Abstract
The behavioral responses to attractive and aversive odors were examined in blinded adult male cockroaches under tethered-walking conditions. A sex pheromone-like stimulant derived from adult virgin females and artificially synthesized limonene were used as attractive and aversive odor sources, respectively. When a searching animal was stimulated with the attractive female-derived odor, the horizontal deflections of both the antennae were increased, and in most cases the vertical antennal positions were shifted downward. The stimulation also significantly decreased the walking speed of the animal. These behavioral changes imply a careful search in the immediate surroundings. The aftereffect of the sex pheromone was more pronounced on locomotion than on antennal movement. On the other hand, stimulation with the aversive odor (limonene) tended to suppress active antennal movement, and also increased the walking speed. Immediately after the withdrawal of the aversive odor, the active movement of the antennae was resumed, and the walking speed rapidly decreased to a level approximately the same as that of the control period. These results indicate that the responses to the qualitatively opposite types of odor are reciprocal to each other with regard to both antennal movement and locomotion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Searching is a behavior of animals that are motivated to find beneficial resources such as food, mates, nesting sites, and new habitats (Bell 1991). This behavior, which involves both sensory and locomotor functions, consists of two major components, ranging and local search (Bell 1981, 1991). In ranging, animals have no knowledge regarding the location of the resource objects; therefore, they attempt to cover the maximum possible area. In local search, animals perceive the proximity of an attractant in the absence of any directional information and attempt to explore a limited area. Orientation may occur when they finally locate the direction of the object. Insects are the major subjects for studying searching behavior and the subsequent orientation mainly because of their agricultural importance and neurobiological interest. In particular, odor-mediated orientation in insects has been extensively investigated to date with regard to behaviors such as feeding, host or mate location, and colony formation (Bell and Cardé 1984; Baker 1985).
The antennae of an insect, which are the major chemosensitive appendages located on the head, undoubtedly play a crucial role in odor-mediated searching and orientation behaviors. A striking feature of the insect antenna is that it moves actively to scan the surroundings. Its mobility is assumed to be important for the collection of information regarding both chemical and physical environments. For example, in honeybees, active antennal movement is closely related to tactile discrimination and learning (Erber et al. 1997, 1998; Scheiner et al. 2005). In order to achieve successful searching, the manner in which the antennae should be moved spatiotemporally appears to be an important aspect of insect behavior, particularly in invisible environments. However, active antennal movement has been mainly studied from a tactile perspective (Staudacher et al. 2005), and has only rarely been described with regard to olfaction (e.g., Rust et al. 1976; Olberg 1983; Willis and Avondet 2005).
The purpose of this study was to characterize the manner in which walking nocturnal insects (American cockroaches) actively collect olfactory information by using active antennae and self-locomotion. We used an odor derived from virgin females, which putatively contained a volatile sex pheromone, as an attractive stimulant to adult males. Limonene, an essential oil from citrus peel, was used as an aversive odor source. The effects of these qualitatively opposite types of odor stimuli on the antennal movements of cockroaches were investigated by using three-dimensional motion analyses under tethered-walking conditions. The locomotion of the animal was simultaneously recorded in order to examine the effects of the stimulants on the search trajectories. Some of the results reported here have previously appeared in abstract form (Nishiyama et al. 2005).
Materials and methods
Animals
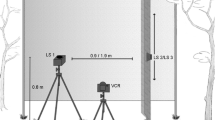
Adult male cockroaches (Periplaneta americana L.) were obtained from a laboratory colony that was maintained at 27°C on a 12:12 LD cycle. Although cockroaches generally exhibit natural search only under dark conditions, it was necessary to conduct the present experiments under light conditions as described below. Therefore, the compound eyes and ocelli of the animals were cauterized in order to eliminate all vision (Okada and Toh 2004). In order to restrain the animal, a thin flexible plastic plate (0.5 × 3 cm) was glued to the pronotum and attached to a clamp stand. Using a fluorescent paint, several spots were marked on the cuticle of both the antennae and the head for the purpose of capturing their motion–two spots were marked on the centers of the ocelli, one on the center of the horizontal boundary between the frons and the clypeus, and two on both the flagella 10 mm from their proximal ends (Fig. 1b). In order to allow for recovery from the operation, the experiments on the treated animals were initiated at least 2 days later.
