Abstract
When confronted with predators, animals are forced to take crucial decisions such as the timing and manner of escape. In the case of the crab Chasmagnathus, cumulative evidence suggests that the escape response to a visual danger stimulus (VDS) can be accounted for by the response of a group of lobula giant (LG) neurons. To further investigate this hypothesis, we examined the relationship between behavioral and neuronal activities within a variety of experimental conditions that affected the level of escape. The intensity of the escape response to VDS was influenced by seasonal variations, changes in stimulus features, and whether the crab perceived stimuli monocularly or binocularly. These experimental conditions consistently affected the response of LG neurons in a way that closely matched the effects observed at the behavioral level. In other words, the intensity of the stimulus-elicited spike activity of LG neurons faithfully reflected the intensity of the escape response. These results support the idea that the LG neurons from the lobula of crabs are deeply involved in the decision for escaping from VDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Simple behaviors provide us with an opportunity to observe the relationship between neuronal activity and animal behavior. Due to the simplicity and reliability of the underlying circuitry, predator avoidance responses are attractive paradigms for studying the neural control of behavior (Simmons and Young 1999). The lateral giant system of the crayfish and the Mauthner neuron system of fishes are among the best-understood behavioral circuits, both in terms of functional anatomy and neural computation (e.g. Edwards et al. 1999; Korn and Faber 2005). These circuits are involved in detecting a mechanical disturbance and triggering an escape reflex, which must initiate quickly (within few millisecond) and reliably without the luxury of elaborate decision-making. In contrast, vision allows the detection of impending threats at greater distances, giving the animal enough time to assess the situation and decide upon a defensive strategy. Some animals, including crabs, exhibit a variety of responses to dangerous visual stimuli such as running away, freezing, or preparing to fight. When animals choose to escape, further decisions must be made regarding the timing and direction of the escape (Hemmi 2005; Oliva et al. 2007). Thus, while still rapidly executed, visually elicited defensive responses usually involve decision-making processes that can hardly occur at the level of a single command neuron.
Semiterrestrial crabs possess a highly developed visual system and display conspicuous visually guided behaviors (e.g. Cannicci et al. 2000; Zeil and Hemmi 2006). Their escape reaction to visual stimuli has been studied extensively in the field (Nalbach 1990; Land and Layne 1995a, b; Hemmi and Zeil 2003; Jennions et al. 2003). Furthermore, stable intracellular recordings can be made in the intact, awake animal (e.g. Tomsic et al. 2003). For these reasons, semiterrestrial crabs offer good opportunities for studying the representation of biologically meaningful visual stimuli at the neurophysiological level (e.g. Johnson et al. 2002; Oliva et al. 2007).
For many years, our laboratory has studied learning-induced changes of the escape response to a visual stimulus in the crab Chasmagnathus. This learning consists of a reduction of the escape response caused by repeated presentations of a visual danger stimulus (VDS). The memory formed is determined by a long-lasting (days) association between the moving stimulus and the context (Tomsic et al. 1998; Pedreira et al. 2002, 2004; Pedreira and Maldonado 2003). Studies have been conducted using chiefly behavioral, pharmacological, and molecular approaches (e.g. Freudenthal and Romano 2000; Pedreira and Maldonado 2003; Merlo and Romano 2007). More recently, we have begun studying neurons from the optic lobe of the crab, which are responsive to VDS that trigger escape behavior (Berón de Astrada and Tomsic 2002; Tomsic 2002). In previous publications, we termed this class of neurons as movement detector neurons, but, for a number of reasons, we have recently renamed them lobula giant (LG) neurons (Medan et al. 2007).
Early studies with Chasmagnathus recognized the existence of more than one type of LG neuron (Berón de Astrada and Tomsic 2002). However, all of them proved to share a common set of features. Morphologically, they have large dendritic arborisations that extend tangentially across the retinotopic mosaic of the lobula. The soma is located beneath the lobula and the axon projects to the midbrain through the protocerebral tract (Berón de Astrada and Tomsic 2002; Sztarker et al. 2005; Medan et al. 2007). Physiologically, LGs are much more responsive to motion stimulation than to stationary changes in light intensity (Berón de Astrada and Tomsic 2002). The firing profile of these cells corresponds well with that of the escape response. The activity of LGs decreases in response to repeated stimulation with a VDS, correlating closely with the diminished behavioral response (Tomsic 2002; Tomsic et al. 2003). These data strongly suggest that LGs play a decisive role in shaping the escape response to visual stimuli. To further explore this notion, we decided to investigate the correspondence between the neuronal and the behavioral responses over a range of very different experimental conditions such as seasonal variations, stimulus differences, and perceptual alterations (monocular or binocular stimulus perception). For every condition we tested, the intensity of the escape consistently matched the level of VDS-elicited activity in LG neurons, thus providing further evidence that LG neurons are part of the decision-making process, to escape from visual danger, in the crab.
