other birds (e.g. Vorobyev et al. 1998). For any colour space of more than two dimensions, a purple colour (a non-spectral colour) results from a mixture of two extreme spectral loci (Wyszecki and Stiles 1982). In the human trichromatic colour space, a purple (colour) corresponds to the mixture between a blue and red wavelength. However, in a tetrachromatic space in addition to the human “blue + red” purple source, two additional sources of purple are possible: a “red + UV” and “green + UV”.
An interesting question is, can we find spectral surfaces in nature that fill these tetrachromatic extra “purple” sources? For example, the spectral locus for the crown iridescences is located in between the red and UV purple line (Fig. 3b). We suggest that this category of hue corresponds to a bird “red + UV” purple (Goldsmith 1990; Thompson et al. 1992, see also Altshuler 2003). Other possible sources of bird “red + UV” purple are also seen in the loci of plumage from the Pekin robin (Leiothrix lutea) (Vorobyev et al. 1998). The behavioural implications of these colours still need to be explored further.
We found that red flowers subtend a higher chromatic contrast to the background than do flowers of other colours, and therefore they could be discriminated more efficiently (but see Schaefer et al. 2007). The higher levels of chromatic contrast subtended by red flowers (Table 1) in the firecrown could be explained by the decreased overlap between the LWS cone mechanism stimulated strongly by red, and the MWS cone mechanisms by the green foliage, probably allowing better wavelength discrimination in this range. However, the extent to which higher levels of chromatic contrast relate to hummingbird flower choices needs to be further evaluated (e.g. the dependency of contrast discrimination from the retinal angle of vision).
To understand further the interplay between the hummingbirds’ retinal mosaic and colour vision abilities, we will need to test the behavioural significance of different chromatic categories for foraging ecology, mating behaviour or sexual selection (Varela et al. 1993; Hart 2001).
Abbreviations
- ERG:
-
Electroretinogram
- LWS:
-
Long-wavelength-sensitive
- MWS:
-
Medium-wavelength-sensitive
- SWS:
-
Short-wavelength-sensitive
- VS:
-
Violet sensitive or UVS ultraviolet-wavelength-sensitive
- λ T0.5 :
-
Wavelength at 50% eye transmittance
References
Aizen MA, Vazquez DP, Smith-Ramirez C (2002) Historia natural y conservación de los mutualismos planta-animal del bosque templado de Sudamérica austral. Rev Chil Hist Nat 75:79–97
Altshuler DL (2003) Flower color, hummingbird pollination, and habitat irradiance in four neotropical forests. Biotropica 35:344–355
Anderson A, Ornborg J, Anderson M (1998) Ultraviolet sexual dimorphism and assortive mating in blue tits. Proc R Soc Lond B 265:445–450
Armesto J, Smith C, Sabag C (1996) The importance of plant–bird mutualisms in the temperate rainforest of southern South America. In: Lawford RG et al (eds) High latitude rainforests and associated ecosystems of the west coast of the Americas. Springer, New York
Badyaev AV (2000) Evolution of sexual dichromatism: contribution of carotenoid- versus melanin-based coloration. Biol J Linn Soc 69:153–172
Bené F (1941) Experiments on the color preference of black-chinned hummingbirds. Condor 43:237–242
Bennett AT, Cuthill IC, Partridge JC, Lunau K (1997) Ultraviolet plumage colors predict mate preferences in starlings. Proc Natl Acad Sci USA 94:8618–8621
Bleiweiss R (1985) Iridescent polychromatism in a female hummingbird: is it related to feeding strategies? Auk 102:701–713
Bleiweiss R (1998) Origin of hummingbird faunas. Biol J Linn Soc 65:77–97
Bleiweiss R (2004) Ultraviolet plumage reflectance distinguishes sibling bird species. Proc Natl Acad Sci USA 101:16561–16564
Bowmaker JK, Heath LA, Wilkie SE, Hunt DM (1997) Visual pigments and oil droplets form six classes of photoreceptor in the retinas of birds. Vision Res 37:2183–2194
Carvalho LS, Cowing JA, Wilkie SE, Bowmaker JK, Hunt DM (2007) The molecular evolution of avian ultraviolet- and violet-sensitive visual pigments. Mol Biol Evol 24:1843–1852
Chávez AE, Bozinovic F, Peichl L, Palacios AG (2003) Reflectance in the genus Octodon (Rodentia): implications for visual ecology retinal spectral sensitivity, fur coloration and urine. Invest Ophthalmol Vis Sci 44:2290–2296
Chen DM, Goldsmith TH (1986) Four spectral classes of cone in the retinas of birds. J Comp Physiol A 159:473–479
Chittka L, Waser NM (1997) Why red flowers are not invisible to bees. Israel J Plant Sci 45:169–183
Fraga RM, Ruffini AE, Grigera G (1997) Interacciones entre el picaflor rubí Sephanoides sephaniodes y plantas del bosque subantártico en el Parque Nacional Nahuel Huapi, Argentina. Hornero 14:224–234
Goldsmith TH (1980) Hummingbirds see near ultraviolet light. Science 207:786–788
Goldsmith TH (1990) Optimization, constraint, and history in the evolution of eyes. Q Rev Biol 65:281–322
Goldsmith TH, Butler BK (2003) The roles of receptor noise and cone oil droplets in the photopic spectral sensitivity of the budgerigar, Melopsittacus undulatus. J Comp Physiol A 189:135–142
Goldsmith TH, Goldsmith KM (1979) Discrimination of colors by the black-chinned hummingbird Archilochus alexandri. J Comp Physiol A 130:209–220