a Experimental setup. The movements of both the antennae were recorded by a pair of stereo video cameras. The locomotion of the animal was detected by an optical PC mouse that was set on the surface of the Styrofoam sphere and the trajectories of the cursor displayed on a PC screen were recorded by another camera. The odor stimuli were presented from the cut end of a plastic tube placed in front of the animal. b Definition of the antennal position. Fluorescent paint spots are depicted by stars. The origin of the 3D Cartesian coordinates was set at the center of each antennal socket (here, only the right antenna is shown). The plane that included the three head spots (shaded triangle) was parallel to the XZ plane. The horizontal position was defined as the azimuth, and the vertical position was defined as the latitude of the flagellum with respect to the origin (O). c Definition of the parameters for locomotion. The frame-by-frame rotational movement of the sphere (line AB) was decomposed into a linear component of forward translation (line CB) and an angular component of horizontal turn (angle AOC). Symbols (plus and minus) show the polarity of the horizontal turn
Experimental apparatus
The treadmill system used in this study was similar to that described previously (Okada and Toh 2000). Briefly, a cockroach was placed on the top of a freely rotating Styrofoam sphere (diameter, 10 cm) that was floated on an upward air stream; this system enables the animal to walk with minimum load (Fig. 1a).
Odor stimuli were provided as air pulses from a plastic tube (inner diameter, 4 mm) whose cut end was positioned 7 cm in front of the animal’s head. A positive pressure of approximately 4 × 105 Pa was applied to the tubing system with an air compressor. The timing of the air pulse was controlled by an electrical pulse generator that was connected to a solenoid valve. The flow rate was adjusted to 6.8 ml s−1. The tubing system was interposed by a sample chamber containing a piece of filter paper (30 × 10 mm) impregnated with the odor source (described below). In order to remove the released odor quickly, an exhaust funnel (diameter 30 cm) was placed behind the animal, which generated a headwind toward the animal. The flow rate was adjusted to 0.3 m s−1 at the top of the Styrofoam sphere. Under these conditions, we examined the structure of the odor plumes by using visible titanium tetrachloride smoke. The smoke released from the tip of the tube was dispersed widely before the animal due to the upward air current generated for floating the treadmill sphere. The odor plume, which may exhibit a complex structure, was almost equally received by both the antennae.
In order to prepare the attractive odor source, a filter paper (No. 2; Toyo Roshi, Tokyo, Japan) was placed in a rearing cage (30 × 20 × 30 cm) in which approximately 20 adult virgin females had been maintained for at least 2 weeks. It is known that stimulants impregnated on filter paper can function similarly to a female sex pheromone (Roth and Willis 1952). We confirmed that the adult males mostly exhibited mating-like behavior (Roth and Willis 1952; Barth 1970) in response to the treated filter paper. As the aversive odor source, commercially purchased d-limonene (Sigma-Aldrich) was used. Its effectiveness was also confirmed as cockroaches exhibited apparent aversion to the limonene odor. Filter papers (30 × 10 mm) were simply soaked in undiluted limonene and packed in the sampling chamber.