Materials and methods
Animals
We used adult male Chasmagnathus granulatus crabs measuring 2.7–3.0 cm across the carapace, weighing ∼17.0 g, collected from the rías (narrow coastal inlets) of San Clemente del Tuyú, Argentina. Animals were transported to the laboratory, where they were lodged in plastic tanks (35 × 48 × 27 cm) at a density of 20 crabs per tank. The tanks were filled upto 2 cm depth with diluted seawater. Water used in tanks and other containers during the experiments was prepared using hw-Marinex (Winex, Hamburg, Germany), salinity 10–14‰, at a pH of 7.4–7.6, and maintained within a range of 22–24°C. The holding and experimental rooms were maintained on a 12 h light/dark cycle (lights on 7:00 a.m.–7:00 p.m.). To prevent the animals from being exposed to stimuli comparable to the VDS prior to the experiment, the tanks were kept in isolation from visual disturbances. Experiments were carried out year-round, between 8:00 a.m. and 7:00 p.m. everyday, beginning 3–4 days following the animals’ arrival in the laboratory and until 2 weeks later. Each crab was used in only one experiment. Experimental procedures are in compliance with the Principles of Animal Care of Laboratory Animals published by the National Institutes of Health.
Visual stimuli
The characteristics of visual stimulation were the same for both behavioral and electrophysiological experiments. Background illumination was provided by a lamp oriented to the roof of the setup, which reached the eye of the animal with an intensity of 200 mW/m2. The decrement in luminance registered at the eye level during the passage of the stimulus was about 35%. The standard VDS used in our laboratory consists of the horizontal displacement of a black rectangular screen above the crab (subtended angles: 10 × 35°), which is driven from one end by a motor at an angular velocity of 82°/s (Fig. 1). In experiment 2, we also used a second VDS with a larger angular size (21 × 43°), which was called the VDS–LAS (large apparent size). For both stimuli the motion cycle, which consisted of a 90° clockwise and anti-clockwise movement, from the starting position and back, was completed in 2.2 s. Each recording trial lasted 9 s, with two cycles separated by 2 s. Thus, the passing screen entered the crab’s visual field twice, from two opposite sides, which ensures a reliable measurement of the crab response during each trial. This moving stimulus evokes a strong directional escape reaction that has been previously characterized by video record analysis (Pereyra et al. 1999, 2000) and has been extensively used in our laboratory to study the modification of the escape response of Chasmagnathus by learning (e.g. Lozada et al. 1990; Tomsic et al. 1993, 1998, 2003; Pedreira et al. 2002, 2004).
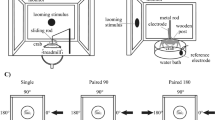
Experimental setups and recording procedures. a Behavioral experiments were conducted in an actometer that translated crab movement into voltage changes. b For intracellular recording, the crab was held in an adjustable clamp. The eyestalks were cemented to the carapace, and a glass microelectrode was advanced through a small hole in the cuticle made at the tip of one eye. c Representative recording (in arbitrary units) of the escape response to the VDS during a trial (two screen motion cycles). d Representative recording of the response of a lobula giant (LG) neuron to the same stimulation. In c and d, the two topsides of the triangles below the traces represent the clockwise and anti-clockwise movements of the stimulus contained in a cycle, and the base of the triangle represents 2.2 s of record
Behavioral recording
The setup used for recording the crab’s behavioral activity has been described previously (Tomsic et al. 2003). Briefly, experiments were conducted in an actometer (Fig. 1a) consisting of a bowl-shaped container with a steep concave wall 12 cm high (23 cm top diameter and 9 cm floor diameter) covered to a depth of 0.5 cm with seawater. The container was connected to a transducer device such that locomotion by the crab inside the container was translated into voltage changes. These signals are proportional to the velocity and number of steps given by each crab during the trial. Thus, when an animal runs during more time or to a higher speed it gives a stronger signal. Voltage signals were then digitized and recorded in a computer (see below).
Electrophysiological recording
Electrophysiological experiments were performed inside a Faraday cage completely covered to prevent outside visual stimuli from reaching the animal. The ceiling and walls of the cage were painted uniformly white. The surface of the vibration-damped table (TMC, Peabody, MA., USA) was painted black. Intracellular recordings from the optic lobe were performed in the awake animal as described by Berón de Astrada et al. (2001). Briefly, the crab was firmly held in an adjustable clamp, which allowed free movements of the walking legs but reduced movements of the chelae (Fig. 1b). The eyestalks were cemented to the carapace at an angle of (∼70° from horizontal, which corresponds to their normal seeing position. A tangential cut performed with a sharp scalpel was made to remove a small piece of thin cuticle (about 500 μm in diameter) from the tip of the eyestalk, without causing damage to the ommatidial area. The glass microelectrode was then positioned and advanced through the opening in the cuticle. Microelectrodes (borosilicate glass, 1.2 mm outer diameter, 0.68 mm inner diameter), were pulled on a Brown–Flaming micropipette puller (P-77, Sutter Instrument, Novato, CA) yielding tip resistances of 40–60 MΩ when filled with 3 M KCl. A bridge-balance amplifier was used for intracellular recordings (Axoclamp 2B, Axon Instruments, Foster City, CA). The output of the amplifier was monitored on an analogue oscilloscope, digitized at 1.5 kHz (Digidata 1200, Axon Instruments, Foster City, CA) and recorded in a computer for subsequent analysis. Experiments were all performed at the membrane resting potential.