Goldsmith TH, Collins JS, Perlman DL (1981) A wavelength discrimination function for the hummingbird Archilochus alexandri. J Comp Physiol A 143:103–110
Goldsmith TH, Collins JS, Licht S (1984) The cone oil droplets of avian retinas. Vision Res 24:1661–1671
Govardovskii VI, Fyhrquist N, Reuter T, Kuzmin DG, Donner K (2000) In search of the visual pigment template. Visual Neurosci 17:509–528
Grant KA, Grant V (1968) Hummingbirds and their flowers. Columbia University Press, NewYork
Greenewalt CH (1960) Hummingbirds. Dover Publications, New York
Hart NS (2001) The visual ecology of avian photoreceptors. Prog Retin Eye Res 20:675–703
Hart NS, Hunt DM (2007) Avian visual pigments: characteristics, spectral tuning, and evolution. Am Nat 169:S7–S26
Hart N, Vorobyev M (2005) Modeling oil droplet absorption spectra and spectral sensitivities of bird cone photoreceptors. J Comp Physiol A 191:381–392
Huth HH, Burkhardt D (1972) Der spektrale sehbereich eines violettohr-kolibris. Naturwissenschaften 59:650
Melendez-Ackerman E, Campbell DR, Waser NM (1997) Hummingbird behaviour and mechanisms of selection on flower color in Ipomopsis. Ecology 78:2532–2541
Miller RS, Miller RE (1971) Feeding activity and color preference of ruby-throated hummingbirds. Condor 73:309–313
Nelder JA, Mead R (1965) A simplex method for function minimization. Comput J 7:308–313
Ödeen A, Håstad O (2003) Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol Biol Evol 20:855–861
Osorio D, Ham AD (2002) Spectral reflectance and directional properties of structural coloration in bird plumage. J Exp Biol 205:2017–2027
Osorio D, Vorobyev M, Jones CD (1999) Colour vision of domestic chicks. J Exp Biol 202:2951–2959
Palacios AG, Goldsmith TH (1993) Photocurrents in retinal rods of pigeons (Columba livia): kinetics and spectral sensitivity. J Physiol (London) 471:817–829
Palacios AG, Srivastava R, Goldsmith TH (1998) Spectral and polarization sensitivity of photocurrents of amphibian rods in the visible and ultraviolet. Visual Neurosci 15:319–331
Pickens AL (1930) Favorite colors of hummingbirds. Auk 47:346–352
Raven PH (1972) Why are bird-visited flowers predominantly red? Evolution 26:674
Schaefer HM, Schaefer V, Vorobyev M (2007) Are fruit color adapted to consumer vision and birds equally efficient in detecting colorful signals. Am Nat 169:159–169
Sheldon BC, Andersson S, Griffith SC, Örnborg J, Sendecka J (1999) Ultraviolet colour variation influences blue tit sex ratios. Nature 402:874–877
Smith-Ramirez C (1993) Los picaflores y su recurso floral en el bosque templado de la isla de Chiloe, Chile. Rev Chilena Hist Nat 66:65–73
Stavenga DA, Smits RP, Hoenders BJ (1993) Simple exponential functions describing the absorbance bands of visual pigment spectra. Vision Res 33:1011–1017
Stiles FG (1976) Taste preferences, color preferences, and flower choice in hummingbirds. Condor 78:10–26
Stiles FG (1982) Aggressive and courtship displays of the anna′s hummingbird. Condor 84:208–225
Stiles FG, Wolf LL (1970) Hummingbird territoriality at a tropical flowering tree. Auk 87:467–491
Thompson E, Palacios A, Varela F (1992) Ways of coloring: comparative color vision as case study for cognitive science. Behav Brain Sci 15:1–74
Van Roessel P, Palacios AG, Goldsmith TH (1997) Activity of long-wavelength cones under scotopic condition in the cyprinid fish Danio aequipinnatus. J Comp Physiol A 181:493–500
Varela FJ, Palacios AG, Goldsmith TH (1993) Color vision of birds. In: Zeigler HP, Bischof HJ Vision, brain, and behavior in birds. MIT Press, Cambridge
Vorobyev M (2003) Coloured oil droplets enhance colour discrimination. Proc R Soc Lond B 270:1255–1256
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of colour threshold. Proc R Soc Lon Ser B 265:1–8
Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC (1998) Tetrachromacy, oil droplets and bird plumage colours. J Comp Physiol A 183:621–633
Waser NM, Chittka L, Price MV, Williams N, Ollerton J (1996) Generalization for pollination systems, and why it matters. Ecology 77:1043–1060
Wyszecki G, Stiles WS (1982) Color science, concepts and methods, quantitative data and formulae, 2nd edn. Wiley, New York
Acknowledgments
We thank Timothy Goldsmith for sharing tables of carotenoid spectra and John Ewer, Juan Bacigalupo for commenting on previous versions of the present article. We would like also to thank two anonymous reviewers for their helpful comments. Luis Felipe Berninzon identified the hummingbird-visited flowers. Servicio Agricola Ganadero (SAG) permission #1392 for animal captures. Animal care and experimental procedures complied with international regulations (NIH publications No 80–23). Funding by Fondecyt #1000396 (AGP) and #3080048 (JCZ), PBCT-CONICYT ACT45 to AGP and FONDAP 1501.0001 (Program 1) to FB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrera, G., Zagal, J.C., Diaz, M. et al. Spectral sensitivities of photoreceptors and their role in colour discrimination in the green-backed firecrown hummingbird (Sephanoides sephaniodes). J Comp Physiol A 194, 785–794 (2008). https://doi.org/10.1007/s00359-008-0349-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-008-0349-8