The movements of both the antennae were simultaneously recorded at 50 frames s−1 by a pair of stereo video cameras (HAS-200R; Ditect, Tokyo, Japan); these cameras were arbitrarily positioned in front of the head. Under the present experimental conditions, the maximum spatial resolution was estimated to be 33 μm pixel−1. A near-UV fluorescent lamp with a peak wavelength of 352 nm (FL20S BLB-A; Toshiba, Japan) was fixed above the cockroach to highlight the fluorescent paint spots on the head capsule and both the antennae. Each trial comprised prestimulation, stimulation, and poststimulation periods of 30, 10, and 30 s, respectively. Each of the prestimulation and poststimulation periods was further divided into three consecutive test periods, each of 10 s duration. Thus, arranged in temporal order, a single trial consisted of the following seven test periods: Before3, Before2, Before1, During, After1, After2, and After3. The image data (3,500 × 2 frames) were stored on a PC as a specific file format of a motion-capturing and 3D analysis program (DippMotion XD; Ditect). After data acquisition, the software detected the five painted spots frame-by-frame and automatically registered their 2D coordinates in each of the paired stereo images. In cases in which the software failed to detect the spots in certain frames, the coordinates of these spots were manually plotted on the images by the experimenter. Transformation of the spots into 3D coordinates was achieved by a direct linear transformation (DTL) algorithm (Abdel-Aziz and Karara 1971) installed in DippMotion XD. The calibration process was conducted by using a specific acrylic cube of a known size (5 × 5 × 5 mm).
The locomotion of the animal was monitored by an optical mouse set at the equator of the treadmill sphere as described previously (Okada and Toh 2006). The graphics of the locomotor trajectory on a PC display were filmed by an additional video camera and stored in the same PC together with the images of the antennal and head movements. The latency of the graphic display was estimated to be 40–60 ms; this latency was negligible in the present analyses.
Data analysis
The antennal position was calculated with reference to the head capsule. A right-handed 3D coordinate system was employed to represent the position of the spot on the flagellum, where the XZ plane was parallel to the plane formed by the three head spots (Fig. 1b). The origin of the coordinate was set at the center of each antennal socket. Since this center could not be marked as a spot, its coordinate was estimated by referring to the three head spots. In a still image, the approximate position of the center of the antennal socket could be manually determined by the experimenter. The distances from the three head spots to the putative origin were measured in 50 sets of still images (a total of 100 images) for each test, and their averages were calculated. Thus, the origin of each antenna was determined frame-by-frame by computing the point that is located at the same distances from the three head spots. The horizontal deflections of the antennae were defined as the azimuth, and the vertical deflections were defined as the latitude of the flagellum with respect to the origin (Fig. 1b). Regarding the horizontal component, the right and left deflections to the head were defined as positive and negative, respectively. Regarding the vertical component, the dorsal and ventral deflections to the head were defined as positive and negative, respectively.
Each of the frame-by-frame displacements of the sphere was divided into two components, the horizontal turn and the forward translation (Fig. 1c, Okada and Toh 2006). Briefly, the anterior edge of the pronotum on the animal’s midline (Fig. 1c, O) was considered as the reference for the horizontal turns. When a particular point on the sphere moved from A to B, point C was established at the intersection between the line parallel to the body axis passing through point B and the arc centered on the reference O. Here, the forward translation corresponds to the line CB, and the horizontal turn corresponds to the angle AOC. The right turn was defined as positive, and the left turn as negative. The spatial resolution achieved was estimated to be 1.5° pixel−1 for a turn and 1.3 mm pixel−1 for a translation. Virtual trajectories projected on a 2D plane were created for each trial by integrating the frame-by-frame vectors.
Differences between the two data groups were examined statistically using the Mann–Whitney U test, the Wilcoxon test, or the Ansari–Bradley test. The results were judged to be significant when P < 0.05. In the cases for multiple comparisons, the level of significance was modified by the Bonferroni correction.
Results
Antennal movements
The recordings were started only when typical searching with active antennal movements and frequent zigzag turns was observed. Immediately after the release of the female-derived odor toward the searching animals, antennal movements were activated in most cases, and the walking speed was decreased. Such behavioral changes were not observed in the control experiments where only an air pulse lacking odor stimuli was used; this clearly indicated that the response was specific to the female-derived odor. Typical examples of the spatial trajectories of the antennae are shown in Fig. 2; these were derived from the three successive test periods—Before1, During, and After1 (sample period, 10 s each). Before the stimulation, the working ranges (differences between the maximum and minimum values) in the horizontal and vertical components were both 83°. The central positions (medians) were located at 19° (horizontal, H) and 41° (vertical, V). During stimulation, the antennae tended to cover wider areas in the horizontal plane, and their vertical positions were entirely shifted downward when compared to those in Before1. The horizontal working range increased to 110°, and the median of the vertical position decreased to 34°. As shown in Fig. 2, some animals exhibited an aftereffect of the stimulation in the horizontal component–the working range remained as wide as 107° even after the cessation of the stimulation (After1).