Experimental protocols
Both behavioral and electrophysiological experiments began after the animal remained visually undisturbed for 10 min in the setup. In electrophysiological experiments, an impaled cell was easily identified as an LG neuron by its differential responses to motion versus a pulse of light (Berón de Astrada and Tomsic 2002). Intracellular dye injections proved this a highly reliable method for the identification of LG neurons (Medan et al. 2007). Neurons were usually impaled within a few minutes of probing. A black curtain was then lowered in the front part of the cage and the adaptation period began. Because we were interested in LGs involved with the escape response of Chasmagnathus elicited by environmentally relevant overhead VDS (Tomsic et al. 1993; Layne et al. 1997), we limited our study to those neurons with receptive fields comprising the dorsal portion of the eye. Only one neuron per animal was evaluated.
In the first experiment (experiment 1), we explored seasonal variations in the response of LG neurons and escape behavior to the VDS. Data were collected year-round, between 2002 and 2005, and were pooled into two groups: spring/summer corresponding to data obtained between October and March; and fall/winter corresponding to data obtained between April and September (note that the experiments were carried out in the Southern hemisphere).
Experiment 2 was aimed at investigating the effect of stimulus apparent size on LG activity and escape behavior using the standard VDS and the VDS–LAS described above. We also assessed the effect of stimulus apparent size on habituation at both the behavioral and neuronal responses. Habituation was produced by repeated VDS stimulation, which consisted of 15 trials separated by inter-trial intervals of 3 min (i.e., a 45 min training session).
The purpose of experiment 3 was to compare the behavioral and neural responses of animals exposed to the VDS binocularly or monocularly. To test the escape response in monocular crabs, one of the eyes was covered with black paint a day prior to the experiment. Early studies proved that this method effectively precludes vision since crabs with the two eyes painted did not react to visual stimulation. When the paint was removed, animals recovered normal visual responses (Sztarker 2000). In half of the animals, the left eye was painted, while in the remaining half it was the right eye. The response of LG neurons to monocular stimulation was evaluated using the method described by Sztarker and Tomsic (2004). Briefly, two identical VDS–LAS, one on each side of a partition that separated the visual field of both eyes, were used to stimulate the eyes independently. Recordings were always taken from the optic lobe in the right eyestalk. Half of the recordings were made upon stimulating the right eye and the balance using the left eye. We found no differences between right and left stimulation, so the data were pooled together to form the monocular group. Binocular stimulation was assessed in the absence of the partition, using one or the other VDS.
Behavioral and electrophysiological data were collected almost simultaneously year-round during the daylight (8:00 a.m.–7:00 p.m.). Additionally, data in experiment 2 were obtained by recording a similar number of animals tested with the standard VDS or the VDS–LAS each day. A similar procedure was followed to obtain the data of experiment 3. Thus, comparisons within groups of experiments 2 and 3 were controlled for seasonal or circadian influences.
Data analysis
The stimulus control and the data recording system used in behavioral and electrophysiological experiments were identical, allowing us to effectively compare between both types of responses. The escape response was transduced and recorded as a train of voltage changes, with peaks corresponding to running steps of the escape reaction. Figure 1c, d respectively, show examples of an escape response and of a response of an LG neuron to a first presentation with the VDS. The behavioral response in a trial was estimated by integrating the area beneath the voltage deflections recorded during the periods of visual motion stimulation (cycle 1 + 2). Likewise, the neuronal response in a trial was estimated by the number of spikes recorded during the stimulation periods (cycle 1 + 2). On repeated trial experiments, successive responses were normalized to the first response of the series. Error bars in the figures are s.e.m.s; asterisks indicate level of significance (*P < 0.05, **P < 0.01, ***P < 0.005, two-sample t-test, two sides p-value).
Results
Seasonal effects on the escape and LG responses to VDS
Years of experience working with Chasmagnathus indicate that the escape response to visual stimuli varies with seasons, being stronger during summer than during winter months. However, the seasonal variation has never been systematically studied. To confirm this impression, we analyzed responses to the standard VDS collected in the lab year-round between 2002 and 2005. Results show that the intensity of escape responses recorded during the spring/summer period were significantly higher than in the fall/winter period (Fig. 2a). Similarly, the response of LG neurons was significantly more intense during spring/summer than in fall/winter (Fig. 2b). Thus, the higher intensity of escape to visual stimuli during the spring/summer period correlates with the stronger response evoked in LG neurons.