Spatial trajectories of antennae (left panel) and their distributions (right panel) before, during, and after the stimulation with female-derived odor. These are the examples of the left antenna. In each trajectory, a total of 500 data points (sampling period 10 s) were connected by β-spline curves. The graphs in the right panel show the medians (filled squires), the working ranges (outer small bars) and the 25th and 75th quartiles (inner small bars) for the corresponding test periods. In During, the horizontal working ranges and the quartile deviations widened, and the vertical positions shifted downward, compared with those in Before1. The effect on the horizontal component persisted even after the cessation of the stimulation (After1). The arrows at the top indicate the antennal orientation: M medial; L lateral; D dorsal; V ventral
The medians and quartile deviations were examined as indices of the behavioral changes of the antennae through the whole test period (70 s in total) in eight animals (Fig. 3). Since the spontaneous antennal movement in the prestimulation periods largely varied depending on the specimen and even within a single specimen, the two parameters were given by angular changes from the values in Before1. For the three consecutive test periods (Before1, During, and After1), the data sets (10 s; i.e., 500 points each) were statistically analyzed with reference to Before1 using the Mann–Whitney U test and the Ansari-Bradley test in order to survey the position and the distribution of the antennae, respectively. The horizontal central position (median) was significantly altered during the stimulation period in 7 of 8 cases for the right (R) antennae and in 6 of 8 cases for the left (L) antennae (Mann–Whitney U test with Bonferroni correction, P < 0.025; Fig. 3a). However, the direction of the shift (medial/lateral) was inconsistent among the antennae. A similar tendency was observed immediately after the stimulation period (Fig. 3a, After1). On the other hand, regarding the vertical component, significant differences were detected between Before1 and During in 8 (R) and 6 (L) cases, respectively, and their medians were shifted downward in most (6) cases for both antennae (Fig. 3b, During). This response was maintained in After1 for half (4) the cases for both antennae. The horizontal distribution of the antennal position was significantly changed in 8 (R) and 7 (L) cases (Ansari-Bradley test with Bonferroni correction, P < 0.025; Fig. 3c). Of these significant cases, the quartile deviation was increased in most (6) cases for both antennae. This response lasted until After1 mainly in the left antennae (5 cases) rather than the right antennae (2 cases). On the other hand, the change in the vertical distribution appeared to be inconsistent (Fig. 3d).
Temporal transitions of the median and the quartile deviation of the antennal movement in responses to the attractive female-derived odor. Each trial for 70 s was divided into seven consecutive test periods from Before3 to After3 (each of 10 s duration). Each plot indicates the angular change from the value in the corresponding Before1. The stimulation period (During) is represented by the shaded region. Symbols for eight different animals are indicated on the right; the open and filled symbols represent the right and left antennae, respectively. Asterisks show the significant differences from the corresponding values in Before1. In the medians for the vertical component, the antennae pointed toward a more ventral direction during the attractive stimuli (b, During). The horizontal quartile deviations became wider in response to the stimuli in most cases (c, During)
The aversive odor stimulation with limonene seemingly decreased the antennal movement. In the typical examples shown in Fig. 4, the horizontal and vertical working ranges before limonene stimulation were 105° and 109°, respectively (Before1). The central positions of the horizontal and vertical components were 22° and 32°, respectively. During the stimulation with limonene odor (During), the horizontal working range was decreased to 93°, but the vertical working range was unchanged (109°). This was due to the occasional large amplitude excursions of the antennae. Instead, concerning the working range, the information provided by the quartile deviation is often more reliable. In fact, the quartile deviation of the vertical deflection was decreased from 28° to 14° here. Immediately after the cessation of the aversive stimulation, active oscillation of both antennae resumed. In this example (After1), the distributions of both the horizontal and vertical components tended to be rather wider than those in Before1, up to 128° (H) and 121° (V) in their working ranges, respectively.
Antennal trajectories and their distributions before, during, and after the stimulation with limonene. These examples were obtained from the left antenna. Representations in this figure are the same as those presented in Fig. 2. The aversive odor stimulation instantaneously decreased the antennal activities (compare During with Before1). This inhibitory effect also appeared in the quartile deviations of the horizontal and vertical components. The antennal activities were immediately restored after the cessation of the stimulation (After1)
The temporal transitions of the medians and the quartile deviations in 8 individuals are shown in Fig. 5. The horizontal position was significantly changed by the aversive odor in 5 and 7 cases for the right (R) and left (L) antennae, respectively (Mann–Whitney U test with Bonferroni correction, P < 0.025). Of these significant cases, the antennae shifted to a more medial position in 4 (R) and 5 (L) cases (Fig. 5a, During). In contrast, the vertical position significantly shifted toward a more ventral position during the stimulation in 7 (R) and 6 (L) cases (Fig. 5b). Statistical tests for the horizontal distribution detected significant differences between Before1 and During periods in 6 (R) and 4 (L) cases (Ansari-Bradley test with Bonferroni correction, P < 0.025). However, the mode of the changes (widened/narrowed) was relatively inconsistent (Fig. 5c). For the vertical distribution, the quartile deviation decreased during the stimulation in most cases (7 cases for both antennae). Statistical differences were detected in 5 cases for the right antenna, but only in 3 cases for the left antennae (Fig. 5d, During). The effect of limonene appeared to be exclusively instantaneous, and prolonged antennal responses were rarely observed after the cessation of the stimulation (see Fig. 5a–d, After1).
Temporal transitions of the median and the quartile deviation of the antennal movement in responses to the aversive limonene odor. Representations in this figure are the same with those presented in Fig. 3. The medians for both the horizontal and vertical components were mostly shifted to more medial and ventral positions, respectively (a, b During). The vertical quartile deviations decreased during the aversive odor stimulation in most cases (d During)
Locomotion of the animals
We next examined the effects of the attractive and aversive odor stimulations on the locomotion of the animals. Figure 6a depicts an example of virtual trajectory before, during, and after stimulation with the female-derived odor. The corresponding time courses for both cumulative translation and cumulative turn are shown in Fig. 6b and c, respectively. Before the stimulation, the cockroaches walked at an approximately constant speed with zigzag turns and occasional pauses. On stimulation with the attractive odor, the animals quickly decreased their walking speed by approximately one-half (Fig. 6b). Most animals continued slow walking even after the cessation of the stimuli for several seconds. Thereafter, the original walking speed was gradually regained. The values of the two locomotor parameters, translation and turn angle, are summarized in Fig. 7a, b. Here, the translation was simply cumulated for 10 s in each of the three consecutive test periods (Before1, During, and After1), and the turn angle was presented as an integral of the absolute turn angles for the same period. The translations during and immediately after the stimulation (During and After1) were significantly decreased when compared with those in Before1 (Fig. 7a, Wilcoxon test with Bonferroni correction, P < 0.0167, n = 8). On the other hand, there was no significant difference in the absolute turns between any pairs of the three test periods (Fig. 7b).