Seasonal changes in the behavioral and neuronal response to the VDS. Animal escape and neuronal (LG) responses to a single VDS presentation were collected year-round between 2002 and 2005. Only one behavioral or neuronal response per animal was taken into account. Data were separated into two groups: the spring/summer and the fall/winter group. a Escape response. The behavioral response in a trial was estimated by integrating the area beneath the voltage deflections recorded during the periods of visual motion stimulation for each animal. Y axis represents the mean response score in arbitrary units (see “Methods”). b Response of lobula giant (LG) neurons. The neuronal response in a trial was estimated as the number of spikes recorded during the periods of stimulation. Y axis represents the mean number of action potentials (a.p.). In this and following figures results are showed as mean ± SEM *P < 0.05, **P < 0.01, ***P < 0.005
For a number of reasons, the behavioral changes cannot be attributed to a general reduction in activity caused by cold weather. First, the experiments were carried out after the animal had spent at least 3–4 days in laboratory environment with controlled temperature and light cycles. In addition, the changes in reactivity are specific to visual danger stimulation since no seasonal changes in behavior are apparent when the escape is elicited by a mild electrical shock (D. Tomsic, unpublished observations; Rakitin et al. 1991). Considering LG neurons, the circannual difference in the response was not related to changes in the membrane resting potential of the cells (spring/summer group = −58.0 ± 1.1 mV; n = 73 vs. fall/winter group = −57.6 ± 1.1 mV; n = 91).
Effects of intensity and repetition of VDS on the escape and LG responses
In a study aimed at characterizing the stimulus attributes used by crabs to visually identify a predator, Layne et al. (1997) demonstrated that absolute object size is not important, and that the “scariness” of an object depends on angular size, i.e., the amount of retina that it covers. Here, we evaluated the effect of stimulus apparent size on both the behavioral and neuronal response. Figure 3a shows that the VDS–LAS (see “Methods”), elicited more robust escape responses than the standard VDS. This effect was coincident with the responses of LG neurons, which showed a larger number of evoked spikes to the VDS–LAS (Fig. 3b).
Behavioral and neuronal responses to VDS with different apparent size. The standard VDS ordinarily used in our laboratory (see Fig. 1 and “Methods”) subtends 35° (longer axis) at the crab’s eye position. The other stimulus subtended a larger angle (43°) and was called VDS–LAS (large apparent size). Both stimuli rotated 90° back and forth at the same angular velocity. a and b correspond respectively to the mean behavioral and neuronal responses to the first presentation of the VDS. c and d correspond, respectively, to the mean behavioral and neuronal responses per trial. The session consisted of 15 trials separated every 3 min. Data were individually normalized to the value at the first trial. Further details as in Fig. 2
Repeated stimulation often results in habituation to a specific stimulus. In previous studies, we have shown that changes in the frequency of stimulation affected the rate of habituation of both the behavioral and LG responses in a remarkably similar manner (Tomsic et al. 2003). The rate of habituation is also known to vary according to the intensity of the stimulus; the more intense the stimulus, the weaker the habituation rate (e.g. Thompson and Spencer 1966; Groves et al. 1969). According to this prediction, the VDS that provoked the larger escape response of the crabs (VDS–LAS), would have a slower rate of habituation. Our results confirm this prediction. As illustrated in Fig. 3c, 15 training trials with the standard VDS caused a faster and deeper reduction of the escape response than a similar amount of training with the VDS–LAS. More importantly, the dynamics of these response changes were reflected by the changes in the response of LG neurons (Fig. 3d).
Effect of monocular and binocular perception of the VDS on the escape and LG responses
Previous studies in Chasmagnathus have shown that monocularly deprived crabs exhibit reduced escape response to the VDS (Hermitte et al. 1995). We have also shown that LG neurons are all binocular. Moreover, the responses of LGs to a VDS presented to the eye corresponding to the recording site (ipsilateral eye) are almost identical to the responses obtained by presenting the stimulus to the opposite eye (contralateral eye) (Sztarker and Tomsic 2004). In the present experiment, we wanted to investigate whether the reduction in the escape intensity of monocular crabs can be related to a reduction in the strength of response of LG neurons. We measured both the behavioral escape and the LG response in animals that were allowed to perceive the VDS with one or both eyes (see “Methods”). Animals were deprived of sight in either the left or the right eye. We found no differences between the two conditions, and then we pooled these data. Monocularly deprived crabs displayed a significantly weaker response than animals with binocular vision (Fig. 4a). More relevant, the reduction in escape intensity of monocularly deprived crabs was accompanied by a reduction in the number of LG spikes (Fig. 4b).
Behavioral and neuronal responses to binocular or monocular VDS stimulation. In behavioral experiments, monocular stimulation was obtained by covering one of the crab’s eye (either right or left) with black paint. In electrophysiological experiments, monocular stimulation was obtained by separating the visual field between the eyes, and by presenting the VDS either to the eye in the recording side (ipsilateral side) or to the contralateral eye (LGs are binocular neurons). Because there were no differences between results obtained by left or right eye stimulation, the data were pooled. Only one behavioral or neuronal response per animal was considered. a Escape response. b Response of lobula giant (LG) neurons. Other details as in Fig. 2
Discussion
We assessed the correspondence between behavioral and neuronal responses to VDS under widely different experimental conditions. Throughout the experiments the strength of activity in the LG neurons correlated with the intensity of the escape response. Thus, the escape behavior appears to be encoded largely in the response of the LGs.