Virtual trajectories of the animal’s locomotion before, during, and after stimulation with the attractive (a) and aversive (d) odors, and the corresponding time courses of the cumulative translation (b, e) and the cumulative turn (c, f). Note that the cockroaches always received headwind under this condition. The filled circles in the trajectories are the points at which the recordings were started. The arrows indicate the direction of walking. The shaded areas in the time courses (b, c, e, f) correspond to the stimulation periods. The slope of translation flattened during the stimulation with female-derived odor (b), while it became steeper in the case of limonene stimulation (e); this indicated that the walking speed was increased and decreased by the attractive and aversive stimulations, respectively. Note that a sharp turn was observed at the offset of attractive stimulation (a star) as well as at the onset of aversive stimulation (d star)
Comparisons of two locomotor parameters among the three consecutive test periods (Before1, During, and After1) for attractive or aversive odor stimulation. Vertical columns and bars indicate the medians and the 25th and 75th quartiles, respectively (n = 8). Significantly different pairs are linked and marked with asterisks. a, b The attractive stimulation with female-derived odor significantly decreased the translations in During and After1. c, d The values of translation and absolute turn in During were significantly increased by the aversive odor (limonene) stimulation
The locomotor response in the cockroaches when they were stimulated with the aversive limonene odor was clearly different from that when they were stimulated with the attractive female-derived odor (Fig. 6, Limonene). Immediately after the limonene odor was presented to a searching animal, it began walking faster (Fig. 6d). At the onset of the stimulation, most animals exhibited back steps and sharp turns (Fig. 6d, e). Although Fig. 6d shows that the animal continuously made right turns during the stimulation, other animals exhibited zigzag turns. After the stimulus offset cockroaches rapidly decreased their walking speed to that of the control period (Fig. 6e), and exhibited zigzag turns again (Fig. 6f). The statistical analysis revealed that both the translation and absolute turn in During were significantly increased when compared with those in Before1 and After1 (Wilcoxon test with Bonferroni correction, P < 0.0167, n = 8; Fig. 7c, d).
We also examined the relationships of various pairs of parameters between the locomotor activity and the antennal movement by comparing the correlation coefficients of their time courses (data not shown). Significant correlations between the turn component and the horizontal antennal position were consistently observed under any conditions. In addition, the translation component, i.e., walking velocity seemed to be correlated with the vertical antennal position. However, this correlation was not observed during and after the attractive odor stimuli. The other pairs mostly showed non-significant couplings. Otherwise, consistently significant correlations occasionally appeared in a unilateral manner. There was a general tendency that consistently significant correlations were more frequently observed during the aversive odor stimuli than the attractive stimuli. These results suggest that the antennae in the animals motivated by the attractive odor may behave independently from their locomotor activities, and vice versa for the aversive odor.
Discussion
In the present experiments, an exhaust system was consistently used for the quick delivery and removal of the released odor; this inevitably caused a headwind toward the cockroach. The wind velocity was set at 0.3 m s−1, as measured above the treadmill sphere. It is known that in P. americana a wind at a speed of >0.24 m s−1 may induce a negative anemotaxis with relatively straightforward walking downwind (Bell and Kramer 1979). Although we were unable to determine whether the headwind caused negative anemotaxis in the present open-loop setup, locomotion with vigorous antennal movements, zigzag turns, and intermittent pauses were still observed as the normal searching behavior in most animals. This suggests that natural adaptation to the wind might occur to some extent. In the outdoor environment, the individuals of P. americana appeared to behave naturally under conditions of light and unstable wind at <0.5 m s−1 (Seelinger 1984). Thus, we surmise that the effect of the artificial headwind itself on the searching behavior of the animals was minimal.
The attractive stimulation with female-derived odor widened the horizontal deflection of both the antennae and simultaneously caused a downward shift of their vertical positions. These changes in the antennal movements would be regarded as a simple searching for mates located at the immediate vicinity in the same plane. However, our findings are somewhat different from the results of a previous study. Rust et al. (1976) reported that the antennae of adult male cockroaches (P. americana) exhibited outward and upward shifts as responses to both sex and aggregation pheromones. In this study, the authors simply exposed the animal to air that putatively contained female-derived sex pheromone and did not use an exhaust apparatus or a wind tunnel. The discrepancy in the results might therefore be due to a difference in the experimental design.