Neuronal correlates with seasonal changes of the escape response
A wide variety of behaviors, including courtship, feeding activity, and defensive reactions, are affected by seasonal variations. Some season-dependent changes in behavior are known to correspond with changes in the nervous system. A good example of this is the neurogenesis associated with the mating season in songbirds (e.g. Nottebohm 1989; Alvarez-Buylla and Kirn 1997). However, there are few examples of seasonal changes in behavior that have been traced to functional changes in individual neurons. In toads, seasonal variations in prey-catching behavior are related to changes in the responsiveness of particular classes of ganglion and tectal neurons involved in the detection of prey (Ewert and Siefert 1974a, b). During winter, when the capture behavior declines compared to other seasons, the number of stimulus-elicited spikes in tectal T5-2 neurons is reduced. The advantage of this type of neuronal modulation could be in the saving of energy by silencing unneeded parts of the neural network (Ewert and Siefert 1974a, b; Ewert 1980).
In the crab, the escape response to the VDS is significantly weaker during colder months (Fig. 2). There are several reasons why escape behavior might change seasonally. In winter, the metabolic rate of Chasmagnathus reaches a minimum, as measured by oxygen consumption (Rosas et al. 1992). Muscle lipids, rather than glycogen, become the main energy substrate (Kucharski and Da Silva 1991). Also, crabs tend to remain for longer periods in their burrows (D’Incao et al. 1988). Accordingly, the reduction in the escape response can be explained in terms of a physiological adaptation to save energy. Whatever the reason may be, the seasonal changes that persist after an adaptation period of several days in the laboratory appear to be rather specific for visual stimulation. No such changes were observed in response to other type of stimuli, such as a mild electric shock (D. Tomsic, unpublished observations; Rakitin et al. 1991).
Virtually nothing is known about seasonal changes of visual sensitivity in invertebrates (Fleissner and Fleissner 2006). This lack of knowledge starkly contrasts with the large amount of studies on circadian changes in the visual system. Evidence of a daily rhythm has been found in the electroretinogram of the crayfish and also in visual interneurons as sustaining fibers in which light responses vary in a circadian manner (Aréchiga and Wiersma 1969; for a review see Aréchiga and Rodríguez-Sosa 2002). However, to our knowledge the variation in the response of LG neurons represents the first account of seasonal changes in visual sensitivity recorded at the neuronal level in crustaceans (see below).
There are several known light- and clock-driven mechanisms that lead to diurnal changes in visual sensitivity in crustaceans. The migration of accessory retinal pigments (in distal pigment cells, reflecting cells and also inside their retinula cells), the effective size of the rhabdom and the quantity of opsins are few among them (Aréchiga and Rodríguez-Sosa 2002; Fleissner and Fleissner 2006). Some of these mechanisms might also be involved in the seasonal changes of the visual responses described in the present paper. In fact, a seasonal rhythm of the red pigment concentrating-hormone has been described in the crayfish (Rodríguez-Sosa et al. 1997). Although the study did not evaluate visual sensitivity, this hormone is known to control the migration of pigments in the retina.
The mechanisms described above would produce general adjustments in the visual sensitivity. However, as in the prey-catching behavior of the toad described above, the seasonal change of behavior found in the crab could be accounted for a modulation on the activity of a particular group of higher order neurons. In such a case, the reduction of the LGs and the escape response could be an adaptation related to the lower risk of avian predation experienced by Chasmagnathus during the winter months (Bachmann and Martinez 1999). Whether the seasonal effect is circumscribed to visually evoked escape behaviors (i.e., to stimuli signaling danger) or to a general change resulting from variations in the visual sensitivity, remains to be explored, for instance, by evaluating the optomotor response.
Neuronal correlates with stimulus changes of the escape response
The intensity with which a behavioral defensive response is evoked depends on the perceived threat of the stimulus. The concept of the stimulus releasing value was introduced by Tinbergen in his classical investigations using dummies (e.g. Tinbergen and Perdeck 1950; Tinbergen 1951). The study of stimulus releasing values provides a framework for understanding the features of a stimulus that are most relevant to the animal, and a method for investigating the sensitivity of particular neurons to those features. The strategy for identifying neurons involved in the prey-catching behavior of the toad was to look for elements that were sensitive to stimulus changes that also affected the behavioral response. Using this technique, Ewert et al. (1978) found that the response of a group of tectal (T5-2) neurons correlated with the strength of prey-catching behavior. This correlation implicated T5-2 neurons as central elements in the prey recognition and catching behavior of toads (reviewed in Ewert 1980, 1987, 1997).
Experiments in crabs, varying the size and distance of a VDS, have shown that it is the angular and not the absolute size that determines the intensity of the escape response (Layne et al. 1997). Here, we found a correlation between escape intensity and LG spike activity based on variations in the angular size of the stimulus (Fig. 3a, b). Moreover, as predicted from the habituation theory, repetitions with the more intense (effective) stimulus resulted in a rate of habituation that was much slower than that produced by the weaker stimulus (Fig. 3c). This differential rate of habituation was reflected in the rate of change of LG neurons (Fig. 3d). These results suggest that the releasing values of the VDS are encoded in the strength of response of LG neurons.
Neuronal correlates of escape responses elicited by binocular or monocular VDS perception
In a previous study, we found that all the LG neurons recorded from the lobula of the crab possess a binocular visual field. Surprisingly enough, the LG responses obtained by presenting the VDS separately to the ipsilateral side (the recording side) or to the contralateral side were almost identical, although ipsilateral responses occurred 25 milliseconds earlier. Moreover, we found that the habituation curves from repeated VDS presentation in the ipsilateral and contralateral sides were identical. These results conclusively show that, at least in the dorsal visual field (so far the only tested area), LG neurons fully integrate binocular information (Sztarker and Tomsic 2004). Behavioral experiments from this paper show that the escape response to the VDS elicited in monocular subjects is approximately 50% weaker than in binocular animals (Fig. 4a), while electrophysiological experiments with LG neurons in response to monocular or binocular VDS stimulation also exhibit this effect (Fig. 4b). These results indicate that information from both eyes is combined to produce the behavioral binocular response, and that this integration takes place in LG neurons. Therefore, differences in the level of escape responses caused by perceptual alterations (in this case, binocular or monocular sight) can be accounted for by differences in the response of LG neurons.
Are there other interneurons related to visually-elicited defensive responses in crustaceans?
The results discussed thus far emphasize the importance of the LG neurons in the escape behavior to visual stimuli in crabs. But what about other classes of large neurons connecting the optic lobe with the midbrain? Could their activity also reflect the escape performance? In addition to the LGs, we have previously investigated several classes of large neurons from the crab’s optic lobe, such as sustaining fibers, dimming fibers, and others (Berón de Astrada et al. 2001; Sztarker and Tomsic 2004), none of which met the criteria to be classified as integral to the escape behavior. For instance, while the escape response declines upon repeated VDS presentations, the responses of sustaining and dimming neurons remain unchanged (Tomsic et al. 2003). In contrast, peristimulus time histograms of LG responses to a single VDS trial show that the firing profile of these cells corresponds well with that of the behavioral reaction (Tomsic et al. 2003; Oliva et al. 2007). Upon repeated stimulus presentations, the rate of decline of LG responses closely matches the habituation profiles obtained with different frequency or intensity of stimulation (Tomsic et al. 2003 and results of experiment 2 in this paper). Furthermore, learning-induced long-term changes in LGs reflect the persistent memory-mediated reduction of the escape response (Tomsic et al. 2003). Finally, LG neurons, but not sustaining, dimming or other classes of neurons so far studied in the optic lobe, integrate binocular information (Sztarker and Tomsic 2004) in a way that reflects the performance of escaping (experiment 3 of this paper). These characteristics distinguish LG neurons as central elements, where decisions regarding escape from visual danger are likely made. Still, there is an important caveat for this assumption. Although intracellular staining revealed that the axon of all LG neurons exits the optic lobe to the midbrain (Medan et al. 2007), the actual connection with the motor output is still unknown. However, the neuronal reaction anticipates the behavioral response by about 120 milliseconds (Tomsic et al. 2003; Oliva et al. 2007), a delay similar to that found in the locust between the reaction of the lobula giant movement detector neurons (LGMD) and the avoidance response to visual stimuli. The spikes of each LGMD are transmitted faithfully to a second identified neuron, the descending contralateral movement detector (DCMD), which transmits these spikes to the thoracic motor centers (Santer et al. 2006). Considering the homologies between the visual nervous system of insects and crustaceans (Strausfeld 2005; Sztarker et al. 2005), as well as the similarities found in the response of LG neurons of the crab and the LGMD neurons of the locust (Oliva et al. 2007), these systems may be wired similarly. If so, LGs of the crab would make contact with descending neurons that activate the motor centers.
LG neurons and their role in decision-making processes
To cope with visually dangerous situations crabs display different defensive strategies such as running away, freezing, or preparing to fight. These alternative responses involve decision-making processes based on complex information about the stimulus movement respect to the crab (e.g. size, speed, direction, etc.). It is unlikely that all this information is processed in a single neuron, nor is it likely that the system contains a limitless number of neurons, each specifically tuned to a unique stimulus. A more parsimonious manner of classifying the stimulus and translating the information into adaptive motor responses would be to use sets of neurons with different feature sensitivities, which through distinct combinations of their relative activation may encode separate motor programs (Ewert 1987, 1997). A recent study using a variety of different smaller stimuli revealed that the group of LGs is composed of various subtypes of neurons with different motion sensitivity (Medan et al. 2007). However, all subtypes show strong responses to the VDS used here. Therefore, the escape performance appears to be explained by the collective contribution of the entire assembly of LG neurons, rather than by the activity of a particular class.