The walking speed was remarkably decreased during stimulation with the female-derived odor. This response could be interpreted as a careful local search for useful resources. In general, the locomotion of a relatively quiescent male is activated on perceiving a female sex pheromone; however, the motivated males decrease their walking speed on approaching the pheromone source (Bell and Tobin 1981; Willis and Avondet 2005) or under turbulent wind conditions (Willis and Avondet 2005). Therefore, decreasing the walking speed may contribute to a reliable final approach toward useful resources or the determination of the appropriate direction in which to steer under unstable wind conditions.
On the cessation of the attractive stimulation, most animals took a sharp turn. Similar turns of male cockroaches were described at the loss of a sex pheromone or on encountering a boundary between clean air and a pheromone plume; this response was interpreted as a preprogrammed response (Tobin 1981; Willis and Avondet 2005). After the loss of the attractive odor, the aftereffect was relatively more pronounced in locomotion than in the antennal movement—the translation (i.e., walking speed) remained slow over 10 s after the cessation of the stimulation (Fig. 7). This could be considered as a type of context-dependent local ranging designed to retrieve lost resources.
The aversive odor stimulation with limonene appeared to decrease the vertical movement of the antenna, although statistical significance could not be determined in the majority of cases for the left antennae, probably because of the small sample size. It also remarkably increased the walking speed to approximately twice that of the control period. Limonene, a monocyclic monoterpenoid, is a major essential oil from citrus peel and is generally a toxin or a repellent for insects (Coats et al. 1991). The response to limonene may be a type of simple avoidance behavior to an aversive stimulus source such as a wind-induced escape response (Camhi 1980). In fact, the antennae of an escaping cockroach are almost always fixed and pointed forward (our personal observation). This is consistent with the present observation that animals exhibited medial and ventral shifts of the antennal position during aversive stimuli (Fig. 5a, b). Sharp turns together with back steps were often observed at the onset of limonene stimulation. Although the cockroaches always had to face upwind in the present open-loop system, it would be safe for them to steer downwind in order to escape from a repellent odor under such circumstances. This could explain the significant increase in the absolute turn during the stimulation period (Fig. 7d).
Immediately after the cessation of limonene stimulation, the cockroaches rapidly restored their walking speed to that of the control period and resumed vigorous oscillation of the antennae. The response was basically instantaneous, and the aftereffects were rarely recognized; this behavior was quite different to that observed in the case of stimulation with the attractive female-derived odor. It is likely that cockroaches incur minimal costs in avoidance of repellent odors.
It is known that the horizontal antennal position corresponds with the turn direction of the body in walking cockroaches (McCoy 1985; Okada et al. 2002) and crickets (Horseman et al. 1997), as well as in crustaceans (Zeil et al. 1985). In the present study, we examined the relationships of various pairs of parameters between the antennal movement and the locomotor activity. Consistent correlations were detected in the pair of the horizontal antennal position and the turn component under any conditions as predicted. However, besides this pair, the antennae in the animals motivated by the attractive odor may behave rather independently from their locomotor activities, and vice versa for the aversive odor. This hypothesis will be tested in further behavioral studies.
This study demonstrated that there are clear differences in both the antennal movement and locomotion of animals in responses to two qualitatively opposite types of odor. The results obtained may indicate a simple procedure in which cockroaches appropriately alter the searching style depending on the quality of olfactory information. In order to generalize this hypothesis, further behavioral analyses of the responses to a variety of attractive and aversive odor stimulants, which take into consideration both their chemical components and quantitative aspects, will be required.