References
Alvarez-Buylla A, Kirn JR (1997) Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol 33:585–601
Aréchiga H, Rodríguez-Sosa L (2002) Distributed circadian rhythmicity in the crustacean nervous system. In: Wiese K (Ed) The crustacean nervous system. Springer, Berlin, pp 113–122
Aréchiga H, Wiersma AG (1969) Circadian rhythm of responsiveness in crayfish visual units. J Neurobiol 1:71–85
Bachmann S, Martinez MM (1999) Feeding tactics of the american oystercatcher (Haematopus palliatus) on Mar Chiquita coastal lagoon, Argentina. Ornit Neurotr 10:81–84
Berón de Astrada M, Sztarker J, Tomsic D (2001) Visual interneurons of the crab Chasmagnathus studied by intracellular recordings in vivo. J Comp Physiol A 187:37–44
Berón de Astrada M, Tomsic D (2002) Physiology and morphology of visual movement detector neurons in a crab (Decapoda: Brachyura). J Comp Physiol A 188:539–551
Cannicci S, Barelli C, Vannini M (2000) Homing in the swimming crab Thalamita crenata: a mechanism based on underwater landmark memory. Anim Behav 60:203–210
D’Incao F, Ruffino ML, Silva KG (1988) In: Notas preliminares sobre a ecologia de Chasmagnathus granulata (Dana, 1851) na barra de Rio Grande (Decapoda, Grapsidea) resumos XV congressos Brasileiros de zoologia, Curitiva, p 93
Edwards DH, Heitler WJ, Krasne FB (1999) Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci 22:153–161
Ewert JP (1980) Neuroethology: an introduction to the neurophysiological fundamentals of behavior. Springer
Ewert JP (1987) Neuroethology of the releasing mechanism: prey-catching behavior in toads. Behav Brain Sci 10:337–405
Ewert JP (1997) Neural correlates of key stimulus and releasing mechanism: a case study and two concepts. Trends Neurosci 230:332–339
Ewert JP, Siefert G (1974a) Neuronal correlates of seasonal changes in contrast-detection of pray catching behavior in toads (Bufo bufo L.). Vision Res 14:431–432
Ewert JP, Siefert G (1974b) Seasonal change of contrast detection in the toad’s Bufo bufo (L.) visual system. J Comp Physiol A 94:177–186
Ewert JP, Borchers HW, Wietersheim A (1978) Question of prey feature detectors in the toad’s Bufo bufo (L.) visual system: a correlation analysis. J Comp Physiol A 126:43–47
Fleissner G, Fleissner G (2006) Endogenous control of visual adaptation in invertebrates. In: Warrant E, Nilson D-E (Eds) Invertebrate vision. Cambridge University Press, Cambridge, pp 127–166
Freudenthal R, Romano A (2000) Participation of Rel/NF-kappaB transcription factors in long-term memory in the crab Chasmagnathus. Brain Res 855:274–281
Groves PM, Lee D, Thompson RF (1969) Effects of stimulus frequency and intensity on habituation and sensitization in acute spinal cat. Physiol Behav 4:383–388
Hemmi JM, Zeil J (2003) Robust judgment of inter-object distance by an arthropod. Nature 421:160–163
Hemmi JM (2005) Predator avoidance in fiddler crabs: 1. Escape decisions in relation to the risk of predation. Anim Behav 69:603–614
Hermitte G, Aggio J, Maldonado H (1995) Failure of interocular transfer in two types of learning in the crab Chasmagnathus. J Comp Physiol A 177:371–378
Jennions MD, Backwell PR, Murai M, Christy JH (2003) Hiding behavior in fiddler crabs: how long should prey hide in response to a potential predator? Anim Behav 66:251–257
Johnson AP, Horseman BG, Macauley MW, Barnes WJ (2002) PC-based visual stimuli for behavioural and electrophysiological studies of optic flow field detection. J Neurosci Methods 114:51–61
Korn H, Faber DS (2005) The Mauthner cell half a century later: a neurobiological model for decision-making? Neuron 47:13–28
Kucharski LCR, Da Silva RSM (1991) Effect of diet composition on carbohydrate and lipid metabolism in an estuarian crab, C. granulata (Dana, 1851). Comp Biochem Physiol 99:215–218
Land MF, Layne J (1995a) The visual control of behaviour in fiddler crabs: I. Resolution, thresholds and the role of the horizon. J Comp Physiol A 177:81–90
Land MF, Layne J (1995b) The visual control of behavior in fiddler crabs: II. Tracking control systems in courtship and defense. J Comp Physiol A 177:91–103
Layne J, Land M, Zeil J (1997) Fiddler crabs use the visual horizon to distinguish predators from conspecifics: a review of the evidence. J Mar Biol Ass UK 77:43–54
Lozada M, Romano A, Maldonado H (1990) Long term habituation to a danger stimulus in the crab Chasmagnathus granulatus. Phys Behav 47:35–41
Medan V, Oliva D, Tomsic D (2007) Characterization of lobula giant neurons responsive to visual stimuli that elicit escape behaviors in the crab Chasmagnathus. J Neurophysiol 98:2414–2428