References
Abdel-Aziz YA, Karara HM (1971) Direct linear transformation from comparator co-ordinates into object space coordinates. In: Proceedings of the symposium on close-range photogrammetry. American Society of Photogrammetry, Falls Church, pp 1–18
Baker TC (1985) Chemical control of behavior. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol. 9 Pergamon, Oxford, pp 621–672
Barth RH (1970) The mating behavior of Periplaneta americana (Linnaeus) and Blatta orientalis Linnaeus (Blattaria, Blattinae), with notes on three additional species of Periplaneta and interspecific action of female sex pheromone. Z Tierpsychol 27:722–748
Bell WJ (1981) Pheromones and behaviour. In: Bell WJ, Adiyodi KG (eds) The American cockroach. Chapman & Hall, London, pp 371–397
Bell WJ (1991) Searching behavior: the behavioural ecology of finding resources. Chapman & Hall, London
Bell WJ, Kramer E (1979) Search and anemotactic orientation of cockroaches. J Insect Physiol 25:631–640
Bell WJ, Tobin TR (1981) Orientation to sex pheromone in the American cockroach: analysis of chemo-orientation mechanisms. J Insect Physiol 27:501–508
Bell WJ, Cardé RT (1984) Chemical ecology of insects. Chapman & Hall, London
Camhi JM (1980) The escape system of the cockroach. Sci Am 243:158–172
Coats JR, Karr LL, Drewes CD (1991) Toxicity and neurotoxic effects of monoterpenoids in insects and earthworms. ACS Symp Ser 449:305–316
Erber J, Pribbenow B, Grandy K, Kierzek S (1997) Tactile motor learning in the antennal system of the honeybee (Apis mellifera L). J Comp Physiol A 181:355–365
Erber J, Kierzek S, Sander E, Grandy K (1998) Tactile learning in the honeybee. J Comp Physiol A 183:737–744
Horseman BG, Gebhardt MJ, Honegger HW (1997) Involvement of the suboesophargeal and thoracic ganglia in the control of antennal movements in crickets. J Comp Physiol A 181:195–204
McCoy MM (1985) Antennal movements of the American cockroach Periplaneta americana. PhD thesis, University of Kansas
Nishiyama K, Okada J, Toh Y (2005) Antennal movement during odor-source searching in the American cockroach. Zool Sci 22:1475 (Abstr)
Okada J, Kanamaru Y, Toh Y (2002) Mechanosensory control of antennal movement by scapal hair plates in the American cockroach. Zool Sci 19:1201–1210
Okada J, Toh Y (2000) The role antennal hair plates in object-guided tactile orientation of the cockroach (Periplaneta americana). J Comp Physiol A 186:849–857
Okada J, Toh Y (2004) Spatio-temporal patterns of antennal movements in the searching cockroach. J Exp Biol 207:3693–3706
Okada J, Toh Y (2006) Active tactile sensing for localization of objects by the cockroach antenna. J Comp Physiol A 192:715–726
Olberg RM (1983) Pheromone-triggered flip-flopping interneurons in the ventral nerve cord of the silkworm moth, Bombyx mori. J Comp Physiol A 152:297–307
Roth LM, Willis ER (1952) A study of cockroach behavior. Am Midland Nat 47:66–129
Rust MK, Burk T, Bell WJ (1976) Pheromone-stimulated locomotory and orientation responses in the American cockroach. Anim Behav 24:52–67
Scheiner R, Schnitt S, Erber J (2005) The functions of antennal mechanoreceptors and antennal joints in tactile discrimination of the honeybee (Apis mellifera). J Comp Physiol A 191:857–864
Seelinger G (1984) Sex-specific activity patterns in Periplaneta americana and their relation to mate-finding. Z Tierpsychol 65:309–326
Staudacher E, Gebhardt MJ, Dürr V (2005) Antennal movements and mechanoreception: neurobiology of active tactile sensors. Adv Insect Physiol 32:49–205
Tobin TR (1981) Pheromone orientation: role of internal control mechanism. Science 214:1147–1149
Willis MA, Avondet JL (2005) Odor-modulated orientation in walking male cockroaches Periplaneta americana, and the effect of odor plumes of different structure. J Exp Biol 208:721–735
Zeil J, Sandeman R, Sandeman DC (1985) Tactile localization: the function of active antennal movements in the crayfish Cherax destructor. J Comp Physiol A 157:607–617
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (C) from JSPS (17570063) and the Yamada Science Foundation to JO.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishiyama, K., Okada, J. & Toh, Y. Antennal and locomotor responses to attractive and aversive odors in the searching cockroach. J Comp Physiol A 193, 963–971 (2007). https://doi.org/10.1007/s00359-007-0249-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-007-0249-3