Merlo E, Romano A (2007) Long-term memory consolidation depends on proteasome activity in the crab Chasmagnathus. Neurosci 147:46–52
Nalbach HO (1990) Discontinuous turning reaction during escape in soldier crabs. J Exp Biol 148:483–487
Nottebohm F (1989) From bird song to neurogenesis. Sci Am 260:74–79
Oliva D, Medan V, Tomsic D (2007) Escape behavior and neuronal responses to looming stimuli in the crab Chasmagnathus granulatus (Decapoda: Grapsidae). J Exp Biol 210:865–880
Pedreira ME, Maldonado H (2003) Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron 38:863–869
Pedreira ME, Pérez-Cuesta L, Maldonado H (2002) Reactivation and reconsolidation of long-term memory in the crab Chasmagnathus: protein synthesis requirement and mediation by NMDA-type glutamatergic receptors. J Neurosci 22:8305–8311
Pedreira ME, Pérez-Cuesta L, Maldonado H (2004) Mismatch between what is expected and what actually occurs triggers memory reconsolidation or extinction. Learn Mem 11:579–585
Pereyra P, González Portino E, Maldonado H (2000) Long-lasting and context-specific freezing preference is acquired after spaced repeated presentations of a danger stimulus in the crab Chasmagnathus. Neurobiol Learn Mem 74:119–134
Pereyra P, Saraco M, Maldonado H (1999) Decreased response or alternative defensive strategies in escape: two different types of long-term memory in the crab Chasmagnathus. J Comp Physiol A 184:301–310
Rakitin A, Tomsic D, Maldonado H (1991) Habituation and sensitization to an electrical shock in the crab Chasmagnathus. Effect of background illumination. Phys Behav 50:477–487
Rodríguez-Sosa L, De la Vega MT, Vergara P, Aréchiga H (1997) Seasonal rhythm of red pigment-concentrating hormone in the crayfish. Chronobiol Int 14:639–645
Rosas C, Sanchez A, Escobar E, Soto L, Bolongaro-Crevenna A (1992) Daily variations of oxygen consumption and glucose hemolymph level related to morphophysiological and ecological adaptations of crustacea. Comp Biochem Physiol 101:323–328
Santer RD, Rind FC, Stafford R, Simmons PJ (2006) Role of an identified looming-sensitive neuron in triggering a flying locust’s escape. J Neurophysiol 95:3391–3400
Simmons P, Young D (1999) Nerve cells and animal behavior, (2nd Ed). Cambridge University Press
Strausfeld NJ (2005) Evolution of crustacean optic lobes and origins of chiasmata. Arthropod Struct Dev 34:235–56
Sztarker J (2000) Interneuronas monoculares y binoculares: indicios funcionales de la organización circuital del sistema visual en el cangrejo Chasmagnathus. Licenciate thesis, Universidad de Buenos Aires
Sztarker J, Strausfeld NJ, Tomsic D (2005) Organization of optic lobes that support motion detection in a semiterrestrial crab. J Comp Neurol 493:396–411
Sztarker J, Tomsic D (2004) Binocular visual integration in the crustacean nervous system. J Comp Physiol A 190:951–962
Thompson RF, Spencer WA (1966) Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73:16–43
Tinbergen N (1951) The study of instinct. Clarendon Press, Oxford
Tinbergen N, Perdeck AC (1950) On the stimulus situation releasing the begging responses in the newly hatched Herring Gull chick (Larus argentatus Pont.). Behaviour 3:1–38
Tomsic D (2002) Visual learning in crabs investigated by intracellular recordings in vivo. In: Wiese K (ed) The crustacean nervous system. Springer, Berlin, pp 328–335
Tomsic D, Berón de Astrada M, Sztarker J (2003) Identification of individual neurons reflecting short- and long-term visual memory in an arthropod. J Neurosci 23:8539–8546
Tomsic D, Massoni V, Maldonado H (1993) Habituation to a danger stimulus in two semiterrestrial crabs. Ontogenic, ecological and opioid system correlates. J Comp Physiol A 173:621–633
Tomsic D, Pedreira ME, Romano A, Hermitte G, Maldonado H (1998) Context-US association as a determinant of long-term habituation in the crab Chasmagnathus. Ani Learn Behav 26:196–209
Zeil J, Hemmi JM (2006) The visual ecology of fiddler crabs. J Comp Physiol A 192:1–25
Acknowledgments
We would like to thank John Tuthill for corrections to this manuscript. This work was supported by postdoctoral fellowships from the National Research Council of Argentina (CONICET) to J.S. and from the following research grants to D.T.: Universidad de Buenos Aires, grant number X 173; ANPCYT, grant number PICT 12300/02.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sztarker, J., Tomsic, D. Neuronal correlates of the visually elicited escape response of the crab Chasmagnathus upon seasonal variations, stimuli changes and perceptual alterations. J Comp Physiol A 194, 587–596 (2008). https://doi.org/10.1007/s00359-008-0333-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-008-0333-3